Abstract
The disease-programming effects of a maternal low-protein (MLP) diet in rat pregnancy have been suggested to be attributable of hyperhomocysteinaemia. The aim of the present study was to determine whether MLP feeding impacted upon maternal and day 20 fetal homocysteine concentrations, with ensuing effects upon oxidant/antioxidant status. Sixty-four pregnant rats were fed either MLP diet or control diet before termination of pregnancy at days 4, 10, 18 or 20 gestation (full-term gestation 22 d). Maternal plasma homocysteine concentrations were similar in control and MLP-fed dams at all points in gestation. Fetal plasma homocysteine was similarly unaffected by MLP feeding at day 20 gestation. Activities of superoxide dismutase and glutathione peroxidase were similar in livers of mothers and fetuses in the two groups. Whilst catalase activity was not influenced by diet in maternal liver, MLP exposure increased catalase activity in fetal liver at day 20. Oxidative injury (protein carbonyl concentration) was lower in the livers of MLP-fed animals at day 18 gestation (P<0·05), but significantly greater at day 20. Hepatic expression of methionine synthase was similar in control and MLP-fed dams at all stages of gestation. Expression of DNA methyltransferase 1 in fetal liver was altered by maternal diet in a sex- and gestational age-specific manner. In conclusion, MLP feeding does not impact upon maternal or fetal homocysteine concentrations prior to day 20 gestation in the rat. There was no evidence of increased oxidative injury in fetal tissue that might explain the long-term programming effects of the diet.
Keywords: Programming, Pregnancy, Homocysteine, Antioxidants
Epidemiological associations between risk of heart disease and diabetes in adult life and indices of fetal growth have prompted the suggestion that early life events programme physiological and metabolic processes (Gluckman & Hanson, 2004). Experimental studies of rodents using a wide variety of nutritional manipulations in pregnancy confirm that nutritional programming plays an important role in the aetiology of disease (Langley-Evans et al. 1996b; Gambling et al. 2003; Khan et al. 2003). One such approach has been the feeding of a maternal low-protein (MLP) diet in rat pregnancy. Offspring of MLP-fed rat dams exhibit hypertension (Langley & Jackson, 1994), disturbed glucose handling (Langley et al. 1994), impaired renal development (Langley-Evans et al. 1999) and have a shorter lifespan (Sayer et al. 2001).
Studies with the MLP diet have generally used casein as the sole protein source, and as such have required additional dietary methionine to provide requirements for sulphur. Rees (2002) suggests that the level of methionine provided exceeds the capacity of the maternal liver to convert it to cysteine via the trans-sulphuration pathway. This leads to accumulation of toxic homocysteine which will have the capacity to cause oxidative injury unless recycled to methionine via methionine synthase or homocysteine-betaine methyltransferase activities (Young et al. 2004). Disturbances of the methionine–homocysteine cycle may impact upon the provision of methyl donors for methylation of DNA, which is proposed as a mechanism for heritable programming of gene function (Waterland & Jirtle, 2003; McMillen & Robinson, 2005).
This view is supported by evidence from a number of studies. Studies in which the MLP diet is supplemented with folic acid (Lillycrop et al. 2005) or with glycine (Jackson et al. 2003) generally show that the effects of low-protein feeding can be reversed. Lillycrop et al. (2005) have shown that MLP feeding impacted upon both the expression and methylation status of specific genes. However, in contrast, Bogdarina et al. (2004) found no evidence that maternal protein restriction impacted on the methylation of the promoter for hepatic glucokinase, despite programmed changes in gene expression. The case for a gross imbalance in methionine–homocysteine metabolism, whilst supported indirectly by the observed effects of folate or glycine supplementation, is yet to be confirmed. Indeed Brawley et al. (2004) reported no differences in maternal homocysteine concentrations in low-protein-fed pregnant rats compared to controls.
The present study aims to assess the potential contribution of elevated plasma homocysteine to programming of physiological and metabolic processes by focusing primarily on the fetal period. This avoids the potential contribution of secondary mechanisms that may act to influence development in the postnatal period. In addition to considering the impact of MLP feeding on maternal homocysteine, the study assesses the possibility that the pro-oxidant effects of homocysteine impact upon oxidant–antioxidant balance within both maternal and fetal tissue. Our hypothesis was that MLP feeding would elevate homocysteine, increase oxidative injury in both the maternal and fetal compartment, and impact upon expression of DNA methyltransferase 1 (DNMT1), which is one of the enzymes responsible for the maintenance of DNA methylation patterns during development (Newell-Price et al. 2000). DNMT1 was selected for study as the other DNA methyltransferases (3L, 3a and 3b) are expressed only in the embryonic period and are responsible for de novo methylation. Their role in the maintenance of imprinted methylation patterns is of little significance.
Materials and methods
Chemicals and reagents
All chemicals and reagents were of reagent grade and purchased from Fisher Scientific (Loughborough, UK).
