Abstract
An anti-Anthrax Vaccine Adsorbed (anti-AVA) standard human reference serum pool, AVR414, has been prepared, and the total and protective antigen (PA)-specific immunoglobulin G (IgG) were quantified. AVR414 was prepared by plasmapheresis of healthy adults who had received a minimum of four subcutaneous injections of AVA. Mass values (in milligrams per milliliter) for total IgG and IgG subclasses 1 to 4 were determined by radial immunodiffusion. Anti-PA-specific IgG assignment (in micrograms per milliliter) was done by consensus of two complementary approaches: homologous enzyme-linked immunosorbent assay (ELISA) with affinity-purified anti-PA IgG as a calibrator and summation of mean PA-specific IgG subclass concentrations determined by IgG subclass-specific ELISA using the United States National Reference Preparation for Human Serum Proteins as a standard. The total IgG concentration assigned to AVR414 reference serum was 8.33 mg/ml. IgG subclass concentrations were the following: for IgG1, 4.48 mg/ml; for IgG2, 3.35 mg/ml; for IgG3, 0.37 mg/ml; and for IgG4, 0.30 mg/ml. The assigned mass value for total anti-PA-specific IgG was 141.2 μg/ml. Anti-PA-specific IgG subclass concentrations were the following: for IgG1, 79.6 μg/ml; for IgG2, 35.3 μg/ml; for IgG3, 3.2 μg/ml; and for IgG4, 25.3 μg/ml. Human reference serum pool AVR414 will have direct application in the standardization of anthrax serological assays, in reagent qualification, and as a standard for quantification of PA-specific IgG in humans who have been vaccinated with or otherwise exposed to Bacillus anthracis PA.
The immune response to anthrax toxin protective antigen (PA) is central to protection against anthrax (19, 20). Immunoglobulin G (IgG) is the most abundant immunoglobulin in human serum and provides the dominant immune response to protein antigens after vaccination with multiple injections (16, 16a). Measurement of anti-PA IgG antibody is therefore an appropriate marker of human immune responses to Bacillus anthracis infection and anthrax vaccines. A lack of assay standardization and qualified reagents has been a major obstacle to the comparative analysis of human serological responses to clinical anthrax and anthrax vaccines. Compounding this problem are variations in antigen selection, preparation, and purity; variations in assay methodology and end point determination between laboratories; the diversity of antibodies in polyclonal serum; and the absence of a suitable standard reference serum (32). In 2001, the Centers for Disease Control and Prevention (CDC; Atlanta, Ga.) initiated the Anthrax Vaccine Research Program to determine the feasibility of reducing the number of priming series doses of the licensed Anthrax Vaccine Adsorbed (AVA or BioThrax; BioPort Corp., Lansing, Mich.) (17, 26, 27) from six to three and changing the route of administration from subcutaneous (s.c.) to intramuscular (28) without reducing the vaccine's immunogenicity. The Anthrax Vaccine Research Program required the development of precise, accurate, specific, and sensitive serological assays for the quantification of anti-PA IgG responses in humans (32). Fundamental to the consistency of such assays is the availability of a standard reference serum and qualified control reagents together with standardized assay technologies and methods for end point determination (29). In the present study, we report the preparation and assignment of mass values for total and PA-specific IgG and IgG subclasses for an anti-AVA human reference serum, AVR414. The performance characteristics of AVR414 as a standard reference reagent for quantification of anti-PA IgG responses in human serum and the assignment of PA-specific IgG mass values to positive quality control (QC) sera and standards (AVR801) for use in anthrax serological assays are also demonstrated.
MATERIALS AND METHODS
Preparation of anti-AVA human standard reference serum.
The anti-AVA human reference serum AVR414 (CDC standard anthrax reference sera AVR414 and AVR801 may be obtained free of charge under a suitable materials transfer agreement by application to C. P. Quinn, CDC) was prepared by pooling equal volumes of serum from each of three healthy adult CDC volunteers who had received a minimum of four s.c. injections of AVA with the licensed regimen (at 0, 2, and 4 weeks and 6, 12, and 18 months with two yearly boosters). Serum selection was based on anti-PA IgG titers in the range of 3,200 to 6,400 as determined by an anti-PA IgG enzyme-linked immunosorbent assay (ELISA) (32). Plasmapheresis of selected donors and subsequent serum conversion were done at the Emory Transfusion Medicine Program, Emory University School of Medicine (Atlanta, Ga.) and the Scientific Resource Program at the CDC, respectively, by TPE DUAL-NEEDLE operation using a Spectra apheresis system as described by the manufacturer (Cobe BCT, Inc., Blood Component Technology, Lakewood, Colo.). The plasma units were stored frozen at −70°C, thawed overnight at 4°C prior to use, and converted to serum by the injection of 4.0 ml of sterile glass microbeads (B. Braun Instruments, Burlingame, Calif.) suspended in 1.5 M CaCl2-2.0 M ɛ-amino-caproic acid (Sigma, St. Louis, Mo.). Clots were allowed to form overnight at 4°C and were then removed by centrifugation at 2,200 × g for 15 min at 4°C. The serum from each unit was recovered by aspiration and stored separately in 500-ml sterile polycarbonate containers (Nalge Nunc International, Rochester, N.Y.). The level of residual anticoagulants was not determined (32). The anti-AVA human standard reference serum AVR414 was stored frozen in 3-ml aliquots at −70°C.
