Abstract
Anaplasma phagocytophilum is an obligate intracellular bacterium that infects neutrophils and causes human granulocytic anaplasmosis. Infection induces neutrophil secretion of interleukin-8 or murine homologs and perpetuates infection by recruiting susceptible neutrophils. We hypothesized that antibody blockade of CXCR2 would decrease A. phagocytophilum tissue load by interrupting neutrophil recruitment but would not influence murine hepatic pathology. C3H-scid mice were treated with CXCR2 antiserum or control prior to or on day 14 after infection. Quantitative PCR and immunohistochemistry for A. phagocytophilum were performed and severity of liver histopathology was ranked. Control mice had more infected cells in tissues than the anti-CXCR2-treated group. The histopathological rank was not different between treated and control animals. Infected cells of control mice clustered in tissue more than in treated mice. The results support the hypothesis of bacterial propagation through chemokine induction and confirm that tissue injury is unrelated to A. phagocytophilum tissue load.
Anaplasma phagocytophilum (7) is a tick-borne obligate intracellular bacterium that causes human granulocytic anaplasmosis (HGA) and infections of other mammals such as horses, dogs, ruminants, and small mammals (4). The bacteria infect neutrophils (2), where they live in cytoplasmic vacuoles to form clusters called morulae. HGA is an acute, febrile illness with myalgia, headache, malaise, and other manifestations (3). The duration of illness is usually brief, but infections range from mild to severe or fatal.
Mouse models are effective tools for studying the pathogenesis of A. phagocytophilum infection (5). Although infected mice do not develop clinical signs, there is a progressive development of histopathological lesions similar to those in humans and in other HGA models (6). Martin et al. (9, 10) showed that cytokines and chemokines play a role in A. phagocytophilum pathogenesis and that tissue pathology is not caused by direct A. phagocytophilum-mediated injury but results from immunopathologic mechanisms. In addition, A. phagocytophilum-infected neutrophils produce large quantities of chemokines in vitro (8), and Akkoyunlu et al. (1) showed that infection is perpetuated by CXC chemokine-induced recruitment of susceptible neutrophils. Whether this mechanism impacts HGA pathogenesis is unclear, since A. phagocytophilum load does not correlate with histopathological lesions. We hypothesized that antibody blockade of the CXC chemokine receptor CXCR2 would diminish A. phagocytophilum load (1) but would not influence tissue histopathology in the mouse model.
(Part of this paper was presented as a poster at the annual meeting for the American Society of Rickettsiology—Bartonella as an Emerging Pathogen Group 2001 Joint Conference, Big Sky, Mont., 2001 [abstract no. 68].)
MATERIALS AND METHODS
C3H-scid mice (SCID) were purchased from the Frederick Cancer Research Center (Frederick, Md.) and from Jackson Laboratory (Bar Harbor, Maine). C3H/HeJ mice were obtained from Jackson Laboratory. Procedures involving mice were approved in a protocol by the Institutional Animal Care and Use Committee at The Johns Hopkins University School of Medicine. The initial experimental design involved two groups of mice under two treatments to test the hypothesis that CXCR2 receptor antibody administration diminishes infected neutrophil tissue infiltration but not histopathology (1). For the initial experiments, A. phagocytophilum (NCH-1) was cultured in HL-60 cells, and 2 × 106 infected cells in 1 ml of RPMI 1640 medium were intraperitoneally injected into SCID mice. The challenge inoculum was 100 μl of A. phagocytophilum-infected SCID mouse blood (5); when pooled, 8% of neutrophils contained morulae for an approximate inoculum of 2.4 × 104 infected neutrophils. For confirmatory experiments, A. phagocytophilum Webster strain was propagated in HL-60 cells, and when >50% of the cells were infected; SCID and C3H/HeJ mice were inoculated intraperitoneally with 500 μl that contained 106 infected cells.
