Abstract
Previous research has shown a relationship between impulsive personality and the subjective and reinforcing effects of d-amphetamine. Impulsive personality, however, is comprised of multiple dimensions. The association between different dimensions of impulsive personality and the subjective and reinforcing effects of d-amphetamine is unknown. The objective of this study was to assess the independent contributions of the “sensation-seeking” and “impulsivity” dimensions of the impulsive sensation-seeking subscale of the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ) to the subjective and reinforcing effects of d-amphetamine. Forty healthy emerging adults varying in scores on the sensation-seeking and impulsivity dimensions of the ZKPQ participated in a double-blind, placebo-controlled, randomized study comprised of four two-day blocks. Each two-day block consisted of a sample day and self-administration day. Subjective effects and physiological measurements were taken prior to, and hourly for 3 h following, dose administration. On sample days participants were given eight capsules containing 0, 1, or 2 mg d-amphetamine. On self-administration days participants were able to earn capsules containing the same dose of d-amphetamine that was administered on the previous sample day by responding on a Modified Progressive Ratio Task. The “sensation-seeking” dimension was positively associated with drug taking on the Modified Progressive Ratio Task, subjective effects (e.g. ‘good effect’), and heart rate. However, no clear relationship between the “impulsivity” dimension and outcome measures was observed. In conclusion, these data suggest that the narrow sensation-seeking dimension of impulsive sensation-seeking is associated with initial drug liking and drug taking behavior and may be a key predictor of drug use initiation.
Keywords: sensation-seeking, subjective effects, reinforcement, self-administration
Introduction
Impulsivity has been broadly described as “actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation that often result in undesirable outcomes” (Evenden, 1999). Impulsivity is typically conceptualized as a multi-dimensional construct, although there is no consensus on the underlying factor structure. Whiteside and Lynam (2001) have suggested, based on factor analysis of existing personality measures of impulsivity, that the construct may contain four dimensions: urgency, lack of premeditation, lack of perseverance, and sensation-seeking. Alternatively, Gullo, Loxton, and Dawe (2014) suggest that, based on underlying neurobiology, impulsivity is best conceptualized as having two factors: one factor associated with “incentive salience arising from the limbic ‘impulsive’ system and another factor associated with impaired response inhibition arising from the prefrontal ‘executive’ system.” This two-factor conceptualization is also consistent with other two-factor personality models (e.g., Elliot, 2006; Pickering & Gray, 1999; Zuckerman et al., 1993).
Personality questionnaire measures of impulsivity have been associated with multiple risky behaviors, including sexual risk taking (Hoyle, Feifar, & Miller, 2000), gambling (Chambers & Potenza, 2003), risky driving (Jonah, 1997), and drug use (de Wit, 2009). In regards to drug use specifically, multiple different measures of impulsive personality have been associated with different progressive stages of drug use, including initiation, escalation, and the onset of compulsive drug use (e.g. Verdejo-Garcia, Lawrence, & Clark, 2008). For example, more impulsive individuals (as measured by the Barratt Impulsivity Scale) initiate alcohol use at a younger age than less impulsive individuals (von Diemen, Bassani, Fuchs, Szobot, & Pechansky, 2008), and those who use drugs recreationally but do not meet criteria for a drug use disorder have higher impulsive personality questionnaire scores (as measured by the Barratt Impulsivity Scale and the Sensation Seeking Scale Form V) compared to non-drug users (Moreno et al., 2012). Pedersen (1991) followed a sample of 553 individuals between the ages of 16-18 years for 20 months and found that impulsive personality (as measured by the Sensation Seeking Scale Form V) predicted drug and alcohol use behaviors at the end of the follow-up period. Multiple studies have confirmed that individuals who meet criteria for a drug use disorder have higher impulsive personality questionnaire scores, assessed by multiple measures, compared to individuals who do not meet criteria for a drug use disorder (for review see de Wit, 2009). Furthermore, the efficacy of prevention interventions for health behaviors has been enhanced by targeting and tailoring for individual differences in impulsive personality (as measured by the Sensation Seeking/Impulsive Decision Making scale) (Zimmerman et al., 2007). To date, however, little attention has been given to whether dimensions of impulsive personality may be differentially associated with stages of drug use. Identification of which impulsive personality factors are most closely associated with initiation, escalation and/or compulsive drug use could support refined tailoring of prevention and treatment efforts and thereby further enhance intervention efficacy.
When investigating dimensions of impulsive personality associated with different stages of drug use, an important consideration is whether processes that might underlie drug use vary by stage of drug use. For example, impulsive individuals may initiate drug use because of increased likelihood to engage in novel behaviors more generally, reduced sensitivity to potential adverse consequences of drug use, or enhanced sensitivity to drug reinforcement. One way to study the processes underlying initial and early drug use, and particularly sensitivity to reinforcement, is to examine drug taking in a laboratory setting. Sensitivity to drug reinforcement predicts the likelihood of developing drug use problems (Jaffe & Jaffe, 1989; Fischman & Foltin, 1991). In addition, clinical laboratory studies have indicated that individuals scoring high on measures of impulsive personality (e.g. Sensation Seeking Scale Form V, Impulsive Sensation Seeking subscale of the Zuckerman-Kuhlman Personality Questionnaire, and Multidimensional Personality Questionnaire Brief Form) are more sensitive to the reinforcing and subjective effects of drugs (de Wit, Uhlenhuth, Pierri, & Johanson, 1987; Stoops et al., 2007; Kelly et al., 2006; Fillmore, Ostling, Martin, & Kelly, 2009, Kirkpatrick, Johanson, & de Wit, 2013). Together, these results suggest that drug use may be more likely among individuals high in impulsive personality due, in part, to enhanced sensitivity to drug reinforcement.
