Abstract
Leukotriene B4 (LTB4) is a rapidly synthesized, early neutrophil chemoattractant that signals via its cell surface receptor, BLT-1, to attract and activate neutrophils during peritonitis. BLT-1-deficient (BLT-1−/−) mice were used to determine the effects of LTB4 on neutrophil migration and activation, bacterial levels, and survival after cecal ligation and puncture (CLP). Male BLT-1−/− or wild-type (WT) BALB/c mice underwent CLP. Tissues were harvested for determination of levels of bacteria, myeloperoxidase (MPO), LTB4, macrophage inflammatory protein 2 (MIP-2), and neutrophil (polymorphonuclear leukocyte [PMN]) numbers at 4 and 18 h after CLP. PMN activation was determined by an assessment of phagocytosis ability and CD11b expression. Survival was also determined. BLT-1−/− mice had decreased numbers of PMNs in the peritoneum at both 4 and 18 h after CLP but increased numbers of PMNs in the blood at 18 h compared with WT mice. Liver and lung MPO levels were significantly higher in BLT-1−/− mice at both 4 and 18 h after CLP, with increased bacterial levels in the blood, the liver, and peritoneal fluid at 4 h. Bacterial levels remained higher in peritoneal fluid at 18 h, but blood and liver bacterial levels at 18 h were not different from levels at 4 h. PMN phagocytosis and CD11b levels were decreased in BLT-1−/− mice. LTB4 levels were similar between the groups before and after CLP, but MIP-2 levels were decreased both locally and systemically in BLT-1−/− mice. Survival was significantly improved in BLT-1−/− mice (71%) compared with WT mice (14%) at 48 h post-CLP. Thus, LTB4 modulates neutrophil migration into the mouse peritoneum, but not the lung or liver, after CLP. Despite higher bacterial and PMN levels at remote sites, there was increased survival in BLT-1−/− mice compared to WT mice. Decreased PMN activation may result in less remote organ dysfunction and improved survival.
Bacterial peritonitis, with its attendant local and remote complications, continues to be a cause of high morbidity and mortality in the surgical patient (4, 5, 7). Therapeutic strategies have focused on local host defenses in the peritoneum and preservation of remote organ function. While activation and influx of leukocytes into a septic focus are essential, remote tissue neutrophil (polymorphonuclear leukocyte [PMN]) sequestration has been implicated in the genesis of organ injury and failure in models of ischemia-reperfusion, trauma, and endotoxemia (2, 15, 17). Arachidonic acid metabolites, proteases, and oxygen radicals released by activated neutrophils have also been shown to be the final agents that induce damage to endothelial cells, resulting in capillary leakage (29).
The events that lead to a local inflammatory response to intraperitoneal infection are characterized by the recognition of the site of infection by inflammatory cells, the specific recruitment of subpopulations of leukocytes (macrophages, neutrophils, mast cells, natural killer cells, and lymphocytes) into the affected area, and the subsequent clearance of the infecting organism (3, 24). These interactions involve both cell-to-cell contact via numerous cell surface receptors and the production of a network of peptide or lipid mediators acting as chemoattractants or signaling molecules (16). Migration between endothelial cells then proceeds along a chemokine gradient to the site of infection.
There are several groups of chemoattractants for leukocytes, including N-formylated peptides, leukotriene B4 (LTB4) complement components (C3a and C5a), and the CC and CXC chemokines. These chemoattractants trigger directional migration of inflammatory leukocytes and cell polarization with redistribution of adhesion molecules, and they stimulate intracellular calcium mobilization, cytosolic granule release, and other forms of cellular activation (23).
Leukocyte responses are likely to be dependent on the interaction of their receptors with multiple chemokines and other chemoattractants, depending on the specific tissue involved as well as the type and location of infection. Knockout mice for the high-affinity cell surface receptor of LTB4 (BLT-1-deficient [BLT-1−/−] mice) have been generated, and zymosan-elicited peritoneal exudate cells of these animals were found to be unresponsive to LTB4 yet responsive to C5a and platelet-activating factor in vitro, as measured by Ca2+ influx and chemotaxis (14). There was no PMN influx in response to intraperitoneal LTB4 in the knockout mice, which indicated that BTL-1 was the specific receptor for neutrophil chemoattraction by LTB4 (14). Furthermore, leukocytes isolated from the BLT-1−/− animals resisted adherence to venules in response to LTB4 application, which indicated a clear role for specific receptor-mediated LTB4 function in the integrin-mediated firm adhesion of PMNs to the endothelium (14, 27).