For initial experiments, mice were assigned to one of two groups, prevention or treatment, with experimental and control subgroups. The experimental design was intended to evaluate (i) the ability to prevent new infection (prevention) or (ii) the potential for inhibiting the propagation of established infection (treatment). Each experimental and control group consisted of three mice. Mice in the prevention group were treated with 500 μl of goat anti-murine CXCR2 (MIP-2/KC receptor) antiserum (a kind gift of R. M. Strieter, School of Medicine, University of California—Los Angeles) by intraperitoneal injection 2 h before challenge with A. phagocytophilum, and controls received 500 μl of normal goat serum (NGS) 2 h before intraperitoneal challenge (day 0) and on days 2 and 4, followed by necropsy on day 5. The treatment group was challenged on day 0 and then given the CXCR2 antiserum or NGS on day 14 and then four times at 36-h intervals prior to necropsy on day 19 (1). The interval of antibody administration was based upon prior studies that showed a blockade of CXCR2 for at least 48 h (11). Confirmatory experiments were similarly designed, except that four mice were used per group, an additional group of C3H/HeJ mice was tested, the treatment group was excluded, and necropsy was conducted on day 7 (10).
After necropsy, tissues from SCID and C3H/HeJ mice were formalin fixed and paraffin embedded before sections (5 μm) were prepared. Slides were stained with hematoxylin and eosin (H&E) or examined by immunohistochemistry (IHC) by using polyclonal rabbit anti-A. phagocytophilum (6, 9). Because of the ambiguity in detecting splenic inflammation, histopathologic changes were assessed in the liver only and included evaluation for size and density of lesions, cellular content of inflammatory infiltrates, degree of necrosis and/or apoptosis, and number of inflammatory foci. A quantitative assessment of the degree of histopathologic findings was performed by ranking the severity of hepatic lesions, where severe lesions had a higher rank (9). All evaluations were performed with investigators blinded to the identity of mouse treatment status and were conducted separately by two different microscopists (D.G.S. and J.S.D.). The area and volume of tissue were calculated by image analysis (Scion Image; Scion Corporation, Frederick, Md.). The total number of infected neutrophils was counted and tabulated per volume of tissue. Infected neutrophils were also identified by location in splenic tissues by using paired x and y coordinates. Cluster analysis was performed by using a computer algorithm that evaluated distance between the x and y coordinates recorded for each infected cell on a single slide. A cluster was defined as three or more cells separated by no more than 200 or 500 μm (200 μm was chosen as the minimum sensitive distance assessed by microscopy, and 500 μm was chosen as the maximum distance assessed per one high-power field). The clustering metric is defined as the proportion of clustered cells relative to the total number of cells. The 200- and 500-μm threshold values were chosen to assess the sensitivity of the clustering metric and to account for the natural clustering that would occur with dissemination of bacteria from a single infected focus.
To demonstrate changes in bacterial tissue and blood load, a quantitative PCR based upon amplification of the multicopy msp2 gene was performed in confirmatory experiments. In brief, EDTA-anticoagulated blood that was obtained by intracardiac puncture and splenic tissue were acquired. DNA was prepared from approximately 200 μl of blood and 10 mg of splenic tissue by using a DNA minikit (QIAGEN, Valencia, Calif.). DNA concentrations were measured by PicoGreen DNA assay (Molecular Probes, Eugene, Oreg.). Quantitative PCR was conducted in an ABI Biosystems (Foster City, Calif.) TaqMan 7700 instrument, based on a method modified from that of Martin et al. (10) using the msp2 primers msp2con33f (5′-GAAGATGAWGCTGATACAGTA-3′) and msp2con151r (5′-CAACHGCCTTAGCAAACT-3′) and the TaqMan probe msp2con86p (5′-TTATCAGTCTGTCCAGTAACA-3′) labeled with 5′ FAM and 3′ TAMRA. Preliminary experiments demonstrated the ability to detect as few as 1 infected cell/μl (data not shown). Blood samples were tested in duplicate, whereas splenic tissue samples were tested in duplicate twice to confirm repeatability. Results were expressed as the quantity of infected cells per microliter of blood or per milligram of spleen. Since few infected cells could be detected in the liver in initial immunohistochemical and PCR studies (data not shown), the spleen and blood were assayed for bacterial quantitation of the tissue-marginated and circulating pool of infected neutrophils, respectively.