Although impulsive personality has been associated with drug taking in a laboratory setting, there is still ambiguity as to what dimensions are most closely associated with the reinforcing and subjective effects of drugs. In previous laboratory studies (i.e., Fillmore et al. 2009; Kelly et al. 2006, 2009; Stoops et al. 2007), impulsive personality was measured using the two-dimension impulsive-sensation-seeking subscale (ImpSS) of the ZKPQ (Zuckerman, Eysenck, & Eysenck, 1993). That subscale includes “SS” items associated with the sensation-seeking dimension of impulsive personality, which is highly correlated with the SS dimension in the factor-analytic derived UPPS Impulsive Behavior Scale (Whiteside & Lynam, 2001) and the limbic or reinforcing ‘impulsive’ system in the two-factor model (Gullo et al., 2014), as well as “Imp” items associated with the inhibitory prefrontal ‘executive’ system in the two-factor model of Gullo et al. (2014). Thus, it is unclear from previous research whether the Imp or SS dimensions of the ZKPQ may be differentially predictive of drug taking and subjective effects of drugs in a laboratory setting. Gaining a better understanding of how these two dimensions relate to laboratory measures of drug self-administration would provide important information for tailoring future prevention interventions.
The current study was conducted to determine the relative contribution of sensation-seeking (SS) and impulsivity (Imp) items from the ImpSS subscale of the ZKPQ on measures of drug abuse vulnerability (i.e. drug taking behavior and subjective effects using a progressive ratio self-administration procedure) by administering d-amphetamine (a prototypical stimulant with abuse liability) to healthy emerging adults with minimal stimulant use history in a research laboratory setting. Based on previous research (e.g. Fillmore et al. 2009; Kelly et al. 2006, 2009; Stoops et al. 2007) indicating that the overall ImpSS subscale of the ZKPQ is positively correlated with drug taking behavior and subjective effects, it was hypothesized that SS or Imp would be positively associated with drug taking behavior and subjective drug effects.
Methods
Participants
Volunteers responding to online advertisements and flyers placed in the local community completed online questions about general health status and a 19-item Likert-scale variation (item scores of 0 to 4) of the Impulsive Sensation-seeking (ImpSS) subscale from the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ; Zuckerman et al. 1993; McDaniel & Mahan, 2008). On the basis of population- and gender-based median scores on the ImpSS, male and female participants were recruited from four recruitment strata: low Imp/low SS, high Imp/low SS, low Imp/high SS, and high Imp/high SS. Recruitment continued until 10 healthy adults (ages 18–31) from each of the four strata completed the study (N=40, 18 females). This recruitment approach resulted in SS and Imp scores that were both continuously distributed and independent in the study sample, allowing Imp and SS to be examined as independent continuous variables.
Individuals who met study eligibility criteria based on this initial screen were invited to participate in an orientation and medical screening session. Medical screening involved completion of a battery of medical and psychological assessments including the Structured Clinical Interview for DSM-IV (SCID), the UPPS (Whiteside, Lynam, Miller, & Reynolds, 2005), the Beck depression Inventory (Beck, Steer, & Carbin, 1988), a drug-use history questionnaire, and blood chemistry, liver function, and urinalysis tests. Individuals were excluded if they reported any contraindication to the use of amphetamines (e.g. cardiac abnormalities, coronary artery disease), history of significant medical illness (e.g., cardiovascular disease, neurological or psychiatric disorder), current regular use of drugs other than caffeine or alcohol, past week stimulant use (excluding caffeine), past or present diagnosis of substance abuse or dependence, were currently pregnant or breastfeeding, or had any other condition that would increase medical risk for study participation. Each participant's information was examined and approved by the study physician (CA Martin) before beginning study procedures. A total of forty-three volunteers provided informed consent and initiated the study; two of these individuals were dropped from the study due to scheduling conflicts, and one participant dropped because they could not swallow the drug capsules. Data from these three participants were not included in the analyses. All study procedures were approved by the University of Kentucky's Institutional Review Board.
Design
A double-blind, placebo-controlled, randomized design was used to examine the behavioral effects of d-amphetamine between participants varying along the personality dimensions SS and Imp. The study was comprised of four two-day blocks, including one two-day practice block. On the first day of each two-day block (sample day), the behavioral effects of oral d-amphetamine (0, 8, 16 mg) were assessed. On the second day of each two-day block (self-administration day), the reinforcing effects of the dose administered in the previous session were determined using the Modified Progressive Ratio Task, as described below.
Schedule
Participants were instructed to abstain from solid food and caffeine for four h prior to testing each day and were provided with a standard fat- and caffeine-free snack 30 minutes after arrival. Each test day lasted approximately five h. At the start of each day participants answered open-ended questions regarding sleep, medication use, food intake and health status during the preceding 24 h and completed breath (Alcohol Sensor III, Intoximeters, Inc), and urine tests (OnTrak TestStik, Varian, Inc.; Clearview HCG II, Unipath, Ltd) to assess recent cocaine, benzodiazepine, barbiturate, marijuana, amphetamine, and opiate use and pregnancy status. No drug use or pregnancy was detected during the study.
Sample Days
On sample days participants completed baseline assessments 45 minutes after arrival (immediately preceding drug administration), and one, two, and three h after consumption of eight capsules, verified by staff observation. Participants were told to pay careful attention to the drug effects because they would be able to earn capsules containing the same dose on the next session (self-administration day).