The role of BLT-1, the high-affinity receptor for LTB4, in response to polymicrobial peritonitis, however, has not been investigated. Using the cecal ligation and puncture (CLP) model of polymicrobial peritonitis, we tested the hypothesis that the LTB4 receptor would govern neutrophil migration and therefore affect clinical outcome of polymicrobial peritonitis in this model. We showed that PMN recruitment was reduced in BLT-1−/− mice, with a concomitant increase in bacterial load in the peritoneum. Despite this finding and increased remote organ PMN accumulation, there was improved survival in the BLT-1−/− mice, possibly due to reduced activation of PMNs. Our study reinforces the importance of LTB4 during peritonitis and provides novel evidence of interactions between LTB4 signaling through BLT-1 and levels of the CXC chemokine macrophage inflammatory protein 2 (MIP-2).
MATERIALS AND METHODS
Animals.
Six- to eight-week-old BLT-1−/− mice (Ltb4r1tm1Bodd) backcrossed for seven generations onto a BALB/c genetic background (14) and their wild-type (WT) BALB/c controls were used. Animals were housed in a facility approved by the American Association for Accreditation of Laboratory Animal Care and were provided food and water ad libitum. Studies were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the guidelines of the National Institutes of Health and under the supervision of a veterinarian.
CLP.
Mice were anesthetized with inhaled isofluorane (Abbott Laboratories, Chicago, Ill.). CLP was performed by exposing the cecum through a midline laparotomy, ligating with 4-0 silk suture just below the ileocecal junction and ensuring that a standard length of cecum was ligated each time and that there was no bowel obstruction. For survival and 18-h harvest experiments, a single 23-gauge puncture was made in the cecum. For 4-h harvest experiments, two 18-gauge punctures were made in the cecum to yield higher bacterial counts. The cecum was then returned to the peritoneal cavity, and the abdominal incision was closed in layers.
Survival.
Mice were injected with 1 ml of normal saline subcutaneously for volume resuscitation at the time of CLP. Cefoxitin (100 mg/kg of body weight) was administered subcutaneously every 12 h.
Timed harvests.
Mice were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg) at 4 or 18 h after CLP, and tissues were harvested at sacrifice. Peritoneal lavage fluid was obtained to determine bacterial counts, cytokine levels, neutrophil counts, and myeloperoxidase (MPO) levels. Liver, lung, and spleen tissue samples were collected for determination of bacterial counts and MPO levels. Blood was collected by cardiac puncture for evaluation of bacterial and leukocyte counts and PMN activation levels.
Peritoneal lavage.
Peritoneal exudate cells were recovered by peritoneal lavage with 4 ml of sterile, ice-cold, heparinized RPMI 1640 medium (GIBCO/BRL, Bethesda, Md.) and were counted manually by using a hemocytometer.
MPO assay.
MPO activity was used as a measure of neutrophil accumulation. Liver and lung tissue samples (40 to 60 mg) were homogenized in 20 mM phosphate buffer (pH 7.4) and centrifuged at 10,000 × g for 15 min. Tissue cell pellets were then resuspended in 50 mM phosphate buffer (pH 6.0) with 10 mM EDTA and 0.5% hexadecyltrimethylammonium bromide. The solubilized pellets were frozen, thawed, heated for 2 h at 60°C, sonicated for 2 s, and then refrozen. The MPO level was determined spectrophotometrically by using tetramethylbenzidine as the color reagent, as previously described (26).
MIP-2 and LTB4 assays.
Concentrations of MIP-2 were determined in supernatants from peritoneal lavage and in serum by enzyme-linked immunosorbent assays (Biosource International, Camarillo, Calif.) performed according to the manufacturer's instructions. Concentrations of LTB4 were measured from peritoneal lavage supernatant by enzyme immunoassay (Cayman Chemical, Ann Arbor, Mich.) performed according to the manufacturer's instructions.
Bacterial counts.
Homogenized liver and lung tissues (150 to 250 mg of samples in 2 ml of sterile saline), whole blood, and peritoneal lavage fluid were plated in serial log dilutions on tryptic soy or brain heart infusion agar plates. After plating, tryptic soy agar plates were incubated at 37°C aerobically for 24 h, and brain heart infusion agar plates were incubated anaerobically for 48 h. Results were expressed as log CFU per milliliter for blood, log CFU per gram for tissues, and log CFU per mouse for peritoneal lavage fluid. Lower limits of detection were 1.52 log CFU/ml for blood, 2.12 log CFU/mouse for peritoneal lavage fluid, and 2.65 log CFU/g for liver.