Animals
Experiments described in the present paper were performed under licence from the Home Office in accordance with the 1986 Animals Act. Rats were housed in plastic boxes on a 12 h light cycle, at a temperature of 20 ± 2°C. The rats had free access to food and water at all times. Sixty-four virgin female Wistar rats (Harlan Ltd, Belton, UK) were mated at weights between 180 and 220 g. Upon confirmation of mating by the appearance of a semen plug on the cage floor, the rats were allocated to be fed either a control diet (180 g casein/kg diet, n 32) or a low-protein diet (MLP; 90 g casein/kg diet, n 32), as described previously (Langley-Evans et al. 1994). The full composition of the diets is described elsewhere (Langley-Evans et al. 1994), but essentially each diet was based on casein mixed with starch–sucrose (2:1, w/w), cellulose, maize oil, and minerals and vitamins formulated to AIN76 specification. The diets were isoenergetic, the difference in energy between the control and MLP diets being made up with additional carbohydrate (starch–sucrose 2:1, w/w). The rats were fed the semi-synthetic diets until pregnancy was terminated at days 4, 10, 18 or 20 gestation. At these time-points the pregnant rats were killed using CO2 asphyxia and cervical dislocation. Maternal blood was collected by cardiac puncture and transferred to heparin tubes. Blood was centrifuged at 13 000 rpm and 4°C for 10 min and the plasma was retained. The maternal liver was rapidly dissected and snap-frozen in liquid nitrogen. Liver and plasma were stored at −80°C for up to 6 months until used for further analyses. Six of the MLP-fed and two control dams failed to carry their pregnancy to the time of cull.
At days 18 and 20 gestation, the uterus was removed and each horn was opened. Fetuses were removed and separated from the placentas and, following weighing, were killed by decapitation for collection of blood samples. Fetal blood samples were pooled from all the animals in the litter. The placentas were trimmed, blotted and weighed. Each fetus had its sex determined by genito-anal distance, and the position in the horn was noted along with the sex of the neighbouring fetuses. The number of resorption sites in the uterus was also noted. The liver, heart and kidneys were dissected from each fetus, weighed to the nearest 0·1 mg and then snap-frozen in liquid nitrogen. Fetal blood was centrifuged at 13 000 rpm and 4°C for 10 min and the plasma was retained. Organs and plasma were stored at −80°C until used for further analyses.
Plasma homocysteine determination
Homocysteine concentrations were measured in day 20 fetal and in all maternal plasma samples using a commercial kit (Diazyme, La Jolla, CA, USA). Insufficient sample was obtained for assay of fetal homocysteine at day 18 gestation. For sample extraction, 20 μl plasma sample or calibrator (2–60 μmol/l S-adenosyl-l-homocysteine) were added to eppendorf tubes containing 150 μl of a mixture of reagents (130 μl adenosine, Tris (2-carboxyethyl)-phosphine hydrochloride, 10 μl Tris buffer, 50 mm-phosphate buffer and 10 μl recombinant S-adenosyl-l-homocysteine hydrolase, phosphate buffer and glycerol) prepared according to the manufacturer’s instructions. After thorough mixing and incubation at 37°C for 30 min, 100 μl adenosine analogue in phosphate buffer were then added to each tube. Tubes were mixed well and incubated at room temperature (18–25°C) for 10 min. Adenosine deaminase (50 μl) was added and tubes were left to incubate at room temperature for a further 15 min. Finally, 100 μl DEAE-Sephadex was added to the tubes, the solution was mixed and incubated at room temperature for 10 min.
The pretreated samples or calibrators (25 μl) were loaded in duplicate on to avidin-coated microtitre plate strips. Diazyme™-12A-biotin conjugate (50 μl) was then added to each microtitre well and the plate was left to incubate for 5 min at room temperature. Horseradish peroxidase–S-adenosyl-l-homocysteine (25 μl) was added to each well and was left to incubate for a further 30 min in the dark, at room temperature. The plate was then decanted and blotted on paper towel. The wells were washed three times with 400 μl wash per well. Tetramethylbenzidine solution (100 μl) was added to each well and left to incubate at room temperature for 10 min. Finally, 100 μl 1 m-phosphoric acid (stop solution) were added to the wells. The plate was then read at 450 nm using a plate reader (Tecan Sunrise, Männedorf, Switzerland) within 15 min of adding the stop solution. Calibration curves were prepared using a four parameter logistic curve fit. The intra-assay variation for this assay was 7·74 % and all samples fell within the dynamic range of the assay.
Determination of mRNA expression
Total RNA was isolated from snap-frozen livers using the TRIzol method (Invitrogen, Paisley, UK). The RNA was treated with DNase (Promega, UK) and subjected to phenol–chloroform extraction and ethanol precipitation. Total RNA (0·5 μg) was reverse-transcribed using MMLV RT (Promega, Southampton, UK). Real-time RT–PCR was performed using an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA, USA). A template-specific primer pair and an oligonucleotide probe (Sigma-Genosys, Poole, UK) specific to each of methionine synthase, DNMT1 and the housekeeping gene β-actin were designed using Primer Express version 1.5 (Applied Biosystems). The full sequences of the primers and probes are shown in Table 1. All primer sets were tested under the Taqman PCR conditions using rat genomic DNA as a template. In all cases a single product of the appropriate size was detected by gel electrophoresis (data not shown). A negative template control and relative standard curve were included on every PCR run. The standard curve was prepared from a pool of sample cDNA at relative dilutions of 0·05, 0·1, 0·2, 0·4, 1·0, 2·5 and 5·0. Relative target quantity was calculated from the standard curve and all samples were normalised to β-actin expression.