Self-administration Days
Typically 24 h after the sample day, participants completed a self-administration day. On self-administration days participants were able to respond on a Modified Progressive Ratio Task (described below) to earn capsules of the same dose administered during the previous sample day. Participants were also informed that regardless of their performance on the Modified Progressive Ratio Task they would have to wait 30 minutes until they received the study drug they had earned. Worth noting is that additional time was provided if participants were actively performing the task after 30 minutes had passed. Participants were given the capsules earned that day immediately after completing the Modified Progressive Ratio Task. As during sample days, participants completed assessment measures preceding drug administration (baseline) and 1, 2 and 3 h after drug consumption. Participants were required to complete full testing sessions if they did not choose to receive any drug that day.
Modified Progressive Ratio Task
Participants were required to click on a computer mouse a progressively larger and predetermined number of times to earn capsules. To earn the first capsule, participants had to respond 50 times, and the number of responses required to earn each subsequent capsule doubled (i.e., 100, 200, 400, 800, 1600, 3200 and 6400 responses). To earn all eight capsules, participants had to respond a total of 12,750 times. The task ended if the participant failed to emit a response after two-minutes. The total number of capsules earned was administered after 30-minutes or upon task completion.
Study Drug
Study drug doses were prepared by the University of Kentucky Investigational Pharmacy in size 00 opaque capsules. Doses were evenly divided into 8 capsules such that on 0, 8 and 16 mg dose days, each capsule contained 0 mg, 1 mg, and 2 mg, respectively. In an initial 2-day practice block, which served to orient participants to study procedures and tasks, placebo capsules (0 mg) were administered. Participants were required to earn 8 capsules on the Modified Progressive Ratio Task during the first self-administration day. Data from the initial two-day practice session were collected but not analyzed. During the final three two-day blocks, doses (0, 8, 16 mg) were tested in random order, balanced across recruitment strata.
Assessments
Assessments typically lasted for 30 minutes and consisted of cognitive and psychomotor performance tasks, visual analog scale ratings of drug effect and heart rate and blood pressure.
Performance Tasks
Participants completed a 3-minute Digit-Symbol Substitution Task (McLeod, Griffiths, Bigelow, & Yingling, 1982), a 250 trial Cued Go/No Go Task that lasted approximately 15-min (Marczinski & Fillmore, 2003) and a 168 trial N-Back Task that lasted approximately 8-min (Gevins & Cutillo, 1993). Task performance data are not included in the primary analyses.
Visual Analog Scales (VAS)
Participants were asked to rate drug effects (i.e., “any effect”, “bad effects”, “good effects”, “high”, “anxious”, “like drug”, “willing to take again”, “my performance is impaired by the drug,” and “my performance is improved by the drug”) by placing a mark on a 100-unit line with the left endpoint labeled “Not at all” and the right endpoint labeled “Extremely”.
Stimulant-Sensitive Items from the Adjective-Rating Scale (ARS)
Participants completed the adjective-rating scale (Oliveto et al., 1992). Two component scores were analyzed: ‘sedated’ (a composite of ARS items related to sedation) and ‘stimulated’ (a composite of ARS items related to stimulation).
Cardiovascular Measures
Heart rate and blood pressure (oscillometric) were recorded using an automated blood pressure monitor (Dinamap Pro 200, General Electric).
Data Analysis
While four strata (i.e., low Imp/low SS, high Imp/low SS, low Imp/high SS, and high Imp/high SS) were used to recruit participants, the resulting SS and Imp scores were continuously distributed across participants. To enhance statistical sensitivity, SS and Imp were converted to z-scores and analyzed as continuous between-subject variables in all analyses (see participant distributions in Figures 2-4). Mixed models were fitted for data from self-administration (Modified Progressive Ratio Task only) and sample days (VAS, ARS, and cardiovascular measures). Self-administration variables from the Modified Progressive Ratio Task were analyzed with dose (placebo, 8mg, and 16 mg) as a within-subject factor and participant as a random factor. Data from measures used on sample days (VAS, ARS, and cardiovascular measures) were analyzed using dose and time (baseline, 1, 2, and 3 h post dose) as within-subject factors and participant, participant by dose, and participant by time as random factors. Main and interaction effects were estimated in all mixed models. Post-hoc analyses were conducted when significant effects of SS or Imp by dose by time were indicated (SS or Imp by dose on the Modified Progressive Ratio Task). Post-hoc analyses were comprised of t-tests between estimations of the independent variable in question (i.e. each level of dose, or each point in time) provided by the overall mixed model. Analyses were conducted with the SAS statistical software package, version 9.3 (SAS Institute Inc., Cary, NC). For clarity, F values and significance (p) are provided in the tables. Significance threshold for all analyses was set at p ≤ 0.05.
Figure 2.
Change in number of capsules earned compared to placebo. Impulsivity and sensation-seeking z-scores and their association with change in capsules earned relative to placebo on the Modified Progressive Ratio Task (lines of best fit).
