Abstract
Agrobacterium, Sinorhizobium, and Ochrobactrum are genera closely related to Brucella but, in contrast to the latter, are not pathogenic for humans and animals. We studied by an indirect enzyme-linked immunosorbent assay (ELISA) the reactivities of brucellosis sera against cytosolic (CYT) and membrane (MA) antigens from these nonpathogenic bacteria, and we evaluated the potential usefulness of these cross-reactions for the diagnosis of brucellosis in humans, sheep, cows, and dogs. Canine infection by Brucella canis was detected with high specificity by CYT antigen-based ELISAs (96% for Agrobacterium, 96% for Sinorhizobium, and 91% for Ochrobactrum), while sensitivity was variable (58% for Agrobacterium, 88% for Sinorhizobium, and 84% for Ochrobactrum). In addition, it was possible to diagnose canine disease shortly after exposure to the pathogen (15 days). Similar results for canine brucellosis were obtained with MA antigens. In contrast, normal sera from humans, sheep, and cattle reacted strongly with all the antigens (CYT and MA antigens from the three bacteria), producing high cutoff values and, consequently, low sensitivities. While for some host species the reactivity patterns of normal sera by Western blotting were similar to those produced with sera from infected individuals, the reactivity pattern of bovine sera against Sinorhizobium meliloti antigens exhibited some differential bands for the two groups of sera. These results show that crude fractions from nonpathogenic alpha-proteobacteria can be used to diagnose canine brucellosis but may need to be further separated into simpler fractions to have diagnostic usefulness in ovine, bovine, or human infection. By reducing the biosafety requirements, the use of antigens derived from these nonpathogenic bacteria would simplify the production of diagnostic kits for brucellosis, especially in settings where biosafety level-3 facilities are scarce or absent.
Members of the genus Brucella cause brucellosis, an infectious disease affecting livestock and humans. Brucella spp. belong to the alpha-2 subgroup of Proteobacteria, which also includes Agrobacterium, Rhizobium, and Ochrobactrum, among other genera (13). There are several lines of evidence about the close genetic and antigenic relationship between Brucella and other alpha-proteobacteria. Analysis of the complete set of predicted Brucella suis proteins has revealed that it is very similar to that of species in the Rhizobium/Agrobacterium group. A total of 1,902 B. suis open reading frames are conserved in Mesorhizobium loti, Sinorhizobium meliloti, and Agrobacterium tumefaciens, and 2,408 B. suis open reading frames are conserved in at least one of these three genomes (14). Similar relationships were found when the genome of Brucella melitensis was analyzed (8).
While the natural hosts for Brucella species are animals and humans, some related alpha-proteobacteria usually live in the soil (Ochobactrum spp.), establish symbiotic relationships with plants (Rhizobium spp.), or are phytopathogens (Agrobacterium spp.). Rhizobium, Bradyrhizobium, and Sinorhizobium spp. and related bacteria induce the formation of nitrogen-fixing nodules on their leguminous hosts (10). In contrast, A. tumefaciens attacks wounded plant tissues and induces neoplastic growths called crown galls (15).
In contrast to the high pathogenic potential of most Brucella species, many related alpha-proteobacteria either are not pathogenic for immunocompentent individuals or show only limited pathogenicity. Human infections by A. tumefaciens have been reported almost exclusively in immunocompromised patients with indwelling catheters or prosthetic cardiac valves (1, 2). Similarly, infections by Ochrobactrum anthropi have occurred mostly in patients with debilitating underlying diseases (acute leukemia, human immunodeficiency virus infection) or those under chemotherapy (1, 12). Thus, the risk of Agrobacterium or Ochrobactrum infection for immunocompetent individuals appears extremely low. In addition, there are no reports of human disease caused by Rhizobium or other nodule-forming bacteria (although A. tumefaciens may appear under the name Rhizobium radiobacter in some papers).