Calibration standard for serum immunoglobulins.
The U.S. National Reference Preparation for Human Serum Proteins (USNRP; IS1644, lot 20575L) was obtained in lyophilized form from the CDC (33) and was used as a standard reference in IgG subclass-specific ELISAs for quantification of anti-PA IgG subclasses. The USNRP was reconstituted in 1.0 ml of sterile distilled water to provide final concentrations of 11.28 mg of total IgG/ml, comprising 7.12 mg of IgG1/ml, 2.79 mg of IgG2/ml, 0.60 mg of IgG3/ml, and 0.60 mg of IgG4/ml (33).
Anthrax toxin PA.
Purified recombinant PA with an amino acid sequence concurring with that of the B. anthracis V770-NP1-R anthrax vaccine strain was provided by Stephen H. Leppla (National Institute of Allergies and Infectious Diseases, National Institutes of Health, Bethesda, Md.). Antigen was stored frozen in 100- to 500-μl aliquots (4.75 mg/ml) in 5 mM HEPES, pH 7.3, at −70°C. PA was expressed and purified to homogeneity as described previously (18, 25).
Affinity purification of human IgG.
Total IgG was purified from the AVR414 serum pool with a HiTrap Protein G column (Amersham Biosciences Corp., Piscataway, N.J.). Bound IgG was eluted with 0.1 M glycine-HCl (Sigma), pH 2.75, in 1.0-ml fraction volumes and immediately neutralized by the addition of 0.1-ml volumes of 1.0 M Tris-HCl, pH 9.0 (Life Technologies, Gaithersburg, Md.). Eluted peak fractions were dialyzed against 0.01 M phosphate-buffered saline (PBS), pH 7.4 (Life Technologies). The concentration of purified IgG was determined by radial immunodiffusion (RID) and the Bio-Rad (Hercules, Calif.) protein assay using purified commercially obtained human IgG as a standard (ICN Biomedicals, Inc., Costa Mesa, Calif.).
Affinity purification of PA-specific IgG.
The anti-PA-specific IgG from the protein G-purified AVR414 total IgG was isolated by affinity adsorption to PA immobilized on CNBr-activated Sepharose 4B according to the manufacturer's instructions (Amersham Biosciences Corp.). Affinity-purified total IgG was loaded onto the column (2.5-ml bed volume) and eluted under neutral conditions using ActiSep elution medium according to the manufacturer's instructions (Sterogene Bioseparations, Inc., Carlsbad, Calif.) (7). Fractions (0.5 ml) were collected, dialyzed against several changes of 100 mM HEPES buffer (Life Technologies), pH 7.4, containing 100 mM sodium chloride (Sigma), and then concentrated using Slide-A-Lyzer concentrating solution (Pierce Chemical Company, Rockford, Ill.). The concentration of purified anti-PA-specific IgG was determined by adsorption at 280 nm using an extinction coefficient of 1.35 (9a).
Quantitative anti-PA IgG ELISA.