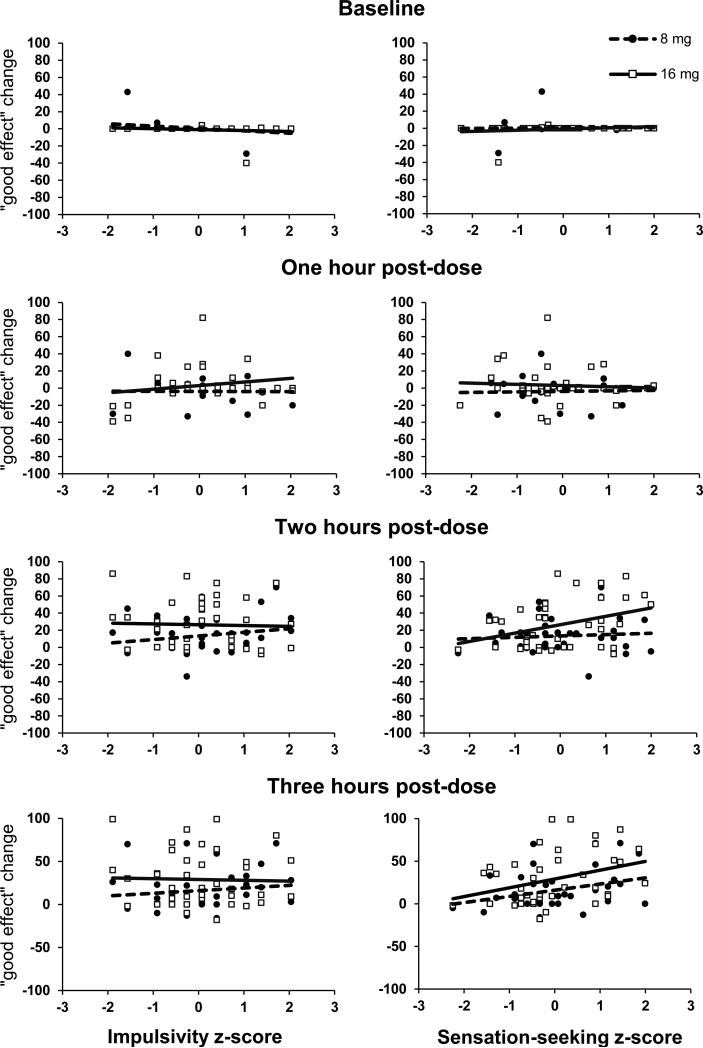
Figure 4.
“Good effect” by session and personality. Association between impulsivity and sensation-seeking z-scores and change in report of “Good Effect” on a 100-point visual analog scale compared to placebo in accordance with time and dose.
Results
Participant Characteristics
Participants were predominantly male (N=22, 55%) and Caucasian (N=36, 90%), had an average age of 22.4 (SD= 3.4) years, and averaged 15.2 (SD=2.2) years of education. Significant correlations were found between SS from the ImpSS and SS on the UPPS, and between Imp on the ImpSS and the UPPS Urgency, Premeditation (lack of) and Perseverance (lack of) factors. No significant correlations between SS or Imp and caffeine, alcohol, or marijuana consumption were observed (Table 1). Thirty-three participants reported no prior use of stimulants other than caffeine (e.g. methylphenidate, amphetamine, cocaine). The other seven participants reported lifetime use of a noncaffeine stimulant, with one reporting use of amphetamine in the past month.
Table 1.
Relationship between sensation-seeking, impulsivity, the UPPS scale, and drug use
| Total N = 40 | Correlation with SS (r) | Correlation with Imp (r) | |
|---|---|---|---|
| UPPS Scale | |||
| Urgency, (mean, SD) | 2.0 (.5) | .05 | .48** |
| Premeditation (lack of), (mean, SD) | 2.2 (.6) | .26 | .52*** |
| Perseverance (lack of), (mean, SD) | 1.9 (.5) | .21 | .59*** |
| Sensation-seeking, (mean, SD) | 3.0 (.5) | .45** | .13 |
| Caffeine, mg/day (mean, SD) | 66.63 ± 100.42 | .12 | −.11 |
| Alcohol, drinks/week (mean, SD) | 2.57 ± 2.76 | .26 | .10 |
| Marijuana, times/month (mean, SD) | .11 ± .36 | .15 | .04 |
| Impulsivity/Sensation-seeking Scale | |||
| Impulsivity (mean, SD) | 25.38 ± 7.32 | ||
| Sensation-seeking (mean, SD) | 9.78 ± 3.05 | ||
SS= sensation-seeking; Imp= Impulsivity
p≤.01
p≤.001
d-Amphetamine Effects
Prototypical stimulant effects of d-amphetamine (indicated by significant dose and dose x time relationships) were apparent on self-report and physiological variables (Table 2). As anticipated, dose-related increases were observed on ratings of ‘Any Effect,’ ‘Good Effect,’ ‘High,’ ‘Like Drug’ and ‘Take Again.’ Effects of the study drug were especially pronounced on “Any Effect” and “Good Effect” (Figure 1). Significant effects of d-amphetamine were found for the sedated (dose-related decreases) and stimulated (dose-related increases) scales of the ARS. Significant increases in heart rate (Figure 1) and blood pressure were also observed.
Table 2.