Table 1.
Primer sequences
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Methionine | gtatcgcccaggctgactatg | cacgtctgcggagcatttg | catccggtaggccaagtgttcgagg |
| synthase, | TM = 58, GC = 57 | TM = 60, GC = 58 | TM = 69, GC = 60 |
| NM_030864 | Length = 21 bp | Length = 19 bp | Length = 25 bp |
| DNA | ggaaggtgagcatcgacgaa | gggatcatccggaatgacc | acgcagtcgcccacctccagag |
| methyltransferase 1, | TM = 60, GC = 55 | TM = 59, GC = 58 | TM = 69, GC = 68 |
| NM_001015006 | Length = 20 bp | Length = 19 bp | Length = 22 bp |
| β-Actin, | ttcaacaccccagccatgt | gtggtacgaccagaggcataca | cgtagccatccaggctgtgttgtcc |
| NM_031144 | TM = 58, GC = 53 | TM = 59, GC = 55 | TM = 68, GC = 70 |
| Length = 19 bp | Length = 22 bp | Length = 25 bp |
TM, melting temperature (°C); GC, percentage of G and C bases.
Antioxidant enzyme activities
The activities of the antioxidant enzymes catalase, glutathione peroxidase (GPx) and superoxide dismutase (SOD) were determined in liver samples. In the present study, SOD activity represents total SOD within the tissue with no separation of the CuZn and Mn forms. Activity of GPx was determined as described previously (Langley-Evans & Sculley, 2005). Catalase was determined using the spectrophotometric method of Aebi (1984), in which the conversion of H2O2 to H2O and O2 is monitored as a change in absorbance at 240 nm. SOD was determined using the method of Winterbourn et al. (1975), in which the light-triggered release of superoxide radicals from riboflavin leads to the formation of a blue complex through reaction with nitroblue tetrazolium. All enzyme activities are expressed as units per mg protein. Protein concentrations were determined using the method of Bradford (1976).
Protein carbonyl concentrations
Tissue protein carbonyl concentration was used as a marker of oxidative injury. Hepatic protein carbonyl concentrations were determined using an adaptation of the method of Levine et al. (1990). Samples were homogenised in 50 mm-potassium phosphate buffer, 5 mm-EDTA, pH 7·4, at a concentration of 100 mg tissue/ml. After centrifugation at 13 000 g for 5 min at 4°C, four aliquots of 100 μl supernatant were removed for duplicate assays. Each aliquot was treated for 5 min with 500 μl 20 % trichloracetic acid solution to precipitate out protein at 4°C. Protein was pelleted by centrifugation at 13 000 g for 5 min. All pellets were resuspended using 2m-HCl. For each sample two aliquots were resuspended using the acid containing 0·1 % 2,4-dinitrophenylhydrazine, which forms complexes with protein carbonyl residues that are characteristic of oxidised proteins. After incubation for 1 h on ice, all samples were reprecipitated with 500 μl 20 % trichloracetic acid. Excess 2,4-dinitrophenylhydrazine was removed from the pellets with three washes using ethyl acetate–acetone (1:1, v/v) and repeated centrifugation at 13 000 g for 5 min. After the final wash pellets were resuspended in 0·8 ml 6 m-guanidine hydrochloride and absorbance was measured at 370 nm. The carbonyl concentration was then determined using the extinction coefficient of 21 000 M−1 cm−1. Data are expressed as nmol carbonyls per mg protein.
Statistical analysis
All data are presented as means and their standard errors. Following assessment of the normality of distributions for all variables using a Kolmogorov–Smirnov test, all data were analysed using two- or three-way ANOVA as appropriate, followed by a least significance difference test as a post hoc test. Fetal and placental weight data were adjusted for litter size, the position in the uterine horn and the sex of the neighbouring fetuses in the horn, as these are factors known to impact on fetal growth in the rat. P<0·05 was accepted as statistically significant.
Results
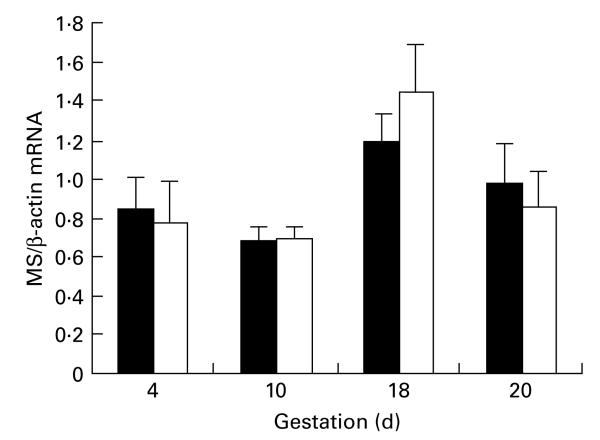
As shown in Table 2, the weight gain of the pregnant animals was unaffected by the feeding of the MLP diet. Litter size (not assessed at days 4 or 10) was not affected by maternal diet at day 18 gestation. Litter size was significantly lower in the MLP group than in the control group at day 20 gestation. Maternal plasma homocysteine concentration tended to decline with advancing gestation (Table 2), but this effect was not statistically significant (P=0·118). There was no statistically significant effect of feeding the MLP diet on maternal homocysteine. Fetal plasma homocysteine concentrations (Table 2) at day 20 were similar to those noted in maternal plasma, but no correlation between maternal and fetal concentrations was observed. Methionine synthase is one of the key enzymes catalysing the regeneration of methionine from homocysteine. No difference in the expression of hepatic methionine synthase was noted between control and MLP-fed dams at any stage of gestation (Fig. 1). Methionine synthase expression was normalised against expression of β-actin, which did not vary with gestation or maternal diet.