The quantitative ELISA for human anti-PA IgG has previously been described in detail (32). Briefly, Immulon 2 HB microtiter plates (Thermo Labsystems, Franklin, Mass.) were coated with 100 μl of purified recombinant PA (2 μg/ml) in 0.01 M PBS, pH 7.4 (Life Technologies). After incubation overnight at 4°C, the plates were washed three times with PBS containing 0.1% Tween 20. Serum samples (and all subsequent antibody added to the plates) were diluted in PBS containing 5% skim milk and 0.5% Tween 20 (pH 7.4), loaded, and transferred in twofold dilutions down the plate. After incubation for 60 min at 37°C, plates were washed as described above. Bound human anti-PA IgG was detected by incubation for 60 min at 37°C with horseradish peroxidase-conjugated mouse monoclonal anti-human IgG Fc PAN clone HP6043 (Hybridoma Reagent Laboratory, Baldwin, Md.) (12). Plates were washed three times, and ABTS Microwell peroxidase substrate system (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was added for incubation for 30 min at 37°C. ABTS peroxidase stop solution (Kirkegaard and Perry Laboratories) was added to stop the assay, and plates were read with an MRX Revelation microtiter plate reader (Thermo Labsystems) at a wavelength of 410 nm with a 610-nm reference filter. The affinity-purified anti-PA IgG (139.0 μg/ml) was used as a calibrator to generate a 7-point standard curve in triplicate over a twofold dilution series in an anti-PA ELISA to assign a PA-specific IgG concentration to AVR414. Experiments to assign mass values were repeated a minimum of three times on three different assay plates by three different operators. The anti-PA IgG concentration for AVR414 was calculated by interpolation from the calibration curve using a four-parameter logistic log (4-PL) model and ELISA for Windows software (version 2.0) (31).
Quantitative IgG subclass-specific ELISA.
Monoclonal antibodies to human IgG1 (HP6069 γ1 Fc), IgG2 (HP6002 γ1 Fc), IgG3 (HP6047, anti-hinge region), and IgG4 (HP6025 γ1 Fc) (12, 34) were purchased from the Hybridoma Reagent Laboratory. For determination of a specific IgG subclass concentration, Immulon 2 HB microtiter plates (Thermo Labsystems) were coated with the appropriate monoclonal anti-subclass capture antibody at a concentration of 2 μg/ml in 0.01 M PBS (Life Technologies), pH 7.4, at 4°C for 16 to 24 h. The USNRP was used to generate 7-point standard curves in triplicate in a threefold dilution series (1:100 to 1:12,800). Purified anti-PA IgG from the human standard serum AVR414 was diluted in serum diluent, added to the first pair of test wells (100 μg/ml in 100 μl per well), mixed, and serially transferred down the plate in a threefold dilution series. Bound IgG subclasses were detected with polyclonal sheep anti-human IgG-horseradish peroxidase (ICN Biomedicals, Inc.). All subsequent reagents, incubation, and washing steps were performed as described above for the human anti-PA IgG ELISA. PA-specific IgG subclass concentrations were derived from a minimum of eight separate determinations. The anti-PA IgG subclass concentrations were calculated as described above (31).
Determination of total IgG and IgG subclasses by RID.
Mass values (in micrograms per milliliter) for total IgG and IgG subclasses in AVR414 were determined by RID using the NL NANORID and BINDARID kits, respectively (The Binding Site, Inc., San Diego, Calif.). Mass values assigned by RID were calculated from a minimum of five separate determinations over three nonconsecutive days using the kit-supplied calibrator as a standard. Concentrations of total IgG and IgG subclasses were interpolated from the standard curves generated on the same plate.
Performance characteristics of AVR414 in anti-PA IgG ELISA.
Performance characteristics established for AVR414 in human anti-PA IgG ELISA included dilution-corrected linearity of the assigned anti-PA IgG value, intermediate precision (repeatability over time) of a 7-point dilution series, and the goodness of fit of these data to a 4-PL model. Evaluation of parallelism between the AVR414 curve and the curves of test serum samples was performed in accordance with the guidelines described previously (30). Briefly, if the within-assay coefficient of variation (CV) is ≤20%, the curves are considered parallel (30). Dilution-corrected linearity of the curve of AVR414 in an anti-PA IgG ELISA was evaluated by comparison of the expected concentrations of anti-PA IgG in AVR414 for seven twofold dilutions (1:100 to 1:6,400; calibration factor, 141.2 μg/ml) with computed concentrations for the same dilutions (SAS version 8.0; SAS Institute, Cary, N.C.). Dilutional linearity was determined from triplicate curves in 34 independent assays (204 observations). The reportable IgG concentrations (in micrograms per milliliter) of three positive QC sera (AVR216, AVR284, and AVR370) were used to evaluate the assay's intermediate precision using AVR414 as a standard. The positive QC sera were obtained from CDC volunteers who had received at least four injections of AVA. The QC sera were tested in duplicate at single dilutions selected to represent high, medium, and low optical density regions of the reference serum standard curve. Intermediate precision was expressed as the CV of the concentration calculated for the standard curve dilutions between different assay plates over time and performed by different operators. The goodness of fit of the standard was evaluated in 503 different observations and expressed as the approximate multiple correlation coefficient (R2) (31). The utility of AVR414 as a human standard reference serum in an anti-PA IgG ELISA was evaluated with a separate vaccinee serum pool (AVR801) and a panel of 14 positive control vaccinee sera with a range of 58.0 to 398.4 μg of anti-PA IgG per ml. AVR801 was prepared by pooling equal volumes of serum from 57 different recipients of AVA. Donors for AVR801 had received a minimum of four s.c. injections of AVA with the licensed regimen. AVR414 was used to assign anti-PA-specific IgG and anti-PA-specific IgG subclass concentrations to AVR801 using the anti-PA IgG and anti-PA IgG1 to IgG4 subclass-specific ELISAs, respectively, as described above. The anti-PA IgG concentration of AVR801 was determined from the mean of 150 individual test results performed by three operators working independently over a 4-month period of time. Anti-PA IgG1 to IgG4 concentrations were determined from the mean of results from nine individual tests for each IgG subclass performed by three operators working independently using AVR414 as the standard reference serum in anti-PA IgG1 to IgG4 ELISAs. The collection and use of human sera were performed in concordance with the CDC and NIH Institutional Review Boards.