PMN activation.
Activation of cells was determined by measuring the ability of PMNs to ingest fluorescently labeled bacteria and by the cell surface expression of the PMN-activation marker CD11b. Fluorescein isothiocyanate (FITC)-labeled Escherichia coli (Molecular Probes, Eugene, Oreg.) was opsonized by incubation with 5% pooled mouse serum in a shaking water bath at 37°C for 30 min. The bacteria were then washed twice in phosphate-buffered saline prior to the addition of PMNs in 1:100 ratios of blood leukocytes to bacteria and incubated for another 30 min in the water bath. The red blood cells were then lysed, and cells and bacteria were washed twice in phosphate-buffered saline, followed by fixation with 1% paraformaldehyde (Polysciences, Warrington, Pa.). Fluorescence was measured in the PMN population unquenched and quenched with trypan blue (Sigma, St. Louis, Mo.) by using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, N.J.) equipped with CellQuest software. Quenching prevents fluorescence of bacteria attached to the outside of the cell, allowing determination of ingested bacterial levels. Results were expressed as mean channel fluorescence (MCF) levels. CD11b levels were also determined by flow cytometry by labeling PMNs in blood and peritoneal exudate with FITC-anti-CD11b antibody (BD Pharmingen, San Diego, Calif.). Isotype-matched control antibodies were also used. Red cells were lysed hypotonically with ammonium chloride, and samples were then washed with phosphate-buffered saline and fixed in 1% paraformaldehyde. Ten thousand cells were acquired, and the data were analyzed on a FACScan flow cytometer and by gating around the PMN population. Results were expressed as MCF levels.
Statistical analysis.
Concentrations of cytokines, neutrophil counts, MCF levels, and MPO levels were compared by analysis of variance (ANOVA), with the Tukey-Kramer honestly significant difference test as a follow-up. The Fisher exact test was used for differences in survival. Bacterial counts and cell percentages were compared nonparametrically by using the Mann-Whitney U test. Cytokine and MPO levels are expressed as means ± standard errors of the mean (SEM). A P value of 0.05 or less was considered significant.
RESULTS
Neutrophil migration was decreased in the peritoneum and increased in remote organs after CLP in BLT-1-deficient mice.
As LTB4 is a PMN chemoattractant previously shown to exert this effect through BLT-1 (14), we expected decreased PMN accumulation at the site of infection in BLT-1−/− mice. This expectation was confirmed by a slight decrease in PMNs in the peritoneum at 4 h after CLP (not significant) and a significant decrease in PMNs by 18 h in BLT-1−/− mice (Table 1). Conversely, BLT-1−/− mice had increased PMNs in the blood at 4 h and significantly increased blood PMN levels by 18 h after CLP, together with significantly increased MPO levels in the lung and liver at both 4 and 18 h after CLP (Table 1). These data reinforce the importance of LTB4 signaling via BLT-1 on PMN migration to the site of infection and also support previous evidence that there is an increase in both peripheral blood and remote organ PMNs if PMN migration to the site of infection is inhibited (22). Increased PMN accumulation is often used as an indication of remote organ damage and dysfunction, which have been shown to significantly contribute to mortality after CLP (28).
TABLE 1.
Comparison of neutrophil migration in WT and BLT-1−/− mice into the peritoneum, blood, liver, and lung during bacterial peritonitisa
| Time point and mouse type | Peritoneal PMN (106) | Blood PMN (106) | Liver MPO (U/g of tissue) | Lung MPO (U/g of tissue) |
|---|---|---|---|---|
| 4 h | ||||
| WT | 1.77 ± 0.10 | 0.41 ± 0.06 | 4.35 ± 0.61 | 74.34 ± 10.50 |
| BLT-1−/− | 1.45 ± 0.18 | 0.52 ± 0.07 | 9.14 ± 0.86b | 154.33 ± 8.06b |
| 18 h | ||||
| WT | 17.12 ± 2.41 | 0.73 ± 0.06 | 4.52 ± 1.12 | 72.20 ± 14.77 |
| BLT-1−/− | 9.37 ± 0.92b | 4.23 ± 0.72b | 11.86 ± 4.19b | 155.30 ± 20.20b |
Results are expressed as means ± SEM. There were eight mice per experimental group.