Table 2.
Maternal weight gain, plasma homocysteine and litter size of rats fed either maternal low-protein (MLP) diet or control diet‡
(Mean values with their standard errors)
| Days gestation |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 |
10 |
18 |
20 |
|||||||||||||
| Control (n 8) |
MLP (n 6) |
Control (n 8) |
MLP (n 8) |
Control (n 7) |
MLP (n 7) |
Control (n 7) |
MLP (n 5) |
|||||||||
| Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | |
| Weight gain (g)§ | 21 | 2 | 26 | 2 | 54* | 3 | 50* | 4 | 142* | 4 | 153* | 7 | 151* | 9 | 154* | 13 |
| Litter size (no. of pups)∥ | 15 | 1 | 16 | 2 | 17 | 2 | 14† | 2 | ||||||||
| Maternal plasma homocysteine (μmol/l) | 12·9 | 1·1 | 14·9 | 1·1 | 12·3 | 0·9 | 12·7 | 3·0 | 8·16¶ | 0·38 | 12·8 | 4·4 | 8·3 | 1·4 | 7·7 | 2·5 |
| Fetal plasma homocysteine (μmol/l) | 7·4 | 0·7 | 7·2 | 1·7 | ||||||||||||
Mean values were significantly different from those of day 4 gestation within the same maternal dietary group: P<0·05.
Mean value was significantly different from that of the control group: P<0·05.
For details of procedures, see pp. 579–580.
Maternal weight gain increased with gestation (P<0·001).
Litter size was influenced by the interaction of maternal diet and gestation (P=0·007).
n 6.
Fig. 1.
Maternal hepatic methionine synthase (MS) mRNA expression. Data are presented as the ratio of MS mRNA expression to β-actin mRNA expression (arbitrary units). For details of procedures, see pp. 579–580. Values are means with their standard errors depicted by vertical bars. For n values, see Table 2. ANOVA indicated a significant effect of gestation (P=0·002). (∎) Control; (◻) maternal low-protein diet.
The activities of the major antioxidant enzymes in the maternal liver varied considerably with increasing gestation (Table 3). GPx activity increased 6-fold between days 10 and 18 gestation and remained elevated at day 20. ANOVA indicated that this activity increased to a lesser extent in MLP-fed dams than in the control group at day 18 gestation (interaction of gestation and maternal diet, P=0·011). Total SOD activity also increased with gestation. A similar pattern was noted for catalase activity. Oxidative damage, as indicated by the presence of protein carbonyls, was observed in all samples and increased with gestation (Table 3). At day 18 MLP-fed rats had significantly lower protein carbonyl concentrations, but by day 20 MLP feeding was associated with significantly more oxidative damage in liver.
Table 3.
Maternal liver antioxidant enzyme activities and protein carbonyl concentrations of rats fed either maternal low-protein (MLP) diet or control diet‡
(Mean values with their standard errors)
| Days gestation |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 |
10 |
18 |
20 |
|||||||||||||
| Control (n 8) |
MLP (n 6) |
Control (n 8) |
MLP (n 8) |
Control (n 7)§ |
MLP (n 7) |
Control (n 7) |
MLP (n 5)∥ |
|||||||||
| Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | |
| GPx (units/mg protein)¶ |
0·259 | 0·015 | 0·221 | 0·009 | 0·276 | 0·092 | 0·242* | 0·078 | 1·800† | 0·146 | 1·493† | 0·060 | 1·463† | 0·064 | 1·593† | 0·080 |
| SOD (units/mg protein)** |
0·681 | 0·063 | 0·604 | 0·063 | 0·661 | 0·039 | 0·608 | 0·039 | 0·893† | 0·056 | 0·788 | 0·040 | 0·755 | 0·032 | 0·836† | 0·068 |
| Catalase (units/mg pro- tein)†† |
223·7 | 19·7 | 171·3 | 14·1 | 172·7 | 20·2 | 179·1 | 9·2 | 441·5† | 71·6 | 312·0† | 13·9 | 298·0 | 35·2 | 373·0† | 37·4 |
| Carbonyls (nmol/mg pro- tein)‡‡ |
0·134 | 0·020 | 0·142 | 0·010 | 0·139 | 0·020 | 0·124 | 0·020 | 0·352† | 0·040 | 0·250†* | 0·015 | 0·284† | 0·030 | 0·408†* | 0·044 |
GPx, glutathione peroxidase; SOD, superoxide dismutase.