Mice in confirmatory experiments were subjected to a battery of clinical chemistry tests on sera in which specific attention was given to the assessment of hepatocyte injury (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma glutamyltransferase, total bilirubin, and albumin; all from Antech Diagnostics, Lake Success, N.Y.) as correlates of histopathologic findings.
Statistical analysis of hepatic rank, immunohistochemical quantitation of infected cells, and quantitative PCR were performed by using paired Wilcoxon tests for nonparametric data, since questions regarding normal distribution of these data existed. The P values for the Wilcoxon tests were calculated with the one-way test chi-square approximation. The significance of difference in clinical chemistry tests was determined with the Student's t test. Cluster analysis significance was determined by the chi-square goodness-of-fit test comparing experimental results with tissue- and cell number-matched computer-generated randomized cell distributions. Correlation between infected cell quantities and histopathologic rank was assessed by using Pearson's coefficient of correlation. P values of less than 0.05 were considered significant.
RESULTS
Initial experiments.
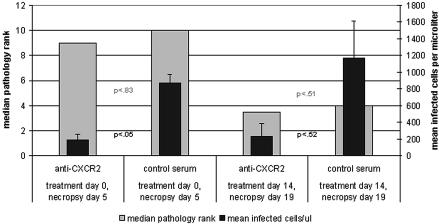
Histopathology rank analysis and IHC comparing the two groups of mice (prevention and treatment) and controls are summarized in Fig. 1. Overall, the hepatic histopathology rank was worse for the prevention group that was examined at day 5 than for the treatment group that was examined at day 19 postinfection (P < 0.004 [Wilcoxon test]). When histopathologic ranks of anti-CXCR2- and control antibody-treated mice were compared, no significant differences were noted in either the prevention (P = 0.83) or treatment (P = 0.51) groups. When animals in both prevention and treatment groups were considered together, more infected cells were observed by IHC in the spleen than in the liver and in those administered NGS compared to antibody-treated mice, although the differences were not significant (P = 0.08 [Wilcoxon test]). However, IHC showed significantly more infected cells in spleens and livers of control antibody-treated animals than in those treated with anti-CXCR2 only when treated on the day of infection (P = 0.05 [Wilcoxon test] for the prevention group versus P = 0.51 [Wilcoxon test] for the treatment group), confirming previous observations in blood (1). Histopathologic rank in liver sections was not correlated (r = −0.34) with the quantity of A. phagocytophilum-infected cells. Typical examples of hepatic histopathology and IHC for A. phagocytophilum-infected cells in liver and spleen are shown in Fig. 2.
FIG. 1.
The comparison of hepatic histopathology ranking and A. phagocytophilum-infected neutrophil tissue load (in spleen) between SCID mice treated with anti-CXCR2 or control antibody at the time of infection or 14 days postinfection (initial experiments only). Higher ranks correspond to higher degrees of severity. Differences in histopathologic rank did not differ significantly between anti-CXCR2-treated and NGS control antibody-treated animals in prevention and treatment groups. P values for differences between splenic organism load in prevention and treatment groups are shown. Error bars indicate standard deviations. P values (paired Wilcoxon test for nonparametric data) between columns compare infected cell load (black type) and histopathology ranks (gray type). Experiments were done in duplicate. Shown are the results of the initial experiment; confirmatory experiment data were similar.
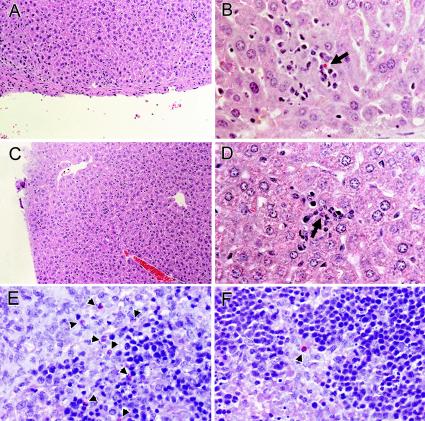
FIG. 2.