The P value was <0.05 for WT versus BLT-1−/− mice by ANOVA.
LTB4 production was not inhibited in BLT-1−/− mice during CLP.
LTB4 is an early mediator of inflammation and is known to be one of the earliest proinflammatory mediators produced following inflammatory stimuli such as infection (13, 30). LTB4 levels were low in the peritoneums of healthy mice that had not undergone CLP, but were increased at 4 h after CLP to similar levels in WT and BLT-1−/− mice (3.1 ± 0.2 and 2.8 ± 0.7 ng/ml, respectively). This finding indicates that the mouse BLT-1 deficiency does not lead to inhibition of LTB4 production or overcompensation by increased LTB4 production during bacterial peritonitis. As expected, LTB4 levels were significantly decreased by 18 h after CLP to near pre-CLP levels, with no differences between results for WT and BLT-1−/− mice (0.17 ± 0.08 and 0.14 ± 0.04 ng/ml, respectively). The regulation of LTB4 production during CLP does not, therefore, appear to be affected in BLT-1−/− mice, providing the potential for LTB4 to signal via an alternative receptor.
MIP-2 levels were regulated locally and systemically by signaling through BLT-1.
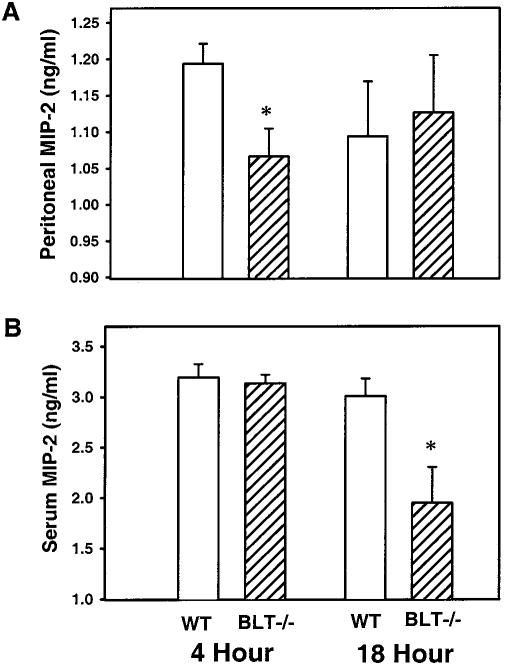
MIP-2, a murine CXC chemokine analogous to human interleukin 8, is known to be another major PMN chemoattractant during bacterial infection (22). We therefore investigated whether differences in PMN accumulation observed for BLT-1−/− mice were also secondary to alterations in MIP-2 levels. Peritoneal and serum MIP-2 was not detected in healthy animals that had not undergone CLP (data not shown), but levels of MIP-2 were significantly increased in both WT and BLT-1−/− mice at both 4 and 18 h after CLP compared with levels in healthy mice. However, levels of peritoneal MIP-2 in BLT-1−/− mice at 4 h after CLP were significantly decreased compared with levels in WT mice at 4 h after CLP (Fig. 1A). Similar significant decreases in MIP-2 levels were detected in the sera of BLT-1−/− mice at 18 h after CLP (Fig. 1B). These data suggest that there is regulation of MIP-2 production via signaling of LTB4 through the BLT-1 receptor. This novel pathway of regulation may represent a potential method of increasing the duration of action of LTB4 during CLP, and it also adds another potential layer of regulation of the overall immune response to bacterial infection.
FIG. 1.
Levels of MIP-2 in the peritoneums (A) and sera (B) of WT and BLT-1−/− mice after CLP. Peritoneal lavage fluid and serum samples were collected from mice at 4 and 18 h after CLP, and MIP-2 levels were determined by enzyme-linked immunosorbent assay. There were eight mice per experimental group per time point, and the results shown were combined from two separate experiments. Results are expressed as the means ± SEM. *, P < 0.05 for WT versus BLT-1−/− mice by ANOVA.
Bacterial clearance was impaired in BLT-1−/− mice during CLP.