Mean values were significantly different from those of the control group: P<0·05.
Mean values were significantly different from those of day 4 gestation within the same maternal dietary group: P<0·05.
For details of procedures, see pp. 579–580.
n 6 for GPx and SOD.
n 4 for GPx and SOD.
Activity of GPx was influenced by gestation (P<0·001) and the interaction of diet and gestation (P=0·011).
Total SOD activity was influenced by gestation (P<0·001).
Catalase activity was influenced by gestation (P<0·001) and the interaction of diet and gestation (P=0·030).
Protein carbonyl concentration was influenced by gestation (P<0·001) and the interaction of diet and gestation (P<0·001).
As shown in Table 4, the maternal diet had no significant impact upon fetal weight at either day 18 or day 20, after adjustment for litter size, position in horn and the sex of neighbouring fetuses. Placental weight, however, was significantly lower in the MLP-fed animals than controls at day 18 gestation and at day 20 gestation (female fetuses only). Fetal/placental ratio increased significantly between days 18 and 20 gestation. This increase was less marked in female fetuses, but not affected by the maternal diet. Liver and heart weight relative to body weight was constant throughout gestation and similar in control and MLP-treated pregnancies (Table 4). Kidney weight relative to body weight increased between days 18 and 20, with the same magnitude of increase noted in both groups of animals.
Table 4.
Fetal and placental weight and fetal organ weight of rats fed either maternal low-protein (MLP) diet or control diet§
(Mean values with their standard errors)
| Fetal wt (g)∥ |
Placental wt (g)¶ |
Fetal/placental ratio** |
Liver (% body wt) |
Heart (% body wt) |
Kidney (% body wt)†† |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestation (d) | Maternal diet | Sex | n | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem |
| 18 | Control | Male | 49 | 1·48 | 0·04 | 0·50 | 0·01 | 3·01 | 0·06 | 7·09 | 0·14 | 0·65 | 0·02 | 0·40 | 0·01 |
| Female | 52 | 1·47 | 0·04 | 0·49 | 0·01 | 3·08 | 0·08 | 7·17 | 0·15 | 0·63 | 0·02 | 0·38 | 0·01 | ||
| MLP | Male | 52 | 1·44 | 0·04 | 0·46* | 0·01 | 3·25 | 0·10 | 7·36 | 0·17 | 0·62 | 0·02 | 0·38 | 0·01 | |
| Female | 58 | 1·44 | 0·03 | 0·45* | 0·01 | 3·24 | 0·08 | 7·55 | 0·21 | 0·63 | 0·02 | 0·39 | 0·01 | ||
| 20 | Control | Male | 60 | 3·60† | 0·08 | 0·60† | 0·02 | 6·23† | 0·20 | 5·75 | 0·18 | 0·61 | 0·02 | 0·48† | 0·01 |
| Female | 50 | 3·60† | 0·09 | 0·65† | 0·02 | 5·86†‡ | 0·24 | 6·04 | 0·19 | 0·64 | 0·01 | 0·52† | 0·01 | ||
| MLP | Male | 38 | 3·80† | 0·12 | 0·61† | 0·01 | 6·36† | 0·24 | 6·17 | 0·22 | 0·59 | 0·01 | 0·49† | 0·01 | |
| Female | 33 | 3·55† | 0·12 | 0·61†* | 0·01 | 5·87†‡ | 0·21 | 6·23 | 0·24 | 0·59 | 0·02 | 0·51† | 0·01 | ||
Mean values were significantly different from those of the control group at same age: P<0·05.
Mean values were significantly different from those of day 18 gestation within the same maternal dietary group: P<0·05.
Mean values were significantly different between males and females of same age and dietary group: P<0·05.
For details of procedures, see pp. 579–580. Fetal and placental weight data are adjusted for litter size and position in the uterine horn and analysed using litter of origin as a covariate.
Fetal weight was influenced by gestation (P<0·001).
Placental weight was influenced by gestation (P<0·001) and maternal diet (P=0·007).
Fetal/placental ratio was influenced by gestation (P<0·001) and the interaction of sex and gestation (P=0·056).
Kidney to body weight ratio varied with gestation (P<0·001).
DNMT1 is the enzyme responsible for the maintenance of DNA methylation patterns during replication of DNA and hence cell division (Newell-Price et al. 2000). The expression of DNMT1 mRNA was determined in the livers of male and female fetuses at days 18 and 20 gestation (Fig. 2). There was no significant effect of diet, sex or gestation upon expression of this gene, although there was an interactive effect of all three factors (P=0·027). This suggested that the maternal diet influenced expression of this gene in a sex- and gestational age-specific manner. Diet effects on DNMT1 expression were confined to day 18 gestation, and here the trends for effects on males and females appeared to go in opposite directions (P=0·002 for diet × sex interaction at day 18). At day 18, in females, DNMT1 mRNA expression was elevated by MLP exposure (P<0·05), whilst in males MLP tended to lower expression, although this was not statistically significant (P=0·085). Expression of DNMT1 mRNA was normalised against β-actin mRNA expression, which did not vary between groups.
Fig. 2.