Comparison of hepatic pathology (A and C) and localization of A. phagocytophilum-infected cells in livers (B and D) and spleens (E and F) of SCID mice treated with either anti-CXCR2 or control antibody before challenge. Note scattered inflammatory lesions and the presence of scattered infected cells (arrows) in liver and an increased concentration and clustering of infected cells (arrowheads) in the spleens of control antibody-treated SCID mice (E) compared with anti-CXCR2-treated SCID mice (F). These panels show typical examples revealed by these methods in duplicate studies. Tissues from anti-CXCR2-treated (A, B, and F) and control antibody-treated (C, D, and E) animals are shown. Magnifications, ×64 (H&E [A and C]), ×252 (B and D), and ×400 (immunoalkaline phosphatase with rabbit anti-A. phagocytophilum [E and F]).
Confirmatory experiments.
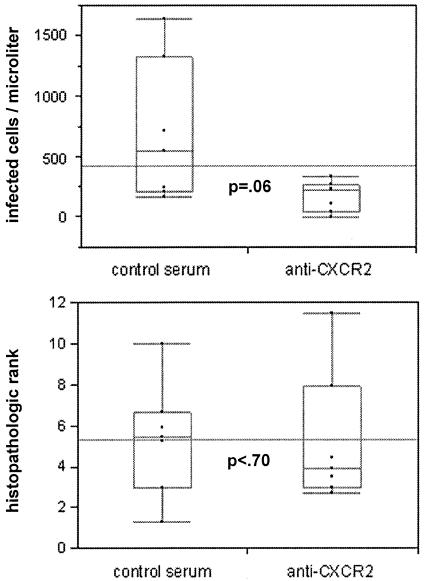
In confirmatory experiments investigating prevention only (antibody administered before infection), quantitative PCR results revealed significantly fewer infected cells in the blood and spleens of antibody-treated mice than in NGS-treated mice (P = 0.0209 [Wilcoxon test]); however, this difference was not detected to be significant by IHC (P = 0.2482 [Wilcoxon test]). Although nearly significantly different when examined by IHC (P = 0.066 [Wilcoxon test]), antibody-treated C3H/HeJ mice had fewer infected cells than did NGS-treated control mice, although this difference was not evident with quantitative PCR (P = 0.29 for blood, P = 0.48 for spleen [Wilcoxon test]). No differences in histopathologic ranking were detected between groups, regardless of whether the mouse strain was C3H/HeJ or SCID, confirming the initial experiments. When data were pooled with those of the prevention group from initial experiments, a nearly significant difference was detected by IHC, with more infected cells in splenic tissues of NGS-treated mice than in CXCR2 antibody-treated mice (P = 0.06 [Wilcoxon test]). No differences were detected for histopathologic rank in the pooled data (P < 0.70 [Wilcoxon test]). Figure 3 shows the pooled data of the initial and confirmatory experiments and demonstrates the between-experiment reproducibility and the lack of association between histopathologic rank and immunohistochemical bacterial load.
FIG. 3.
Box plot diagrams showing pooled initial and confirmatory experimental data comparing splenic bacterial load (IHC) and hepatic histopathologic rank between SCID mice treated with CXCR2 and mice treated with control antibody on day 0. Results of bacterial load and rank are shown for each mouse; the long horizontal line shows the median of the combined observations, the horizontal box plot line shows the median for each antibody treatment group, the box contains the second and third quartiles, and the error bars show the first (bottom) and fourth (top) quartiles; P values were calculated with Wilcoxon nonparametric tests.
Cluster analysis.