Subjective effects by dose, session, and personality
| VAS Variables | SS | Imp | Dose | SS × Dose | Imp × Dose | Dose × Time | SS × Dose × Time | Imp × Dose × Time |
|---|---|---|---|---|---|---|---|---|
| Visual Analog Scale | ||||||||
| Anxious | 1.86 | .55 | 5.97** | .17 | .13 | 1.45 | .46 | .50 |
| Any Effect | .86 | 1.31 | 23.98*** | 1.31 | .68 | 18.37*** | 2.54* | 2.78* |
| Bad Effect | .46 | 4.23* | 2.95† | .44 | .02 | 3.04** | .92 | .51 |
| Good Effect | .25 | .76 | 17.80*** | 2.68† | .43 | 13.39*** | 4.14*** | 3.16** |
| High | .57 | .66 | 8.25*** | 1.14 | .15 | 4.73*** | 1.04 | .22 |
| Impaired | .01 | .44 | 2.02 | .18 | .78 | 2.57* | 1.36 | 1.18 |
| Improved | .15 | .20 | 17.92*** | .92 | .28 | 6.76*** | 1.82 | .96 |
| Like Drug | 6.03* | 7.01** | 20.98*** | 3.02† | .44 | 3.80** | .81 | 1.13 |
| Take Again | 2.70 | 2.35 | 6.95** | 1.81 | .10 | 3.30** | 1.33 | .63 |
| Adjective Rating Scale | ||||||||
| Stimulated | .24 | .25 | 12.79*** | .99 | .87 | 11.89*** | 1.44 | 1.54 |
| Sedated | .01 | .58 | 11.27*** | .17 | .04 | 4.42*** | .63 | 1.08 |
| Modified Progressive Ratio Task | ||||||||
| Capsules Earned | .13 | .62 | 1.17 | 5.27** | 1.45 | -- | -- | -- |
| Cardiovascular Measures | ||||||||
| Heart Rate | .06 | .01 | 14.14*** | 1.34 | .21 | 11.03*** | 3.01** | .91 |
| Diastolic Blood Pressure | 1.23 | .60 | 12.88*** | .39 | 1.31 | 3.84** | 1.48 | .26 |
| Systolic Blood Pressure | .62 | .12 | 28.17*** | 2.5 | 1.51 | 4.46*** | 1.76 | .95 |
All values displayed are F values. VAS= Visual Analog Scale, SS=sensation-seeking, Imp=impulsivity
p≤10
p≤.05
p≤.01
p≤.001
Figure 1.
Mean profiles over time by drug dose. Effects of d-amphetamine on subjective ratings of ‘Any Effect’ and ‘Good Effect’ on a 100-point visual analog scale and heart rate in accordance with dose and time.
Personality by d-Amphetamine Effects
Sensation-seeking status was significantly related to the number of capsules earned [F(2,72)=5.27, p=.007] on the Modified Progressive Ratio Task. Placebo capsules earned were negatively associated with SS (b = −.66). The number of 1 mg capsules earned was positively associated with SS (b = .62), and this slope was marginally different from placebo [t(72)=1.94, p=.06]). The number of 2 mg capsules earned was also positively associated with SS (b=1.04), and this slope was significantly different from placebo (t(72)=3.23, p=.002). Imp scores were not significantly associated with capsules earned on the Modified Progressive Ratio Task. To display these effects graphically, changes from baseline capsules earned for both active doses with associated lines of best fit are presented in Figure 2.
Significant three-way interactions were found between dose, time, and both SS and Imp status on ‘Any Effect’ and ‘Good Effect,’ and SS on heart rate; slopes from the associated regression models are presented in Table 3. As expected, slopes were close to zero under placebo conditions. During the period of peak drug effects (2 or 3 h after dose administration) positive slopes were observed between SS and ratings of ‘Any Effect’ and ‘Good Effect’ at the 8 mg and 16 mg dose conditions; in contrast, negative slopes were observed between Imp and ‘Any Effect’ and ‘Good Effect’ ratings at the 16 mg dose 2 and 3 h post dose. In order to more clearly display these relationships, scatter plots of individual participant ratings (change from placebo) are presented with lines of best fit. For both ‘Any Effect’ (Figure 3) and ‘Good Effect’ (Figure 4), 2 and 3 h post-dose, change scores were positively associated with SS two and three h after 8 and 16 mg dose administration. In contrast, two and three h following the 8mg dose there were slight positive relationships between changes from placebo on Good Effect and Any Effect ratings and Imp scores, while two and three h following the 16mg dose there were negative relationships between changes from placebo on Good Effect and Any Effect ratings and Imp scores. A significant three-way interaction between SS, dose, and time was also observed on heart rate. As expected, slopes were close to zero under placebo conditions. During the period of peak drug effect (i.e., 3 h after the 8 mg dose, 2 and 3 h after the 16 mg dose) positive slopes were observed between SS and heart rate, while Imp was not significantly associated with heart rate, dose, and time (Table 3).
Table 3.
Dose × time × personality slopes
| Placebo | 8 mg | 16 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 hour | 2 hours | 3 hours | Baseline | 1 hour | 2 hours | 3 hours | Baseline | 1 hour | 2 hours | 3 hours | |
| Variable | ||||||||||||
| Any Effect × | ||||||||||||
| Sensation-seeking | −.74 | −3.00 | .70 | .99 | .30 | −3.53 | .34 | 8.76 | −.03 | −4.72 | 9.40 | 10.35 |
| Impulsivity | .44 | .64 | −4.08 | −3.73 | −.25 | 1.26 | 1.48 | −4.17 | .29 | 3.93 | −11.28 | −6.90 |
| Good Effect × | ||||||||||||
| Sensation-seeking | −2.36 | −2.98 | −.77 | −.91 | −.82 | −1.67 | −1.11 | 6.29 | −.29 | −6.30 | 11.42 | 11.75 |
| Impulsivity | 2.36 | −1.00 | −3.24 | −2.48 | −1.14 | −1.49 | .96 | 2.25 | .43 | 4.74 | −9.12 | −8.50 |
| Heart Rate × | ||||||||||||
| Sensation-seeking | −.65 | .65 | −1.45 | −.27 | .75 | −3.54 | .42 | 2.29 | −.31 | −1.67 | 2.89 | 2.10 |
| Impulsivity | 1.29 | −.63 | 1.11 | −.52 | −1.43 | 1.06 | .05 | −.20 | −.64 | −1.01 | .89 | 1.37 |
All values displayed are b values. Bold values indicate a significant dose × time × personality interaction.