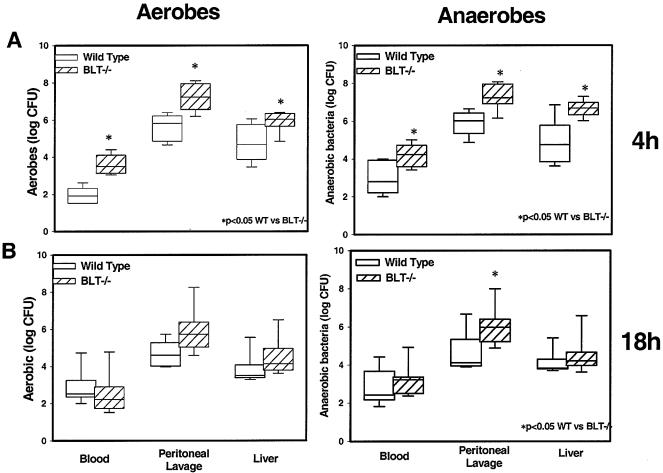
It was expected that bacterial clearance at the site of infection would be impaired secondary to impaired accumulation of PMNs in BLT-1−/− mice and would be increased systemically as well as in remote organs secondary to increased PMN migration to these sites in BLT-1−/− mice, as presented above. Although there was an increase in aerobic and anaerobic bacterial levels in the peritoneums of BLT-1−/− mice at 4 h after CLP, as expected (Fig. 2), there were also increased bacterial levels in the blood and livers at this time point (Fig. 2). By 18 h after CLP, however, there were no significant differences in the recovery of aerobes from any compartment. Recovery of anaerobes at the 18-h time point was significantly greater from the peritoneal cavities of the BLT-1−/− animals, but levels of anaerobes were similar in the blood and livers of the two groups of animals. This result suggests either that another mediator is acting to decrease bacterial clearance remotely or that there is a decrease in the ability of PMNs in BLT-1−/− mice to phagocytose and clear bacteria.
FIG. 2.
Aerobic and anaerobic bacterial levels in the blood, peritoneal lavage fluid, and liver at 4 h (A) and 18 h (B) after CLP in WT and BLT-1−/− mice. Whole blood, peritoneal lavage fluid, and homogenized liver tissue were plated in serial log dilutions on tryptic soy agar (aerobic) or brain heart infusion agar (anaerobic) and incubated at 37°C for 24 to 48 h. There were eight mice per experimental group per time point, and the results shown were combined from two separate experiments. Results are expressed as log CFU per millliliter (for blood), per mouse (for peritoneal lavage fluid), and per gram of tissue (for liver). *, P < 0.05 for WT versus BLT-1−/− mice by the Mann-Whitney U test.
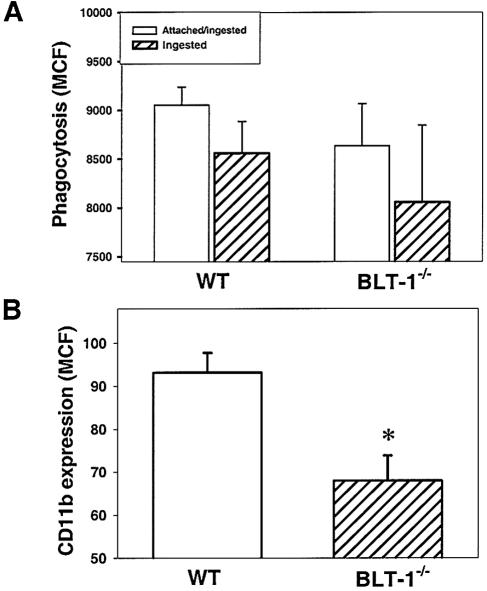
PMN activation was decreased in BLT-1−/− mice after CLP.
PMN activation was determined by measuring PMN phagocytosis ability and cell surface expression of the PMN-activation marker CD11b. There was decreased phagocytosis of FITC-labeled E. coli in BLT-1−/− mice compared with WT mice at 4 h after CLP, although this finding was not statistically significant (Fig. 3A). However, a significant decrease in PMN expression of CD11b was observed for BLT-1−/− mice (Fig. 3B). The macrophage phagocytosis ability of BLT1−/− mice (3,800 ± 800 MCF) was not different from that of WT mice (3,900 ± 500 MCF). These data indicate that LTB4 signaling via BLT-1 may be critical to complete PMN activation during CLP. This signaling pathway may also play a role in PMN migration as CD11b forms part of the integrin cell adhesion molecule CD11b/CD18.
FIG. 3.