In spite of the homologies revealed by computational analyses, few studies have investigated the serological cross-reactivity between proteins from Brucella and those from related alpha-proteobacteria. A notable exception is the study by Velasco et al., who found extensive cross-reactivity between cytosolic proteins from B. melitensis and those from O. anthropi (16). Some cross-reactions were also found at the level of outer membrane proteins and lipopolysaccharide (LPS) (core and lipid A). No similar study has been performed with antigens from other alpha-proteobacteria. Thus, the first goal of the present study was to study serological cross-reactivities between antigens from Brucella and those from A. tumefaciens and S. meliloti.
While serological cross-reactivities between common pathogens can cause difficulties in the differential diagnosis of infections by such pathogens, cross-reactivity can have diagnostic usefulness if one of the cross-reacting species is not pathogenic and is unlikely to enter the host. Under these conditions, cross-reacting antigens could be obtained from the nonpathogenic species and used for the diagnosis of infections caused by the pathogenic agent. The production of antigens for the serological diagnosis of brucellosis involves the handling of live brucellae, which are dangerous pathogens. Large-scale culture of Brucella for antigen production requires biosafety level-3 (BSL-3) facilities (6), which are seldom available in the developing countries where brucellosis is more prevalent. In contrast, antigens from nonpathogenic alpha-proteobacteria can be obtained without the need for special biosafety measures. Therefore, the second goal of this study was to test whether crude cytosolic (CYT) and membrane (MA) fractions from innocuous alpha-proteobacteria can be used to diagnose human and animal brucellosis. To the best of our knowledge, this is the first study in which antigens from nonpathogenic bacteria have been used to diagnose an infectious disease caused by bacteria from a different but related genus.
MATERIALS AND METHODS
Serum samples.
All the human and animal sera included in the study had been used in previous investigations and were positive by enzyme-linked immunosorbent assay (ELISA) against LPS-free CP of Brucella and against Brucella LPS. The preparation of the CP antigen and ELISAs used for testing anti-CP and anti-LPS antibodies are described elsewhere (3, 17).
Ovine sera.
Sera from 42 Brucella-infected sheep (kindly provided by I. Moriyón, Pamplona, Spain) were assayed. Infected groups included 18 animals naturally infected with B. melitensis biovar 1 or 3 (bacterial isolation in lymph nodes, spleen, etc.) and 24 rams with semen cultures positive for Brucella ovis. All infected animals were positive by complement fixation and gel diffusion with a hot-saline extract of B. ovis. Sheep infected with B. melitensis were also positive by the Rose Bengal test. Serum samples from 44 Brucella-free sheep were kindly provided by the Central Veterinary Laboratory, Weybridge, United Kingdom.
Canine sera.
The study included two different groups of canine sera. The first group comprised individual samples from 36 dogs with confirmed (n = 31) or suspected (n = 5) brucellosis. This group included 24 females and 12 males, with ages ranging from 6 months to 8 years. Canine brucellosis was confirmed when Brucella was isolated by blood culture. Suspected brucellosis was defined by either clinical findings compatible with the disease, a history of probable contact with infected dogs, a positive result by the rapid slide agglutination test (2ME-RSAT), or any combination of these.
A second group of sera included serial samples from seven dogs involved in an outbreak of canine brucellosis in a kennel. This cohort included one male and six females, whose ages ranged from 2 to 5 years. B. canis was isolated from blood in two cases and from vaginal discharge in one. The initial serum sample was obtained shortly after the presentation of the index case, at monthly intervals up to 5 months postinfection, and then at 9 months postinfection. Serum samples from 34 Brucella-free dogs were used to establish the cutoff value of each assay.
Bovine sera.
Serum samples from 13 pregnant heifers experimentally infected with B. abortus 544 were analyzed. All these sera showed high titers (>1/80) in the standard tube agglutination test and high titers (>4/64) in the complement fixation test. Serum samples from 36 Brucella-free cows were included to calculate the cutoff of each ELISA. Infected and Brucella-free bovine sera were kindly provided by R. Kittelberger, Upper Hutt, New Zealand.