Figure 3.
“Any Effect” by session and personality. Association between impulsivity and sensation-seeking z-scores and change in report of “Any Effect” on a 100-point visual analog scale compared to placebo in accordance with time and dose.
Discussion
Consistent with our previous clinical laboratory studies (i.e., Kelly et al., 2006, 2009; Stoops et al., 2007), the results of the current study demonstrate that individual differences in ZKPQ impulsive sensation-seeking scores are associated with increased sensitivity to the reinforcing effects (i.e., capsules earned on a progressive ratio task) and subjective (i.e., ‘Good Effect’) effects of d-amphetamine. These data extend the results of previous studies by demonstrating that this association is most closely associated with the SS dimension of the impulsive sensation-seeking scale and not related to the Imp dimension. These results suggest that the biologically based trait of impulsive personality, and particularly the more narrowly focused dimension of SS, confers increased abuse liability for initial amphetamine use, and potentially greater likelihood to continue amphetamine use among healthy emerging adults.
Following administration of 8 or 16 mg d-amphetamine, consistent increases in physiological measures (i.e. heart rate and blood pressure) and subjective effects (e.g. ‘Good Effect’ or ‘Any Effect,’ and stimulated subscale items on the adjective rating scale) were observed. These changes are commensurate with the stimulant effects of d-amphetamine reported in previous studies (e.g. Brauer & de Wit, 1996; Kelly et al., 2006). Furthermore, these effects were dose-dependent (shown in Figure 1), with greater increases in physiological and subjective reports of drug effects following 16 mg compared to 8 mg dosages. This verifies that behaviorally active doses of d-amphetamine, engendering prototypical stimulant effects, were tested in the current study.
It was hypothesized that SS or Imp would be positively correlated with the reinforcing and positive subjective effects of d-amphetamine. This hypothesis was based on previous research demonstrating that when SS and Imp are treated as a single dimension they are positively correlated with the positive abuse-related effects of alcohol (subjective effects, Fillmore et al., 2009) and d-amphetamine (subjective effects, Kelly et al., 2006; reinforcing and subjective effects, Stoops et al., 2007). The current study examined the separate effects of SS and Imp, and found that SS, but not Imp, was associated with the abuse-related behavioral effects of d-amphetamine. Extending the current literature, this study suggests that the SS dimension of impulsive personality may contribute to individual differences in sensitivity to the reinforcing effects of stimulant drugs. Since initial experiences with drugs of abuse have been shown to be predictive of continued drug use (Haertzen, Kocher, & Miyasato, 1983), and experiences with one type of stimulant may generalize to experiences with other types of stimulants (e.g., Sevak, Stoops, Hays, & Rush, 2009), individuals high in SS might be more likely to continue using stimulants of abuse following an initial experience. Commensurate with this idea, other research has suggested that SS is associated with early drug use, evidenced by increased self-reported cigarette, alcohol, and marijuana use among early- and mid-adolescents high in SS (e.g., Martin et al., 2002).
Neurobiological evidence suggests a two-factor model of impulsivity; a sensation-seeking factor associated with “incentive salience arising from the limbic ‘impulsive’ system,” and another factor comprised of impulsivity dimensions other than SS (e.g. the Imp factor from the current study) associated with “impaired response inhibition arising from the prefrontal ‘executive’ system” (Gullo et al., 2014). These results suggest that the SS factor associated with the limbic ‘impulsive system is most closely associated with sensitivity to the reinforcing effects of d-amphetamine. This finding is also consistent with other literature demonstrating that limbic pathways are linked to the reinforcing effects of drugs (e.g. Chiara, 1999).
Sensation-seeking has been targeted in the development of more effective prevention intervention programs focused on individuals who are at greater risk for drug abuse problems. For example, a marijuana prevention program developed specifically for high sensation seekers showed increased program efficacy (Palmgreen, Lorch, Stephenson, Hoyle, & Donohew, 2007). The current study suggests that targeting individuals high in SS for stimulant use prevention intervention efforts may also increase program efficacy. This study further suggests that more narrowly focused SS items may provide a more effective targeting strategy for stimulant abuse prevention programs relative to broader strategies based on impulsivity. Further research, however, is required to determine if individuals high on the SS measure used in this study would progress to problematic use of stimulants, or whether other personality variables might be associated with progression from initial to problematic stimulant use.
In addition to drug taking and subjective effects, SS also predicted d-amphetamine effects on heart rate. The findings from the current study suggest that individuals with higher levels of SS have an increased sensitivity to the physiological effects of stimulant drugs, although worth noting is that the magnitude of SS effect was small, even at the highest dose tested (slopes produced by the model indicated an increase of 2.9 beats per minute for each SD increase in SS at 2 h following taking 16mg of d-amphetamine). However, given the positive and dose-dependent relationship with the cardiovascular effects of d-amphetamine, SS could be associated with individual differences in cardiovascular health risk at higher doses. It should be noted, however, that previous studies examining correlations between SS (as measured by the Impulsive Sensation Seeking Scale) and cardiovascular effects of d-amphetamine have found increases in blood pressure and not heart rate (Stoops et al., 2007; Kelly et al., 2009) or no association between SS and cardiovascular effects of d-amphetamine (Kelly et al., 2006).
