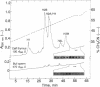
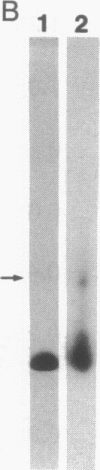
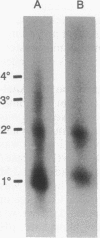
Abstract
CENP-A, a centromere-specific 17-kDa protein, has histone-like properties. However, in contrast to the common somatic histones, CENP-A is quantitatively retained in bull spermatozoa, and we have exploited this fact to purify CENP-A to apparent homogeneity. Partial sequence analysis of the purified protein indicates that CENP-A is a distinctive gene product. Some CENP-A sequences are highly similar to regions of histone H3. Other segments of CENP-A are not related to H3 or any other histone. These unrelated segments are presumably involved in localizing CENP-A to centromeric DNA or in centromere-specific functions of CENP-A.
Full text
PDF
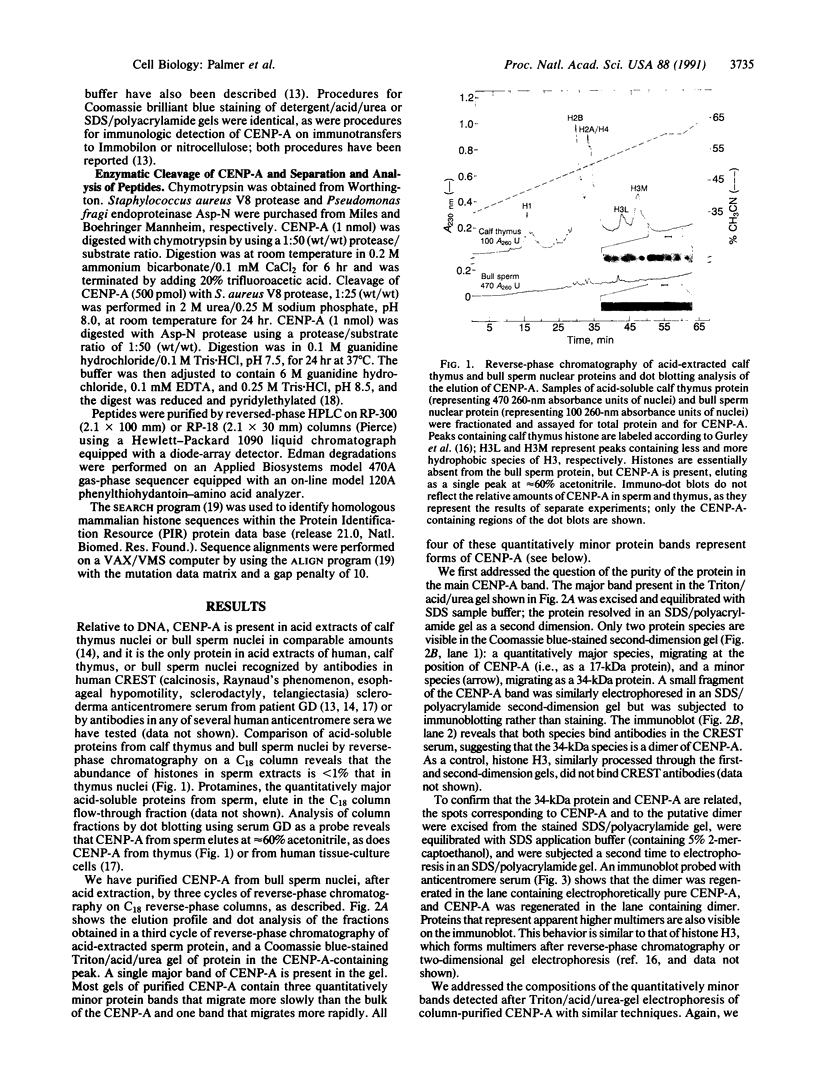

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernat R. L., Borisy G. G., Rothfield N. F., Earnshaw W. C. Injection of anticentromere antibodies in interphase disrupts events required for chromosome movement at mitosis. J Cell Biol. 1990 Oct;111(4):1519–1533. doi: 10.1083/jcb.111.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Maier G., Tilz G., Ponstingl H. A 47-kDa human nuclear protein recognized by antikinetochore autoimmune sera is homologous with the protein encoded by RCC1, a gene implicated in onset of chromosome condensation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8617–8621. doi: 10.1073/pnas.87.21.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M. W., Tan E., Brinkley B. R. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981 Oct;91(1):95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke C. A., Bernat R. L., Earnshaw W. C. CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol. 1990 May;110(5):1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Hooper J. A., Smith E. L. Histone 3. 3. Sequence studies on the cyanogen bromide peptides; complete amino acid sequence of calf thymus histone 3. J Biol Chem. 1973 May 10;248(9):3261–3274. [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan N., Cooke C., Rothfield N. The kinetochore is part of the metaphase chromosome scaffold. J Cell Biol. 1984 Jan;98(1):352–357. doi: 10.1083/jcb.98.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Gorbsky G. J., Sammak P. J., Borisy G. G. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J Cell Biol. 1987 Jan;104(1):9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J., Sammak P. J., Borisy G. G. Microtubule dynamics and chromosome motion visualized in living anaphase cells. J Cell Biol. 1988 Apr;106(4):1185–1192. doi: 10.1083/jcb.106.