Human sera.
Human sera were obtained from 54 patients with active brucellosis who attended the Brucellosis Section of the Hospital F. J. Muñiz (Buenos Aires, Argentina). Human brucellosis was diagnosed on the basis of clinical, serological, bacteriological (Brucella isolation from blood cultures), and epidemiological data. Sera from 31 healthy donors were used to calculate the cutoff of each ELISA.
Bacterial culture and preparation of antigens.
A. tumefaciens, O. anthropi, and S. meliloti (kindly provided by Antonio Lagares, Universidad Nacional de La Plata, La Plata, Argentina) were grown in tryptic soy broth. Cells were killed by addition of 0.4% formaldehyde. Bacterial suspensions were centrifuged at 16,000 × g for 10 min and washed three times with 10 mM Tris-HCl, pH 8 (Tris buffer). The cells were suspended in Tris buffer (0.1 g [wet weight] of cells per ml) and disrupted with a French press (SIM-AMINCO-Espectronic Instruments). Bacterial cells were broken by two passages and then digested with DNase and RNase. Unbroken cells were separated by centrifugation. Cell envelopes were harvested by centrifugation at 105,000 × g for 4 h. The resulting supernatant (CYT) was stored at −20°C until use. To obtain MA antigens, cell envelopes were washed twice in 10 mM Tris-HCl, pH 8.0, and resuspended in the same buffer containing 1% Triton X-100 and 5 mM EDTA. After a 4-h incubation at 37°C with constant agitation, the sample was centrifuged at 105,000 × g for 4 h. The resulting supernatant (MA) was kept at −20°C until use. Protein concentrations in CYT and MA antigens were determined by the bicinchoninic acid method (Pierce).
ELISA.
Maxisorp polystyrene plates (Nunc, Roskilde, Denmark) were sensitized with the corresponding CYT or MA antigen at 0.5 μg per well diluted in phosphate-buffered saline (PBS). For some assays, antigens were incubated overnight at 37°C with 0.05 μg of proteinase K (Promega)/μl before ELISA. Plates were blocked with 200 μl of PBS containing 3% skim milk per well. After a wash with PBS containing 0.05% Tween 20 (PBS-T), sera were dispensed diluted 1:200 in PBS-T containing 1% skim milk. Specific antibodies were detected with horseradish peroxidase-conjugated antibodies against the corresponding species (human, sheep, and dog γ chain specific [Jackson ImmunoResearch Laboratories, Inc.]; cow γ chain specific [Sigma, St. Louis, Mo.]). The reaction was developed by adding ortho-phenylenediamine (2 μg in 0.1 M citrate-phosphate buffer containing 0.03% H2O2) and was stopped with 4 N H2SO4. ELISA plates were read at 490 nm in a Σ960 microplate reader (Metertech Inc., Taipei, Taiwan). To discount nonspecific reactivities, each sample was also tested in wells not coated with the antigen, and the specific optical density (OD) was calculated as ODantigen − ODno antigen. To establish the cutoff value of the assays, serum samples from noninfected controls were tested under the same conditions described above. The cutoff value of each ELISA system was calculated as the mean specific OD of control sera plus 2 standard deviations.
The same ELISA protocol was used for proteinase K-treated antigens, but a smaller number of samples of each host species was included.
Sensitivity and specificity.
The sensitivity of each antigen for each host species was calculated from the results obtained for samples from Brucella-infected individuals as positives/(positives + false negatives). Specificity was calculated from the results obtained for samples from healthy controls as negatives/(negatives + false positives).
Western blotting.