Statistical analysis.
The same mathematical and statistical methods were used for calculations of total and anti-PA-specific IgG and IgG subclasses. The IgG subclass concentrations were assayed separately by ELISA or by RID, and the means were determined, summed, and then compared to the mean concentration of total IgG from the independent assay, ELISA, or RID by using a two-sample t test (23). The t test was computed for various sample size alternatives based on the assumption that sample size might be interpreted as the minimum number for any one subclass, the design number for each subclass, or the total number used for all the subclasses. For the purpose of computing the overall variance (standard deviation) of the subclass sum, the four measurements were assumed to be statistically dependent, with central tendency matched at the mean for the purpose of matching unmatched data.
RESULTS
Total IgG and IgG subclasses.
The concentrations of total IgG in AVR414 determined by RID and by summation of IgG subclasses were 8.33 mg/ml and 8.50 mg/ml, respectively. These values are not statistically significantly different (P = 0.284) (Table 1). The values for individual IgG subclasses in AVR414 determined by RID were the following: for IgG1, 4.48 mg/ml (52.7% of total IgG); for IgG2, 3.35 mg/ml (39.4%); for IgG3, 0.37 mg/ml (4.4%); and for IgG4, 0.30 mg/ml (3.5%) (Table 1).
TABLE 1.
Total IgG and IgG subclass concentrations in anti-AVA standard reference serum AVR414 determined by RID
| Total IgG or IgG subclass | Mean (mg/ml) | SD (mg/ml) | CV (%) | n | % total IgG by subclassa |
|---|---|---|---|---|---|
| IgG1 | 4.48 | 0.09 | 2.0 | 5 | 52.7 |
| IgG2 | 3.35 | 0.22 | 6.6 | 7 | 39.4 |
| IgG3 | 0.37 | 0.02 | 5.4 | 7 | 4.4 |
| IgG4 | 0.30 | 0.00 | 0.0 | 5 | 3.5 |
| Independent observation of total IgG | 8.33 | 0.29 | 3.5 | 12 | 98 |
| Sum of total IgG subclasses | 8.50 | 0.24 | 2.8 | 5 | 100 |
A total IgG concentration of 8.50 mg/ml was used for more-accurate calculation of percentages of total IgG by subclass and based on consideration of equality of each set of data (P = 0.284).
PA-specific IgG and IgG subclasses.
The assignment of the anti-PA-specific IgG concentration in AVR414 was done by consensus of two complementary approaches: an anti-PA IgG ELISA using antigen affinity-purified anti-PA IgG as a calibrator and summation of the mean PA-specific IgG subclass concentrations determined by the IgG subclass-specific ELISA with USNRP as a standard. The anti-PA IgG concentration in AVR414 as determined by ELISA with purified anti-PA IgG was 139.0 μg/ml (Table 2). IgG subclass summation data were obtained from independent determinations in duplicate of 20, 8, 23, and 29 observations for IgG1, IgG2, IgG3, and IgG4, respectively (Table 3). The predominant anti-PA IgG subclass in AVR414 was IgG1, with a concentration of 79.6 μg/ml (55.51% of total IgG). The concentrations of IgG2 and IgG4 were 35.3 μg/ml (24.62% of total IgG) and 25.3 μg/ml (17.64%). The concentration of IgG3 in AVR414 was low (3.2 μg/ml; 2.2% of total IgG). These data indicate that the anti-PA IgG subclasses in AVR414 are primarily IgG1, IgG2, and IgG4 (Table 3). The sum of the individual IgG subclass concentrations (143.4 μg/ml) was compared with the concentration of total anti-PA IgG obtained by anti-PA IgG ELISA (139.0 μg/ml) by two-sample t test (23). There was no significant difference between the two mass values calculated (P = 0.42); a conservative minimum sample size of eight was used for calculating the sum of IgG subclasses. The overall anti-PA IgG concentration was computed as an equally weighted mean of the results from the two methods. The PA-specific IgG component of AVR414 was assigned to be 141.2 μg/ml and was 1.7% of the total IgG (Table 2).