Neutrophil phagocytosis (A) and cell surface expression of CD11b (B) in WT and BLT-1−/− mice at 4 h after CLP. Whole blood was incubated with opsonized FITC-labeled E. coli. Total cell fluorescence of neutrophils (attached and ingested bacteria) and fluorescence after quenching of external fluorescence with trypan blue (ingested bacteria only) were measured by flow cytometry. Neutrophils in whole blood were also stained with FITC-labeled anti-CD11b antibody, and fluorescence was measured by flow cytometry. Results are expressed as MCF levels. There were seven mice per experimental group per time point, and the results shown were combined from two separate experiments. *, P < 0.05 for WT versus BLT-1−/− mice by ANOVA.
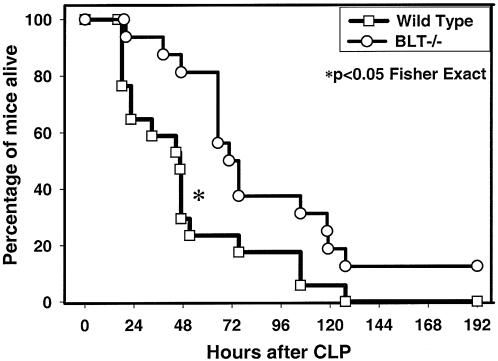
Survival was improved for BLT-1−/− mice after CLP.
Increased levels of bacteria locally and in remote sites have been shown to contribute to mortality after CLP (11). Therefore, given the almost universal increase in bacterial levels in BLT-1−/− mice during peritonitis, as shown above, it was expected that these mice would exhibit an increased mortality rate compared to WT mice. However, the opposite result was observed. Survival was significantly improved for BLT-1−/− mice (71%) compared with WT mice (14%) at 48 h post-CLP (Fig. 4). There was also an ultimate 42% 7-day survival advantage for BLT-1−/− mice (Fig. 4). The improved survival rate may be due to decreased remote organ dysfunction secondary to decreased damage from less-activated PMNs.
FIG. 4.
Survival of WT and BLT-1-deficient (BLT-1−/−) mice after CLP. Mice underwent 23-gauge CLP with saline resuscitation and 100 mg of cefoxitin/kg every 12 h for the duration of the experiment. There were 17 WT mice and 16 BLT-1−/− mice per experimental group. The results shown were combined from three separate experiments. *, P < 0.05 for WT versus BLT-1−/− mice by the Fisher exact test.
DISCUSSION
There has been a recent resurgence of interest in products of the 5-lipoxygenase pathway, with the development of knockout mice rendered leukotriene deficient by disruption of the 5-lipoxygenase gene and, subsequently, of mice deficient in the specific receptor for LTB4 (19, 30). Our data demonstrated a survival benefit from knockout of the specific LTB4 receptor in a model of murine peritonitis produced by CLP, despite impaired neutrophil migration to the site of infection in the peritoneal cavity. LTB4 production in the BLT-1 knockouts was similar to that in the WT mice, indicating a true defect in ligand-receptor interaction. Interestingly, the survival benefit was associated with increased bacterial levels early on after CLP, which likely occurred because of the impaired local host defense response manifested by reduced levels of peritoneal neutrophils. Of special interest is that MIP-2, a potent endogenous chemoattractant, may be regulated in part through the BLT-1 receptor, providing further evidence of cross talk between different chemokines and their receptors. MIP-2 levels in both the peritoneal fluid and blood have been shown to correlate with mortality after CLP (28). It may be that these knockout mice are able to tolerate a higher bacterial load or that lung and liver injury is limited by decreased neutrophil activation in these animals, in which LTB4 cannot engage its receptor. This apparent paradox has been observed previously in STAT4 knockout mice, which had increased survival compared to controls but also had increased levels of bacteria (10).
The 5-lipoxygenase knockout mice have been found to be more susceptible to intratracheal bacterial instillation than the WT mice, but they still recruited PMNs into the lung. However, the knockout mice had defective phagocytosis in vitro that was restored by exogenous LTB4 (1). Further, PMNs treated with an LTB4 receptor antagonist had defective phagocytosis that was restored only with exogenous LTB4, not with other leukotrienes (20). These results signify that LTB4 is important not only to PMN migration but to activation as well. Our data are consistent with these findings in that PMN phagocytosis and CD11b expression were reduced in the BLT-1 knockout mice, and bacterial levels were increased correspondingly. Survival, however, was improved in the BLT-1 knockout mice after CLP, which may be related to the fact that other leukotrienes are not present in the 5-lipoxygenase knockout mice. Although CLP is not normally considered a model of multiple organ dysfunction, there is increasing evidence that death after CLP is often unrelated to absolute bacterial levels and likely occurs as a result of an imbalanced immune response to infection and subsequent multiple organ failure (9, 10). Alternatively, LTB4 may exert anti-inflammatory effects to mediate the immune response to infection through a receptor pathway distinct from BLT-1, such as the peroxisome proliferator-activated receptor pathway (6, 8) via heme oxygenase-1 and nitric oxide production.