It is interesting to note that there were main effects of SS and Imp on ‘Like Drug’ and a main effect of Imp on ‘Bad Effect’ (Table 2). A SS by dose interaction was also observed on ‘Like Drug,’ and post-hoc testing indicated that SS was not related to these ratings under placebo condition, arguing against an independent effect of SS on ‘Like Drug’ ratings. In contrast, Imp was negatively associated with ‘Like Drug’ and positively associated with ’Bad Effect’ under placebo condition, suggesting an independent effect of Imp on these ratings. Individuals who report ‘Bad Effects’ following placebo administration may be less likely to self-administer drugs, although further research on this possibility is necessary.
Previous research has demonstrated an influence of sex on the behavioral effects of stimulant drugs (for review see Lynch, Roth, & Carroll, 2002). We conducted an exploratory analysis to test for sex differences, but detected no main effects of sex, or sex by dose interactions. Furthermore, inclusion of sex as a covariate in the analyses described above did not modify the significant relationships that were reported. These results suggest that the influence of impulsive personality on the behavioral effects of stimulant drugs does not vary by sex. However, it is important to note that this study was not designed to examine sex effects in a controlled manner (e.g., no controls for menstrual cycle phase or contraceptive use), so the absence of sex effects should be interpreted with caution.
Although this study focused on impulsive personality as determined by self-report questionnaires, individual differences in impulsivity can also be assessed by performance. Since personality measures and task performance measures of impulsivity may not be related (Reynolds et al., 2006), it is important to consider whether individual differences in impulsive task performance might similarly predict behavioral response to stimulant drugs. In this study, individual differences in impulsive task performance were determined using the Cued Go/No Go Task (Marczinski & Fillmore, 2003), a common measure of the ‘behavioral inhibition’ factor of impulsive task performance. Using baseline rates of inhibition errors on prepotent ‘go’ trials (i.e., responses following ‘no-go’ signals on cued ‘go’ trials), separate analyses were conducted to determine whether behavioral inhibition was associated with Imp or SS, or d-amphetamine effects. Consistent with previous literature suggesting that many personality and task performance measures of impulsivity are unrelated (Reynolds et al., 2006), behavioral inhibition was not significantly correlated with Imp or SS. Further, no relationship was observed between behavioral inhibition and subjective effects or self-administration of d-amphetamine. Yet, it should be emphasized that this study selectively recruited participants who varied in their levels of SS and Imp on personality questionnaires. A rigorous test of the relationship between impulsive task performance and individual differences in the behavioral effects of stimulant drugs would require a separate study designed to test a range of factors associated with impulsive task performance.
Limitations
There are a few limitations of the current study that should be considered. First, a stratified sampling procedure based on SS and Imp scores was used in order to capture the full range of these personality measures; however, this sample is not representative of typical distributions of these personality measures in the general population. Second, this study was limited to the oral route of administration. While stimulant use also occurs via intranasal, smoking and IV routes, previous research has indicated that prescription stimulant misuse typically occurs via the oral route of administration (Teter et al., 2006). Thus, use of oral administration in the current study is consistent with the typical route of administration among individuals who misuse prescription stimulants. Third, a range of d-amphetamine doses consistent with those used therapeutically was tested (Dexedrine, 2015), however, it is likely that higher doses of amphetamine are used recreationally. Lastly, this manuscript focused on the ImpSS subscale of the ZKPQ. Impulsive personality, however, is measured using a variety of questionnaires, and it is unclear whether results of this study would generalize to other self-report personality measures of impulsivity.
Conclusion
These data extend previous findings by suggesting that, while impulsive personality has been previously associated with the reinforcing and subjective effects of d-amphetamine, it is the SS dimension of impulsive personality that is associated with individual differences in subjective liking and the reinforcing effects of d-amphetamine among healthy emerging adults. These results indicate that SS may be the dimension of impulsive personality most closely associated with initial drug use. As noted earlier, SS has served as a functional tool for tailored prevention programming (e.g., Palmgreen et al., 2007; Noar, 2006). The results of this study support this approach while suggesting further refinement by using the SS specific items used in this study for future prevention programming tailoring.
Public Significance.
This study suggests that the sensation-seeking dimension of impulsive personality is closely associated with the subjective and reinforcing effects of d-amphetamine in healthy emerging adults. Targeting sensation-seeking may be a useful tool for tailoring prevention interventions focused on the initiation of stimulant drug use.
Acknowledgments
Mr. Glenn Robbins and Ms. Cleeve Emurian are thanked for the assistance in data collection for this study.
Financial support
This research was supported by NIH grants_P50 DA05312, UL1TR000117, and K02 DA031766. These funding sources had no other role other than financial support.
Footnotes
Previous Presentations: Preliminary reports of the data in this manuscript have been presented at the 2012 and 2014 annual meetings of the College on Problems of Drug Dependence and the 2012 annual meeting of the Society for Neuroscience.
Contributions
Drs. Lile, and Kelly contributed to the design, analysis, and data collection for this study. Dr. Kryscio contributed to the analysis of these data. Dr. Martin contributed to data collection for this study. Mr. Harvanko contributed to the analysis and first draft of this study. All authors contributed to the preparation of this manuscript and have read and approved the final manuscript.
Conflicts of interest None of the authors have conflicts of interest to report.
References
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biological Psychiatry. 1996;39(1):26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. Journal of Gambling Studies. 19(1):53–84. doi: 10.1023/a:1021275130071. doi:10.1023/A:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chiara G. Drug addiction as dopamine-dependent associative learning disorder. European Journal of Pharmacology. 1999;375(1):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcoholism: Clinical and Experimental Research. 1987;11(1):52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Dexedrine [3 Nov 2015];2015 http://www.pdr.net.