TABLE 2.
Anti-PA-specific IgG concentrations in anti-AVA standard reference serum AVR414 determined by IgG PAN ELISA and by IgG subclass summation
| Anti-PA IgG concentration determination | Anti-PA IgG concentration
|
||
|---|---|---|---|
| Mean (μg/ml) | SD (μg/ml) | CV (%) | |
| IgG PAN ELISAa | 139.0 | 27.4 | 19.7 |
| Summation of anti-PA IgG subclassesb | 143.4 | 13.9 | 9.7 |
| Mean of two methodsc | 141.2 | 15.5 | 11.0 |
Assignment of total anti-PA IgG concentration was based on 147 independent determinations in duplicate.
The sample size for summation was approximated by the effective degrees of freedom (n = 12). Variance-weighted computation was based on the actual sample sizes for each subclass.
The variance and corresponding CV were computed as the squared weighted mean of the observed variances in the repeats of the respective experiments. The effective sample size was calculated as 20.
TABLE 3.
Comparison of anti-PA-specific IgG subclass distributions in the two anti-AVA standard reference sera, AVR414 and AVR801
| IgG subclass | AVR414
|
AVR801
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (μg/ml) | SD (μg/ml) | CV (%) | n | % total IgGa | Mean (μg/ml) | SD (μg/ml) | CV (%) | n | % total IgGa | |
| IgG1 | 79.6 | 9.46 | 11.7 | 20 | 55.5 | 60.2 | 5.19 | 8.62 | 9 | 54.1 |
| IgG2 | 35.3 | 9.73 | 27.6 | 8 | 24.6 | 25.8 | 2.79 | 10.81 | 9 | 23.2 |
| IgG3 | 3.2 | 0.70 | 21.9 | 23 | 2.2 | 4.5 | 0.53 | 11.78 | 9 | 4.0 |
| IgG4 | 25.3 | 2.97 | 11.7 | 29 | 17.6 | 20.8 | 3.11 | 14.95 | 9 | 18.7 |
The total anti-PA IgG concentrations obtained by sum of the mean of the anti-PA IgG subclass concentrations (143.4 μg/ml for AVR414 and 111.3 μg/ml for AVR801) were used for a more accurate calculation of the percentage of total IgG by subclass. This approach was based on consideration of equality of each set of data for comparison of means, with a P value of 0.785 for AVR414 and a P value of 0.467 for AVR801.
ELISA performance characteristics of human reference serum AVR414.
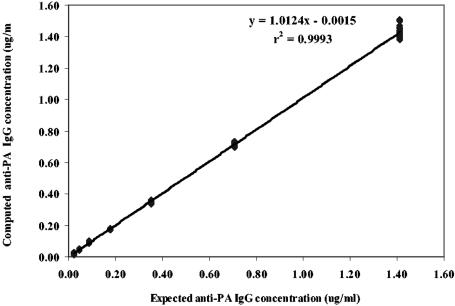
An evaluation of parallelism between the AVR414 curve and the curves of test serum samples showed that the within-assay CV was ≤20% and that the antibody-binding characteristics of standard reference serum AVR414 and the serum samples are similar enough to allow the determination of antibody concentrations in diluted serum samples (30). The interassay precision level of the human anti-PA IgG ELISA was high. The CVs for three positive QC sera were less then 20%: 13.3% for QC1 (AVR216; 163 tests), 17.8% for QC2 (AVR284; 201 tests), and 16.4% for QC3 (AVR370; 215 tests). These values are within the accepted values of 20% for interassay precision (11) and are indicative of a high level of precision for this type of assay (24). The goodness of fit (mean R2) for the AVR414 standard curve calculated over 503 separate assays was 0.998. The intermediate precision of the positive control serum panel expressed as the mean CV was 12.7% (14 separate sera; 378 observations). These values for interassay precision levels are also within the accepted values of 20% for enzyme immunoassays and indicate a high level of precision for the performance of the AVR414 standard in these assays (24). Diluting AVR414 over the range from 1:100 to 1:6,400 and evaluating the resultant plot of measured concentration versus expected concentration by regression analyses of interpolated results from the 4-PL model fit indicated a highly significant linear relationship (mean R2 = 0.999; P < 0.001), with a slope of 1.0 and an intercept through the origin (Fig. 1). Analysis of serum pool AVR801 showed that the anti-PA IgG concentration (109.4 μg/ml) determined by anti-PA IgG ELISA using AVR414 as the standard was not significantly different (P = 0.467) from the anti-PA IgG concentration obtained by the summation of anti-PA IgG subclasses (111.3 μg/ml). The anti-PA IgG subclass concentrations with AVR801 obtained by anti-PA ELISAs using AVR414 as the standard were the following: for IgG1, 60.2 μg/ml; for IgG2, 25.8 μg/ml; for IgG3, 4.5 μg/ml; and for IgG4, 20.8 μg/ml (Table 3). The anti-PA IgG subclass distribution in AVR801 was similar to that in AVR414 (54.1% for IgG1, 23.2% for IgG2, 4.0% for IgG3, and 18.7% for IgG4) (Table 3).