Fetal DNA methyltransferase 1 (DNMT1) mRNA expression. Data are presented as the ratio of DNMT1 mRNA expression to β-actin mRNA expression (arbitrary units) for males (A) and females (B). For details of procedures, see pp. 579–580. Values are means with their standard errors depicted by vertical bars (four to eight observations per group). ANOVA indicated an interaction of gestational age, fetal sex and maternal diet (P=0·027). Mean value was significantly different from that of the control group: *P<0·05. (∎) Control; (◻) maternal low-protein diet.
GPx and SOD activities in the livers of fetuses at days 18 and 20 showed no significant effects of maternal diet (Table 5). Catalase activity increased by 166–317 % between days 18 and 20, but GPx and total SOD activities were similar at both time-points. At day 20, MLP-exposed fetuses had significantly greater hepatic catalase activity than controls. No differences in protein carbonyl concentration were noted between the two dietary groups, but oxidative injury was greater at day 20 than at day 18.
Table 5.
Fetal liver antioxidant enzyme activities and protein carbonyl concentrations of rats fed either maternal low-protein (MLP) diet or control diet§
(Mean values with their standard errors)
| Days gestation |
||||||||
|---|---|---|---|---|---|---|---|---|
| 18 |
20 |
|||||||
| Control (n 14) |
MLP (n 14) |
Control (n 14) |
MLP (n 10) |
|||||
| Mean | sem | Mean | sem | Mean | sem | Mean | sem | |
| GPx (units/mg protein)∥ | 0·096 | 0·010 | 0·103 | 0·087 | 0·171 | 0·013 | 0·204 | 0·013 |
| SOD (units/mg protein)¶ | 0·97 | 0·10 | 0·86 | 0·10 | 1·53 | 0·20 | 1·92 | 0·23 |
| Catalase (units/mg protein)** | 41·57 | 6·44 | 38·07 | 2·78 | 110·73† | 9·78 | 158·88*† | 17·92 |
| Carbonyls (nmol/mg protein)†† | 0·197 | 0·026 | 0·220 | 0·023 | 0·372† | 0·044 | 0·399† | 0·042 |
GPx, glutathione peroxidase; SOD, superoxide dismutase.
Mean value was significantly different from that of the control group: P<0·05.
Mean values were significantly different from those of day 18 gestation within the same maternal dietary group: P<0·05.
For details of procedures, see pp. 579–580. Fetal sex had no impact on antioxidant activities or protein carbonyl concentrations and so data are for males and females combined.
GPx activity rose significantly with gestation (P<0·001).
SOD activity rose significantly with gestation (P<0·001).
Catalase activity rose significantly with gestation (P<0·001) and varied with the interaction of diet and gestation (P=0·020).
Protein carbonyl concentration was influenced by gestation (P<0·001).
Discussion
The feeding of the MLP diet to pregnant rats is an established protocol for the induction of hypertension and renal deficits in later life (Langley-Evans, 2001). As a well-characterised system for studying nutritional programming, it is important to be able to define the mechanism or mechanisms through which MLP feeding exerts its long-term effects. Rees (2002) has proposed that the methionine content of the MLP diet is in excess of requirements, producing an accumulation of homocysteine. This may be envisaged as having toxic actions within maternal and fetal tissues by virtue of its oxidant properties, but may also impact on DNA methylation, since S-adenosylhomocysteine inhibits DNMT1 (Chiang, 1998). One of the most important findings of the present study was the observation that neither maternal nor fetal plasma homocysteine concentrations differed in MLP pregnancies compared to controls. This is in contrast to the findings of Petrie et al. (2002) who reported that low-protein feeding raised maternal homocysteine at day 4 but not day 17 gestation. Consistent with the present study, Brawley et al. (2004) found no difference in homocysteine at day 18 gestation in rats fed MLP diet. The data do not therefore support the hypothesis that MLP feeding promotes gross disturbance in the methionine–homocysteine cycle.
Clearly differences in the finding exist between studies, and this may best be explained by variations in protocol. The present study and those of Petrie et al. (2002) and Brawley et al. (2004) all used identical diets. Whilst we and Brawley et al. (2004) introduced the synthetic diets on day 0 of pregnancy, Petrie et al. (2002) used a habituation protocol whereby dams were fed the diets for 2 weeks prior to mating. The use of a habituation period is known to produce variation in the fetal growth patterns associated with low-protein feeding (Langley-Evans & Nwagwu, 1998), so may also elicit metabolic adaptations in the dams that explain the inconsistent effects upon maternal homocysteine concentrations. It is also possible that there are differences between different strains of rat, as Petrie et al. (2002) studied Rowett Hooded Lister rats, as opposed to the Wistar strain used in the present study. Such strain differences are well-documented in other areas (Saito et al. 2004).