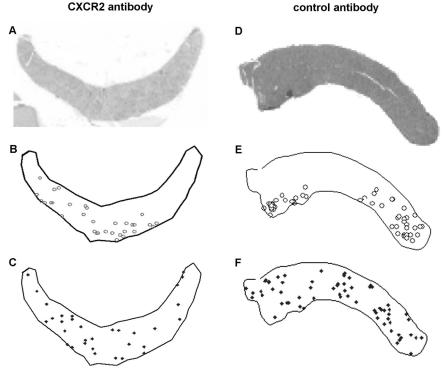
Clustering of infected cells was found to occur at two levels. When a nonstringent cluster (500 μm between cells) was used, a nonrandom distribution was obtained regardless of treatment or prevention groups and regardless of anti-CXCR2 treatment (chi-square test; P < 0.001). When a stringent 200-μm cluster definition was used, mice examined 5 days after infection with anti-CXCR2 treatment showed no significant differences in the clustering of infected cells compared to clustering based on a random distribution (P = 1.0). Figure 4 illustrates the distribution and clustering of infected cells (Fig. 4A and B) compared with one randomly generated distribution. In contrast, control antibody-treated mice still demonstrated significant clustering (P < 0.001).
FIG. 4.
A. phagocytophilum-infected neutrophils cluster in tissues of infected SCID mice compared to randomly generated distributions of infected cells. A CXCR2 antibody-treated SCID mouse (A to C) and a control antibody-treated SCID mouse (D to F) are shown. (A and D) Low-resolution H&E-stained spleens from infected SCID mice; (B and E) the splenic outlines superimposed with coordinates of all infected cells identified by IHC; (C and F) coordinates of a computer-generated random distribution based upon the number of infected cells detected in each mouse. Note the relative lack of clustering of random distributions compared with experimentally generated data and the more intense clustering obtained in the absence of CXCR2 blockade (D to F). Similar maps were generated for each spleen examined in the initial experiments only.
Clinical chemistry.
In the confirmatory experiments, values for alanine aminotransferase, alkaline phosphatase, gamma glutamyltransferase, and total bilirubin were within normal limits for all animals, and no significant differences were detected between NGS-treated control mice and anti-CXCR2-treated mice in both the C3H and SCID mouse groups (P ≥ 0.45). Levels of aspartate aminotransferase were elevated for six of seven NGS control antibody- and seven of eight anti-CXCR2-treated animals, but the values were not statistically different (P = 0.10 for SCID and P = 0.75 for C3H).
DISCUSSION
The neutrophil is the host cell for A. phagocytophilum. Thus, modifications in neutrophil function due to infection could ultimately benefit bacterial survival. Chemokines are key products of human and mouse neutrophils. In particular, interleukin-8 in humans and macrophage inflammatory protein 2 (MIP-2) and KC in mice mediate inflammatory cell recruitment via binding to CXCRs (15) on neutrophils. Other chemokines and cytokines such as MIP-1α, MIP-1β, monocyte chemoattractant protein 1, and RANTES have also been suggested to be important in A. phagocytophilum pathogenesis (8). Klein et al. speculated that chemokines produced after infection of HL-60 cells with A. phagocytophilum played a role in the recruitment of other susceptible cells and enhancement of phagocytosis (8). Analysis of CXCR2 antibody blockade of MIP-2 and KC stimulation in A. phagocytophilum-infected mice further confirms the speculation of Klein and coworkers (8) by demonstrating a decrease in the quantity of infected cells in blood of CXCR2 knockout mice (1). This survival tactic appears to be an important dissemination mechanism for A. phagocytophilum and other intracellular and extracellular bacteria such as Salmonella spp. and Borrelia burgdorferi (12, 14).
Of interest, immunohistologic observations suggested a clustering of infected cells in tissues of animals not treated with anti-CXCR2, a finding consistent with the role of interleukin-8 in the propagation of A. phagocytophilum. A low level of clustering (within 500 μm) was observed regardless of whether CXCR2 was blocked, consistent with focal release of bacteria from infected cells. However, with functional CXC chemokine ligand activity in animals that did not receive CXCR2 antibody, a higher degree of clustering (within 200 μm) was observed, confirming the important role for this chemokine in the propagation of A. phagocytophilum in vivo.