In humans, after major trauma, 5-lipoxygenase products from patient PMN fractions are reduced, corresponding with the appearance of immature band forms (18). This finding implies that cell maturity is important in PMN migration and subsequent amplification of the inflammatory response. Inhibition of the LTB4 receptor with a specific antagonist reduces peritoneal leukocyte influx after CLP and diminishes survival (21). Monocyte chemoattractant-1 in the peritoneum is elevated after CLP, and anti-monocyte chemoattractant-1 antibodies reduce LTB4 levels but not levels of other CXC chemokines. Thus, an endogenous chemokine may regulate leukocyte influx by increasing production of the lipid chemoattractant and vice versa. This possibility implies the presence of coregulatory mechanisms to amplify the peritoneal inflammatory response to infection. Such pathways may involve MIP-2, as well, and our data provided the first evidence of potential cross talk between LTB4 and MIP-2 via the BLT-1 receptor. In a thioglycolate model of peritonitis, both 5-lipoxygenase and C5-deficient mice had diminished peritoneal PMN migration (25). LTB4 inhibition in C5-deficient mice nearly abolished peritoneal PMN migration in this model, which suggested that these two chemoattractants have independent but overlapping roles in PMN chemotaxis. Most chemoattractants are short-lived and inactivated by reactive oxidants.
In summary, we have shown that the specific receptor for LTB4 is required for appropriate early neutrophil migration to the site of infection but not into organs remote from the site of infection. Lack of the BTL-1 receptor is associated with impaired bacterial clearance from polymicrobial peritonitis, yet survival of BLT-1−/− mice was enhanced. This result may be explained by decreased neutrophil activation in remote organs or by engagement of the peroxisome proliferator-activated receptor pathway (6, 8) and an enhanced systemic anti-inflammatory response, both of which could limit remote organ injury. LTB4 has recently been shown to recruit effector T cells via interaction with its receptor BLT-1 on these cells (12), which might also limit tissue injury. In addition, a second low- affinity receptor that is widely expressed, BLT-2, has been described (31). Further study is required to explain the molecular mechanisms at play behind these observations.
REFERENCES
- 1.Bailie, M. B., T. J. Standiford, L. L. Laichalk, M J. Coffey, R. Strieter, and M. Peters-Golden. 1996. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 157:5221-5224. [PubMed] [Google Scholar]
- 2.Botha, A. J., F. A. Moore, E. E. Moore, A. Sauaia, A. Banerjee, and V. M. Peterson. 1995. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J. Trauma 39:411-417. [DOI] [PubMed] [Google Scholar]
- 3.Brisseau, G. F., A. P. Dackiw, P. Y. Cheung, N. Christie, and O. D. Rotstein. 1995. Posttranscriptional regulation of macrophage tissue factor expression by antioxidants. Blood 85:1025-1035. [PubMed] [Google Scholar]
- 4.Cheadle, W. G., M. Mercer-Jones, M. Heinzelmann, and H. C. Polk, Jr. 1996. Sepsis and septic complications in the surgical patient: who is at risk? Shock 6(Suppl. 1):S6-S9. [PubMed] [Google Scholar]
- 5.Christou, N. V., P. S. Barie, E. P. Dellinger, J. P. Waymack, and H. H. Stone. 1993. Surgical Infection Society intra-abdominal infection study. Prospective evaluation of management techniques and outcome. Arch. Surg. 128:193-198. [DOI] [PubMed] [Google Scholar]
- 6.Cunard, R., M. Ricote, D. DiCampli, D. C. Archer, D. A. Kahn, C. K. Glass, and C. J. Kelly. 2002. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J. Immunol. 168:2795-2802. [DOI] [PubMed] [Google Scholar]
- 7.Deitch, E. A., J. L. Vincent, and A. C. J. Windsor (ed.). 2002. Sepsis and multiple organ failure. W. B. Saunders, Philadelphia, Pa.