FIG. 1.
Linear regression of the results of diluting AVR414 from 1:100 to 1:6,400 and evaluating measured concentration versus expected concentration of anti-PA IgG. Data points are the means of results from triplicate determinations.
DISCUSSION
A qualified and characterized standard reagent is a critical component in the development and standardization of serological assays and provides a benchmark for the comparative analysis of new assay technologies (1, 15, 21). In this study, we report the quantification of total IgG and IgG subclasses and the assignment of mass values for total and subclass-specific anti-PA IgG in human standard anthrax reference serum AVR414. The concentration of total IgG assigned for AVR414 (8.33 mg/ml) was within the established range for human serum IgG in healthy adults (8 to 16 mg/ml) (2, 8, 9, 16). The concentrations of IgG1 (4.48 mg/ml) and IgG3 (0.37 mg/ml) in AVR414 were lower than the established range for normal healthy human adults (5.0 to 12.0 mg/ml and 0.5 to 1.0 mg/ml, respectively) (2, 8, 16, 22). The percentages of total IgG represented by IgG1 (52.7%) and IgG3 (4.4%) were also lower than the range in normal adult sera (60.3 to 71.5% and 5.0 to 8.4%, respectively) (8). The concentration of IgG2 (3.35 mg/ml) was within the established range for normal adult sera with a dynamic range of 2.0 to 6.0 mg/ml (2, 8, 22, 36), but the IgG2 percentage of total IgG (39.4%) was higher than the established range (19.4 to 31.0%) (8). The AVR414 standard reference serum pool contains anti-PA-specific IgG of all four subclasses, with concentrations of PA-specific IgG1, IgG2, and IgG4 being the highest (55.5, 24.6, and 17.6% of total anti-PA IgG) and the concentration of IgG3 being the lowest (2.2% of total anti-PA IgG). A possible explanation for the low concentration of anti-PA IgG3 in AVR414 is affinity maturation and isotype switching to IgG2 and IgG4 (4) due to the plasma donors in this study receiving a minimum of four vaccinations of the licensed AVA regimen.
The anti-PA IgG concentrations assigned by the two methods were not significantly different (P = 0.42). This level of probability from two different approaches for mass values assignment is an indication of the robustness and reliability of the assigned units. The utility of AVR414 has been clearly demonstrated in its application to anti-PA IgG mass value assignments in a panel of QC and positive vaccine control sera and the assignment of PA-specific values to an additional standard reference serum pool, AVR801.
It has been our objective to create a standardized platform technology and reagents that will enable the comparative analyses of human anti-PA IgG responses to clinical anthrax and anthrax vaccines. The characterization of the anti-PA-specific total IgG and IgG subclasses of the standard AVR414 described here provides a strong basis for this standardization. AVR414 has been successfully applied in quantitative serological assays to evaluate human humoral antibody immune responses to vaccination with AVA (N. Marano, J. Lingappa, P. Pittman, V. Semenova, S. Leitman, P. Plikaytis, C. Quinn, and B. Perkins, Abstr. 5th Int. Conf. Anthrax, abstr. O609, 2003), for analysis of the immune response to PA in individuals with bioterrorism-associated cutaneous and inhalation anthrax (5, 10, 13, 14, 32, 35, 37), and for evaluation of anti-PA IgG subclass distribution in anthrax vaccinees and confirmed cases of clinical anthrax (V. A. Semenova, P. M. Dull, D. S. Schmidt, T. H. Taylor, E. Steward-Clark, M. M. Ballard, and C. P. Quinn, Int. J. Infect. Dis. vol. 8, abstr. 35.010, p. S111, 2004). For each of those studies, the AVR414 standard reference serum was the pivotal unifying reagent. Qualified reagents, such as AVR414, will facilitate assay technology transfer and reduce interassay and interlaboratory variance (3, 6). In addition, the determination of assay end points using a 4-PL model to describe the standard curve parameters (29) also makes a significant contribution to assay standardization by reducing the number and type of possible errors related to interpolating antibody concentrations from the standard curve (30). Together these are valuable tools in standardizing the evaluation of the human anti-PA IgG immune response in recipients of PA-containing vaccines and in cases of human anthrax.