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner H. H., Lakomek H. J., Bautz F. A. Human anti-centromere sera recognise a 19.5 kD non-histone chromosomal protein from HeLa cells. Clin Exp Immunol. 1984 Oct;58(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Valdez J. G., Prentice D. A., Spall W. D. Histone fractionation by high-performance liquid chromatography. Anal Biochem. 1983 Feb 15;129(1):132–144. doi: 10.1016/0003-2697(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Hadlaczky G., Praznovszky T., Rasko I., Kereso J. Centromere proteins. I. Mitosis specific centromere antigen recognized by anti-centromere autoantibodies. Chromosoma. 1989 Jan;97(4):282–288. doi: 10.1007/BF00371967. [DOI] [PubMed] [Google Scholar]
- Kingwell B., Rattner J. B. Mammalian kinetochore/centromere composition: a 50 kDa antigen is present in the mammalian kinetochore/centromere. Chromosoma. 1987;95(6):403–407. doi: 10.1007/BF00333991. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol. 1989 Nov;109(5):2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. K., O'Day K., Margolis R. L. Biochemical analysis of CENP-A, a centromeric protein with histone-like properties. Prog Clin Biol Res. 1989;318:61–72. [PubMed] [Google Scholar]
- Palmer D. K., O'Day K., Margolis R. L. The centromere specific histone CENP-A is selectively retained in discrete foci in mammalian sperm nuclei. Chromosoma. 1990 Dec;100(1):32–36. doi: 10.1007/BF00337600. [DOI] [PubMed] [Google Scholar]
- Palmer D. K., O'Day K., Wener M. H., Andrews B. S., Margolis R. L. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987 Apr;104(4):805–815. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry. 1984 Sep 25;23(20):4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- Serrano L., Montejo de Garcini E., Hernández M. A., Avila J. Localization of the tubulin binding site for tau protein. Eur J Biochem. 1985 Dec 16;153(3):595–600. doi: 10.1111/j.1432-1033.1985.tb09342.x. [DOI] [PubMed] [Google Scholar]
- Simerly C., Balczon R., Brinkley B. R., Schatten G. Microinjected centromere [corrected] kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J Cell Biol. 1990 Oct;111(4):1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C. The cyclic tyrosination/detyrosination of alpha tubulin. Methods Cell Biol. 1982;24:235–255. doi: 10.1016/s0091-679x(08)60658-5. [DOI] [PubMed] [Google Scholar]
- Valdivia M. M., Brinkley B. R. Fractionation and initial characterization of the kinetochore from mammalian metaphase chromosomes. J Cell Biol. 1985 Sep;101(3):1124–1134. doi: 10.1083/jcb.101.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]