- 8.Devchand, P. R., H. Keller, J. M. Peters, M. Vazquez, F. J. Gonzalez, and W. Wahli. 1996. The PPARα-leukotriene B4 pathway to inflammation control. Nature 384:39-43. [DOI] [PubMed] [Google Scholar]
- 9.Ebong, S., D. Call, J. Nemzek, G. Bolgos, D. Newcomb, and D. Remick. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect. Immun. 67:6603-6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godshall, C. J., A. B. Lentsch, J. C. Peyton, M. J. Scott, and W. G. Cheadle. 2001. STAT4 is required for antibacterial defense but enhances mortality during polymicrobial sepsis. Clin. Diagn. Lab. Immunol. 8:1044-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godshall, C. J., M. J. Scott, J. C. Peyton, S. A. Gardner, and W. G. Cheadle. 2002. Genetic background determines susceptibility during murine septic peritonitis. J. Surg. Res. 102:45-49. [DOI] [PubMed] [Google Scholar]
- 12.Goodarzi, K., M. Goodarzi, A. M. Tager, A. D. Luster, and U. H. von Andrian. 2003. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat. Immunol. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 13.Haribabu, B., R. M. Richardson, M. W. Verghese, A. J. Barr, D. V. Zhelev, and R. Snyderman. 2000. Function and regulation of chemoattractant receptors. Immunol. Res. 22:271-279. [DOI] [PubMed] [Google Scholar]
- 14.Haribabu, B., M. W. Verghese, D. A. Steeber, D. D. Sellars, C. B. Bock, and R. Snyderman. 2000. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J. Exp. Med. 192:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heflin, A. C., Jr., and K. L. Brigham. 1981. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J. Clin. Investig. 68:1253-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane, M. P., and R. M. Strieter. 2000. Chemokine signaling in inflammation. Crit. Care Med. 28:N13-N26. [DOI] [PubMed] [Google Scholar]
- 17.Koike, K., E. E. Moore, F. A. Moore, R. J. Franciose, B. Fontes, and F. J. Kim. 1995. CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion. J. Trauma 39:23-27. [DOI] [PubMed] [Google Scholar]
- 18.Koller, M., M. Wick, and G. Muhr. 2001. Decreased leukotriene release from neutrophils after severe trauma: role of immature cells. Inflammation 25:53-59. [DOI] [PubMed] [Google Scholar]
- 19.Luo, M., S. M. Jones, M. Peters-Golden, and T. G. Brock. 2003. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc. Natl. Acad. Sci. USA 100:12165-12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancuso, P., P. Nana-Sinkam, and M. Peters-Golden. 2001. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 69:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsukawa, A., C. M. Hogaboam, N. W. Lukacs, P. M. Lincoln, R. M. Strieter, and S. L. Kunkel. 1999. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163:6148-6154. [PubMed] [Google Scholar]
- 22.Mercer-Jones, M. A., D. J. Hadjiminas, M. Heinzelmann, J. Peyton, M. Cook, and W. G. Cheadle. 1998. Continuous antibiotic treatment for experimental abdominal sepsis: effects on organ inflammatory cytokine expression and neutrophil sequestration. Br. J. Surg. 85:385-389. [DOI] [PubMed] [Google Scholar]
- 23.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 25.Segal, B. H., D. B. Kuhns, L. Ding, J. I. Gallin, and S. M. Holland. 2002. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47phox: complement, leukotrienes, and reactive oxidants in acute inflammation. J. Leukoc. Biol. 71:410-416. [PubMed] [Google Scholar]
- 26.Suzuki, K., H. Ota, S. Sasagawa, T. Sakatani, and T. Fujikura. 1983. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 132:345-352. [DOI] [PubMed] [Google Scholar]
- 27.Tager, A. M., J. H. Dufour, K. Goodarzi, S. D. Bercury, U. H. von Andrian, and A. D. Luster. 2000. BLTR mediates leukotriene B4-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 192:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walley, K. R., N. W. Lukacs, T. J. Standiford, R. M. Strieter, and S. L. Kunkel. 1997. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect. Immun. 65:3847-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss, S. J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365-376. [DOI] [PubMed] [Google Scholar]
- 30.Yokomizo, T., T. Izumi, and T. Shimizu. 2001. Leukotriene B4: metabolism and signal transduction. Arch. Biochem. Biophys. 385:231-241. [DOI] [PubMed] [Google Scholar]
- 31.Yokomizo, T., K. Kato, K. Terawaki, T. Izumi, and T. Shimizu. 2000. A second leukotriene B4 receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]