Acknowledgments
We acknowledge the contribution of CDC employees and NIH volunteers for their donation of plasma and serum samples, Emory Blood Bank for plasmapheresis of CDC volunteers, Wanda Philips for conversion of plasma to serum, Stephen H. Leppla for provision of anthrax toxin protective antigen, and Susan Leitman (NIH) for providing the plasma for AVR801.
REFERENCES
- 1.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. F. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin. Diagn. Lab. Immunol. 11:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradwell, A. R. 1995. IgG and IgA subclasses in disease, p. 6. The binding site, San Diego, Calif.
- 3.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S. H. Tuner, B. D. Plikaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, B. Swaminathan, G. Arakere, C. Thompson, D. Phipps, D. Madore, and C. V. Broome. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devey, M. E. 1990. Affinity of IgG subclass antibodies, p. 185-194. In Farouk Shakib (ed.), The human IgG subclasses: molecular analysis of structure, function and regulation. Pergamon Press, Inc., Elmsford, N.Y.
- 5.Dewan, P. K., A. M. Fry, K. Laserson, B. C. Tierney, C. P. Quinn, J. A. Hayslett, L. N. Broyles, A. Shane, K. L. Winthrop, I. Walks, L. Siegel, T. Hales, V. A. Semenova, S. Romero-Steiner, C. Elie, R. Khabbaz, A. S. Khan, R. A. Hajjeh, A. A. Schuchat, and the members of the Washington, D.C., Anthrax Response Team. 2002. Inhalational anthrax outbreak among postal workers, Washington, D. C., 2001. Emerg. Infect. Dis. 8:1066-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheesling, L. L., G. M. Carlone, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, H. Käyhty, J. P. Mays, L. Nencioni, C. Peeters, D. C. Phipps, J. T. Poolman, E. Rosenqvist, G. R. Siber, B. Thiesen, J. Tai, C. M. Thompson, P. P. Vella, and J. D. Wenger. 1994. Multicenter comparison of Neisseria meningitidis serogroup C capsular polysaccharide antibody levels measured by standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandics, P., Z. Szathmary, and S. Szathmary. 1990. A novel immunoaffinity chromatography system for the purification of therapeutic proteins. Ann. N. Y. Acad. Sci. 589:148-156. [Google Scholar]
- 8.Hamilton, R. G. 1998. The human IgG subclasses, p. 28. Calbiochem-Novabiochem, La Jolla, Calif.
- 9.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p. 10. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9a.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p. 658-681. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Hsu, V. P., S. L. Lukacs, T. Handzel, J. Hayslett, S. Harper, T. Hales, V. A. Semenova, S. Romero-Steiner, C. Elie, C. P. Quinn, R. Khabbaz, A. S. Khan, G. Martin, J. Eisold, A. Schuchat, and R. A. Hajjeh. 2002. Opening a Bacillus anthracis-containing envelope, Capitol Hill, Washington, D.C.: the public health response. Emerg. Infect. Dis. 8:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson, R. H. 1998. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. 17:469-526. [DOI] [PubMed] [Google Scholar]
- 12.Jefferis, R., C. B. Reimer, F. Skvaril, G. de Lange, N. R. Ling, J. Lowe, M. R. Walker, D. J. Phillips, C. H. Aloisio, T. W. Wells, J. P. Vaerman, C. G. Magnusson, H. Kubagawa, M. Cooper, F. Vartdal, B. Vandvik, J. J. Haaijman, O. Makela, A. Sarnesto, Z. Lando, Z. J. Gergely, E. Rajnavolgyi, G. Laszio, J. Radi, and G. A. Molinaro. 1985. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol. Lett. 10:223-252. [DOI] [PubMed] [Google Scholar]
- 13.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, J. L. Gerberding, and the National Anthrax Epidemiologic Investigation Team. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W.-J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, B. A. Perkins, and members of the Anthrax Bioterrorism Investigation Team. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, A. M., J. T. Whicher, T. B. Ledue, A. Carlstrom, Y. Itoh, and P. H. Petersen. 2000. Effect of a new international reference preparation for proteins in human serum (certified reference material 470) on results of the College of American Pathologists Surveys for plasma proteins. Arch. Pathol. Lab. Med. 10:1496-1501. [DOI] [PubMed] [Google Scholar]
- 16.Kuby, J. 1996. Immunology, p. 124-126. W. H. Freeman and Company, New York, N.Y.