The key unresolved question is whether the increased quantities of A. phagocytophilum that result from induced chemokine production by infected neutrophils contribute to disease or virulence. Martin et al. have presented data that demonstrate a role for immunopathologic injury, regardless of A. phagocytophilum quantity, as the most important aspect in the development of histopathologic lesions in the murine HGA model (9, 10). Such data suggest that a basal quantity of A. phagocytophilum triggers a poorly regulated host immune and inflammatory response. Furthermore, the data suggest that an increasing quantity of A. phagocytophilum in blood or tissues is irrelevant for subsequent histopathology and disease manifestations. The data presented here strongly support this hypothesis, as CXC chemokine-induced recruitment of host cells resulted in increased A. phagocytophilum loads but did not alter the degree of tissue histopathology, further confirmed by the lack of significant increases in serum markers of hepatocyte injury or hepatic inflammation.
These findings could have implications with regard to the course of disease and treatment options. Therapeutic strategies that target the inhibition of inflammation, not typically considered for infectious diseases, may be an option for treatment of HGA. One could speculate that the use of anti-inflammatory agents or corticosteroids inhibits disease, and concurrent use of specific antimicrobial agents would help to control the infection. Quan et al. (13) have suggested that limiting neutrophil chemotaxis and migration in inflammation by the use of chemokine receptor antagonists or monoclonal antibodies may also be another treatment alternative for infectious diseases with prominent inflammation. However, before evaluating other potential treatment options for HGA, further studies will be needed to delineate the mechanisms of inflammation, immune induction, and tissue injury.
Acknowledgments
We thank Patricia Wilcox for technical assistance, Stephen M. Scorpio (Johns Hopkins Applied Physics Laboratory) for assistance in the creation of the cluster analysis program, and the technical staff in the Molecular Microbiology Laboratory, Division of Medical Microbiology, at The Johns Hopkins Hospital.
This work was supported in part by grant number RO1-AI44102-03 (to J.S.D.) and by the National Research Service Award training grant no. RR07002 from the Public Health Service (to D.G.S.). E.F. is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
REFERENCES
- 1.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken, J. S., J. S. Dumler, S. M. Chen, L. L. Van Etta, M. R. Eckman, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the United States: a new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 4.Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 5.Borjesson, D. L., and S. W. Barthold. 2002. The mouse as a model for investigation of human granulocytic ehrlichiosis: current knowledge and future directions. Comp. Med. 52:403-413. [PubMed] [Google Scholar]
- 6.Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180:546-550. [DOI] [PubMed] [Google Scholar]
- 7.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ′HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. (Correction, 52:5-6.) [DOI] [PubMed] [Google Scholar]
- 8.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 9.Martin, M. E., J. E. Bunnell, and J. S. Dumler. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374-378. [DOI] [PubMed] [Google Scholar]
- 10.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-γ and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore, T. A., M. W. Newstead, R. M. Strieter, B. Mehrad, B. L. Beaman, and T. J. Standiford. 2000. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 164:908-915. [DOI] [PubMed] [Google Scholar]
- 12.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158:4838-4845. [PubMed] [Google Scholar]
- 13.Quan, J. M., T. R. Martin, G. B. Rosenberg, D. C. Foster, T. Whitmore, and R. B. Goodman. 1996. Antibodies against the N-terminus of IL-8 receptor A inhibit neutrophil chemotaxis. Biochem. Biophys. Res. Commun. 219:405-411. [DOI] [PubMed] [Google Scholar]
- 14.Vassiloyanakopoulos, A. P., S. Okamoto, and J. Fierer. 1998. The crucial role of polymorphonuclear leukocytes in resistance to Salmonella dublin infection in genetically susceptible and resistant mice. Proc. Natl. Acad. Sci. USA 95:7676-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White, J. R., J. M. Lee, P. R. Young, R. P. Hertzberg, A. J. Jurewicz, M. A. Chaikin, K. Widdowson, J. J. Foley, L. D. Martin, D. E. Griswold, and H. M. Sarau. 1998. Identification of a potent, selective nonpeptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 273:10095-10098. [DOI] [PubMed] [Google Scholar]