Excess homocysteine can be converted to cysteine via the trans-sulphuration pathway or recycled to methionine. In the MLP-fed dam the capacity for trans-sulphuration has been reported to fall rapidly in early gestation, thereby limiting the capacity to remove homocysteine in this manner (Rees & Hay, 2001). Furthermore, in fetal tissues the trans-sulphuration pathway is not active. The observed lack of elevated homocysteine concentrations in the present study may therefore be explained by remethylation. One of the major steps in remethylation is catalysed by methionine synthase. We have shown that, at least at the mRNA level, methionine synthase expression is unaffected by MLP feeding. Whilst at the level of transcription, low-protein feeding does not appear to impact upon this component of the methionine–homocysteine cycle, we cannot exclude diet-induced differences in levels of methionine synthase protein, enzyme activity or flux through the pathway. This is a complex cycle, which is closely related to the equally complex folate cycle.
The present study has not produced evidence of maternal or fetal hyperhomocysteinaemia, which suggests that protein restriction is unlikely to impact upon capacity for remethylation of homocysteine to methionine. Remethylation can also occur via an alternative pathway from betaine. The precursor of betaine is choline and the diets used in the present study provided choline at levels recommended by the American Institute for Nutrition, for rat pregnancy. We cannot exclude the possibility that choline is a limiting nutrient with this form of reduced casein, methionine-supplemented diet, but the observation of unaltered homocysteine concentrations suggests this is unlikely to be the case. The maternal diets also provided folate at levels recommended by the American Institute of Nutrition, but deficits of folate are certainly a possibility in MLP-fed dams. There is evidence that folate (Lillycrop et al. 2005) or glycine (Jackson et al. 2003; Brawley et al. 2004) supplementation of the MLP diet overcomes some of the long-term programming effects. This may stem from direct effects of the diet upon the folate cycle and availability of serine and glycine. With apparent deficits of folate, homocysteine remethylation would be mostly dependent upon the betaine pathway, which would therefore provide the only mechanism for the normalisation of plasma homocysteine concentrations observed in the present study. The alternative to this is that the original premise that the methionine–protein ration is imbalanced (Rees, 2002) is incorrect and the key link between MLP feeding and DNA methylation lies in a different pathway.
DNMT1 is one of a family of DNA methyltransferases and is important during development as it maintains the DNA methylation pattern when DNA replicates during cell division. DNMT1 is essential for normal development and mice deficient for this gene die in utero (Li et al. 1992). Reduced DNA methylation leading to increased expression of specific genes certainly appears to be one mechanism through which MLP feeding programmes physiological processes (Lillycrop et al. 2005). The present study suggests that variation in DNA methylation could arise due to diet-induced changes in the expression of DNMT1. Interestingly, these changes were specific to the day 18 time-point, although could, of course, have been operating at earlier stages of development. Moreover, the effects of MLP exposure on DNMT expression were sex-specific. In females the significant up-regulation of DNMT1 may actually favour increased methylation, rather than the hypomethylation observed by Lillycrop et al. (2005). The trend for decreased DNMT1 expression in males is, however, consistent with reduced DNA methylation. These effects will require further investigation to explore their contribution to sex-specific programming, which has been observed in a number of systems (McMullen & Langley-Evans, 2005). Lillycrop et al. (2005) made their observations on a mixed population of male and female offspring and so it is unclear whether there were sex differences in DNA methylation patterns. The other DNA methyltransferases (3L, 3a and 3b) were not studied in the present experiment. These enzymes, which are only expressed in the embryo, are responsible for de novo DNA methylation (Newell-Price et al. 2000) and may therefore be likely targets for further study in the context of nutritional programming.
The original hypothesis for the present study was that homocysteine may contribute to programming via its oxidative properties. Low-protein feeding is known to lower antioxidant defences, particularly through depletion of tissue glutathione (Huang & Fwu, 1992). The combination of raised homocysteine and lower antioxidant defence could be expected to promote oxidative injury, apoptosis and hence tissue remodelling in the developing fetus. There were no consistent differences in protein carbonyl concentrations in either maternal or fetal tissues. This may in part be explained by the fact that MLP feeding increased fetal activity of catalase, which shares similar substrates with GPx and may therefore compensate for any deficit in glutathione cycle activities. Oxidative injury did vary with diet in maternal liver, but this did not show a consistent pattern with increasing gestation, or appear to be related to homocysteine concentration. Whilst fetal liver showed no evidence of oxidant/antioxidant imbalance associated with MLP feeding up to day 20 gestation, we have previously noted increased oxidation of hepatic proteins at birth (Langley-Evans & Sculley, 2005). This suggests that oxidative processes mediate some programming effects of MLP feeding over the last 2 d of gestation, a period of very rapid fetal growth and development in the rat. There is no evidence in the present study that this is attributable to the oxidant effects of homocysteine and other mechanisms must therefore account for this process.
Fetal and placental weights were evaluated at two points in late gestation. After accounting for important factors that impact on rates of fetal growth in a litter-bearing species, including transfer of steroid hormones between adjacent male and female fetuses in the uterine horn, we found no significant effects of maternal protein restriction upon fetal weight, the fetal/placental ratio or the size of the major truncal organs. We have previously reported that MLP feeding impacts principally on fetal growth in mid-late gestation, with accelerated growth up to day 20 and slowing of truncal growth over the last 2 d of gestation (Langley-Evans et al. 1996a). These effects appear to depend upon the timing of introduction of MLP feeding and the plane of nutrition prior to pregnancy (Langley-Evans & Nwagwu, 1998). Differences between the current findings and those of earlier studies are most likely explained by the fact the present study has more carefully considered effects of gender and position in the uterine horn. Ultimately the offspring of MLP-fed dams are of normal weight at birth in most studies. The present data therefore reinforce the view that nutritional programming occurs independently of fetal growth retardation. This has important implications for the interpretation of epidemiological data linking lower weight at birth, or disproportion at birth to disease in later life.