- 16a.Kuby, J. 1996. Immunology, p. 325-326. W. H. Freeman and Company, New York, N.Y.
- 17.Lansing, Michigan, Department of Public Health. 1978. Anthrax vaccine adsorbed (package insert). Lansing, Michigan, Department of Public Health, Lansing.
- 18.Leppla, S. H. 1991. Purification and characterization of adenylyl cyclase from Bacillus anthracis. Methods Enzymol. 195:153-168. [DOI] [PubMed] [Google Scholar]
- 19.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little, S. F., and B. E. Ivins. 1999. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1:131-139. [DOI] [PubMed] [Google Scholar]
- 21.Mäkelä, O., and F. Péterfy. 1983. Standard sera in solid-phase immunoassays. Eur. J. Immunol. 13:815-819. [DOI] [PubMed] [Google Scholar]
- 22.Maranon, F., M. Casanovas, L. Berrens, J. M. Olles, and M. A. Dieguez. 1994. A competitive enzyme immunoassay subclass for the determination of total IgG subclass levels in human serum. J. Immunol. 15:147-156. [DOI] [PubMed] [Google Scholar]
- 23.Moore, D. S., and G. P. McCabe. 1993. Introduction to the practice of statistics, 2nd ed., p. 499-573. W. H. Freeman and Company, New York, N.Y.
- 24.O'Connel, M. A., B. A. Belanger, and P. D. Haaland. 1993. Calibration and assay development using the four-parameter logistic model. Chemom. Intell. Lab. Syst. 20:97-114. [Google Scholar]
- 25.Park, S., and S. H. Leppla. 2000. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 18:293-302. [DOI] [PubMed] [Google Scholar]
- 26.Pittman, P. R., J. A. Mangiafico, C. A. Rossi, T. L. Cannon, P. H. Gibbs, G. W. Parker, and A. M. Friedlander. 2000. Anthrax vaccine: increasing intervals between the first two doses enhances antibody response in humans. Vaccine 19:213-216. [DOI] [PubMed] [Google Scholar]
- 27.Pittman, P. R., P. H. Gibbs, T. L. Cannon, and A. M. Friedlander. 2001. Anthrax vaccine: short-term safety experience in humans. Vaccine 20:972-978. [DOI] [PubMed] [Google Scholar]
- 28.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 29.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plikaytis, B. D., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and nonparallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plikaytis, B. D., P. F. Holder, and G. M. Carlone. 1996. Program ELISA for Windows user's manual, version 2.00. Centers for Disease Control and Prevention, Atlanta, Ga.
- 32.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. L. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimer, C. B., S. J. Smith, T. W. Wells, R. M. Nakamura, P. W. Keitges, R. F. Ritchie, G. W. Williams, D. J. Hanson, and D. B. Dorsey. 1982. Collaborative calibration of the U.S. National and the College of American Pathologists reference preparation for specific serum proteins. Am. J. Clin. Pathol. 77:12-19. [DOI] [PubMed] [Google Scholar]
- 34.Reimer, C. B., D. J. Phillips, C. H. Aloisio, D. Moore, G. G. Galland, T. W. Wells, C. M. Black, and J. S. McDougal. 1984. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma 3:263-273. [DOI] [PubMed] [Google Scholar]
- 35.Shieh, W. J., J. Guarner, C. Paddock, P. Greer, K. Tatti, M. Fischer, M. Layton, M. Philips, E. Bresnitz, C. P Quinn, T. Popovic, B. A. Perkins, and S. R. Zaki. 2003. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am. J. Pathol. 163:1901-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Söderstrom, T., R. Söderstrom, A. Avanzini, P. Brandtzaeg, G. Karlsson, and L. A. Hanson. 1987. Immunoglobulin G subclass deficiencies. Int. Arch. Allergy Appl. Immunol. 82:476-480. [DOI] [PubMed] [Google Scholar]
- 37.Traeger, M. S., S. T. Wiersma, N. E. Rosenstein, J. M. Maleckit, C. W. Shepard, P. L. Raghunathan, S. P. Pillai, T. Popovic, C. P. Quinn, R. F. Meyer, S. R. Zaki, S. C. Tierney, J. D. Jones, B. A. Perkins, and the Florida Investigation Team. 2002. First case of bioterrorism-related international anthrax in the United States, Palm Beach County, Florida, 2001. Emerg. Infect. Dis. 8:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]