- Elliot AJ. The hierarchical model of approach-avoidance motivation. Motivation and Emotion. 2006;30(2):111–116. doi:10.1007/s11031-006-9028-7. [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146(4):348–361. doi: 10.1007/pl00005481. doi:10.1007/PL00005481. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100(1-2):91–9. doi: 10.1016/j.drugalcdep.2008.09.007. doi:10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. British Journal of Addiction. 1991;86(12):1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87(3):128–43. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Gullo MJ, Loxton NJ, Dawe S. Impulsivity: Four ways five factors are not basic to addiction. Addictive Behaviors. 2014;39(11):1547–56. doi: 10.1016/j.addbeh.2014.01.002. doi:10.1016/j.addbeh.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: Results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug and Alcohol Dependence. 1983;11(2):147–65. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: An exploratory study with d-amphetamine in healthy participants. Experimental and Clinical Psychopharmacology. 2009;17(6):374. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle RH, Feifar MC, Miller JD. Personality and sexual risk taking: A quantitative review. J Personality. 2000;68(6):1203–1231. doi: 10.1111/1467-6494.00132. doi:10.1111/1467-6494.00132. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Jaffe FK. Historical perspectives on the use of subjective effects measures in assessing the abuse potential of drugs. Testing for Abuse Liability of Drugs in Humans. 1989;43 [PubMed] [Google Scholar]
- Jonah BA. Sensation seeking and risky driving: A review and synthesis of the literature. Accident Analysis & Prevention. 1997;29(5):651–665. doi: 10.1016/s0001-4575(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Delzer TA, Martin CA, Harrington NG, Hays LR, Bardo MT. Performance and subjective effects of diazepam and d-amphetamine in high and low sensation seekers. Behavioural Pharmacology. 2009;20(5-6):505–17. doi: 10.1097/FBP.0b013e3283305e8d. doi:10.1097/FBP.0b013e3283305e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: D-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189(1):17–25. doi: 10.1007/s00213-006-0487-z. doi:10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Johanson C-E, de Wit H. Personality and the acute subjective effects of d-amphetamine in humans. Journal of Psychopharmacology. 2013;27(3):256–264. doi: 10.1177/0269881112472564. doi:10.1177/0269881112472564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll MW. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11(1):110–7. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- McDaniel SR, Mahan JE., III An examination of the impss scale as a valid and reliable alternative to the SSS-V in optimum stimulation level research. Personality and Individual Differences. 2008;44(7):1528–1538. [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST). Behavior Research Methods & Instrumentation. 1982;14(5):463–466. [Google Scholar]
- Moreno M, Estevez AF, Zaldivar F, Montes JMG, Gutiérrez-Ferre VE, Esteban L, Flores P. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug and Alcohol Dependence. 2012;124(3):355–362. doi: 10.1016/j.drugalcdep.2012.02.011. doi:10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Noar SM, Zimmerman RS, Palmgreen P, Lustria M, Horosewski ML. Integrating personality and psychosocial theoretical approaches to understanding safer sexual behavior: Implications for message design. Health Communication. 2006;19(2):165–174. doi: 10.1207/s15327027hc1902_8. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: Acquisition, specificity and correlation with self-reports. Journal of Pharmacology and Experimental Therapeutics. 1992;261(3):885–894. [PubMed] [Google Scholar]
- Palmgreen P, Lorch EP, Stephenson MT, Hoyle RH, Donohew L. Effects of the office of national drug control policy's marijuana initiative campaign on high-sensation-seeking adolescents. American Journal of Public Health. 2007;97(9):1644. doi: 10.2105/AJPH.2005.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. Mental health, sensation seeking and drug use patterns: A longitudinal study. Addiction (Abingdon, England) 1991;86(2):195–204. doi: 10.1111/j.1360-0443.1991.tb01769.x. doi:10.1111/j.1360-0443.1991.tb01769.x. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40(2):305–15. [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. The Journal of Pharmacology and Experimental Therapeutics. 2009;328(3):1007–18. doi: 10.1124/jpet.108.147124. doi:10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HJ, LaPlante DA, LaBrie RA, Kidman RC, Donato AN, Stanton MV. Toward a syndrome model of addiction: Multiple expressions, common etiology. Harvard Review of Psychiatry. 2004;12(6):367–374. doi: 10.1080/10673220490905705. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addictive Behaviors. 2007;32(6):1177–88. doi: 10.1016/j.addbeh.2006.08.006. doi:10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: Prevalence, motives, and routes of administration. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2006;26(10):1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. doi:10.1016/j.neubiorev.2007.11. [DOI] [PubMed] [Google Scholar]
- Von Diemen L, Bassani DG, Fuchs SC, Szobot CM, Pechansky F. Impulsivity, age of first alcohol use and substance use disorders among male adolescents: A population based case--control study. Addiction (Abingdon, England) 2008;103(7):1198–1205. doi: 10.1111/j.1360-0443.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. European Journal of Personality. 2005;19(7):559–574. [Google Scholar]
- Zimmerman RS, Palmgreen PM, Noar SM, Lustria MLA, Lu H-Y, Horosewski ML. Effects of a televised two-city safer sex mass media campaign targeting high-sensation-seeking and impulsive-decision-making young adults. Health Education & Behavior. 2007 doi: 10.1177/1090198107299700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The big three, the big five, and the alternative five. Journal of Personality and Social Psychology. 1993;65(4):757. [Google Scholar]