In conclusion, the present study has discounted maternal hyperhomocysteinaemia as a potential basis for altered patterns of DNA methylation in rat offspring exposed to a low-protein diet in utero. MLP feeding did not significantly alter plasma homocysteine concentrations in mothers or fetuses and, similarly, did not produce any changes in maternal hepatic methionine synthase activity. Although MLP feeding compromises antioxidant defences in maternal and fetal tissues, there was no evidence that this process was related to homocysteine. There was a strong suggestion that fetal liver expression of DNMT1 was perturbed by MLP feeding, in a gestation- and sex-specific manner and this will require further investigation to assess whether this provides a route to altered DNA methylation.
Abbreviations
- DNMT1
DNA methyltransferase 1
- GPx
glutathione peroxidase
- MLP
maternal low protein
- SOD
superoxide dismutase
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bogdarina I, Murphy HC, Burns SP, Clark AJ. Investigation of the role of epigenetic modification of the rat glucokinase gene in fetal programming. Life Sci. 2004;74:1407–1415. doi: 10.1016/j.lfs.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, Clough GF, Poston L, Hanson MA. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PK. Biological effects of inhibitors of S-adenosylhomocysteine hydrolase. Pharmacol Ther. 1998;77:115–134. doi: 10.1016/s0163-7258(97)00089-2. [DOI] [PubMed] [Google Scholar]
- Gambling L, Dunford S, Wallace DI, Zuur G, Solanky N, Srai SK, McArdle H. Iron deficiency during pregnancy affects postnatal blood pressure in the rat. J Physiol. 2003;552:603–610. doi: 10.1113/jphysiol.2003.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Fwu ML. Protein insufficiency aggravates the enhanced lipid peroxidation and reduced activities of antioxidative enzymes in rats fed diets high in polyunsaturated fat. J Nutr. 1992;122:1182–1189. doi: 10.1093/jn/122.5.1182. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by maternal low protein diet is reversed by dietary supplementation with glycine. Clin Sci. 2003;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Langley SC, Browne RF, Jackson AA. Altered glucose tolerance in rats exposed to maternal low protein diets in utero. Comp Biochem Physiol. 1994;109:223–229. doi: 10.1016/0300-9629(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats, induced by fetal exposure to maternal low protein diets. Clin Sci. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS, Jackson AA. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil. 1996a;106:307–312. doi: 10.1530/jrf.0.1060307. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Nwagwu M. Impaired growth and increased glucocorticoid-sensitive enzyme activities in tissues of rat fetuses exposed to maternal low protein diets. Life Sci. 1998;63:605–615. doi: 10.1016/s0024-3205(98)00311-7. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin Nutr. 1994;13:319–324. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Sculley DV. Programming of hepatic antioxidant capacity and oxidative injury in the ageing rat. Mech Ageing Dev. 2005;126:804–812. doi: 10.1016/j.mad.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJM, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJM, Sherman RC, Jackson AA. Weanling rats exposed to maternal low protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci. 1996b;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadmann EM. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:382–386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Price J, Clark AJ, King P. DNA methylation and silencing of gene expression. Trends Endocrinol Metab. 2000;11:142–148. doi: 10.1016/s1043-2760(00)00248-4. [DOI] [PubMed] [Google Scholar]
- Petrie L, Duthie SJ, Rees WD, McConnell JM. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr. 2002;88:471–477. doi: 10.1079/BJN2002695. [DOI] [PubMed] [Google Scholar]
- Rees WD. Manipulating the sulfur amino acid content of the early diet and its implications for long-term health. Proc Nutr Soc. 2002;61:71–77. doi: 10.1079/pns2001137. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM. Early changes in maternal sulphur amino acid metabolism caused by maternal protein deficiency. Pediatr Res. 2001;50(Suppl.):29A. [Google Scholar]
- Saito K, Sakai N, Kim HS, Ishizuka M, Kazusaka A, Fujita S. Strain differences in diazepam metabolism at its three metabolic sites in Sprague-Dawley, Brown Norway, Dark Agouti, and Wistar strain rats. Drug Metab Dispos. 2004;32:959–965. [PubMed] [Google Scholar]
- Sayer AA, Dunn RL, Langley-Evans SC, Cooper C. Prenatal exposure to a maternal low protein diet shortens life span in rats. Gerontology. 2001;47:9–14. doi: 10.1159/000052764. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C, Hawkins R, Brian M, Carrell R. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337. [PubMed] [Google Scholar]
- Young L, Rees WD, Sinclair KD. Programming in the preimplantation embryo. In: Langley-Evans SC, editor. Programming of Chronic Disease Through Fetal Exposure to Undernutrition. CABI Publishing; Wallingford: 2004. pp. 333–352. [Google Scholar]