CYT and MA antigens were electrophoresed in a 12.5% polyacrylamide gel in the presence of sodium dodecyl sulfate and were electrotransferred to nitrocellulose sheets by conventional methods. After being blocked with Tris-buffered saline (TBS) containing 0.1% Tween 20, the nitrocellulose sheets were rinsed with TBS containing 0.05% Tween 20 and cut into strips. Each strip was incubated for 1 h at room temperature with serum diluted 1:200 in TBS containing 0.05% Tween 20. After a subsequent incubation with species-specific peroxidase-conjugated antibodies (see “ELISA” above), the reaction was developed with 4-chloro-α-naphthol (3 mg/ml) and H2O2 (0.03%) in TBS.
Statistical analysis.
Differences in median ODs between groups were analyzed with the Kruskal-Wallis test and Dunn's multiple comparison test by using the software included in GraphPad Prism (version 3.0; GraphPad, Inc., San Diego, Calif.).
RESULTS
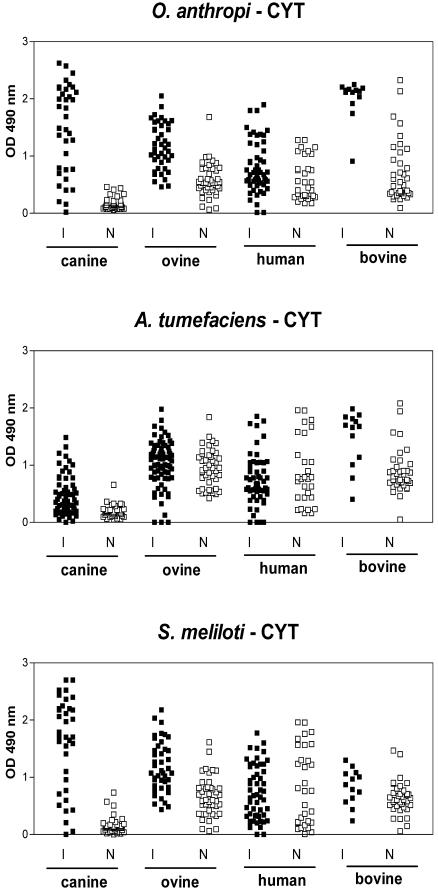
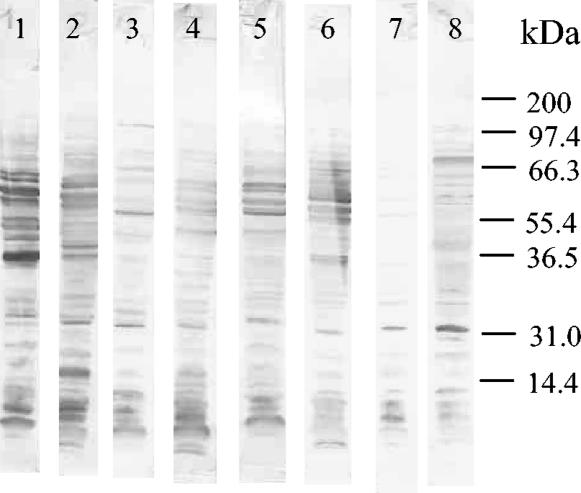
Sera from patients and animals infected with different Brucella species, all of which were positive against CP and LPS from Brucella by ELISA (3, 17), were assayed for reactivity against CYT and MA antigens from other alpha-proteobacteria. As shown in Fig. 1, most sera from Brucella-infected dogs exhibited strong reactions against CYT antigens from O. anthropi and S. meliloti but weaker reactions against antigens from A. tumefaciens. Similar results were obtained with MA antigens (data not shown). For CYT antigens, median reactivities of sera from infected and control dogs were 1.590 and 0.144 (P < 0.001) for O. anthropi, 1.707 and 0.130 (P < 0.001) for S. meliloti, and 0.373 and 0.167 (not significant) for A. tumefaciens. For MA antigens, median reactivities were 1.534 and 0.506 (P < 0.001) for O. anthropi, 1.134 and 0.454 (P < 0.001) for S. meliloti, and 0.805 and 1.053 (not significant) for A. tumefaciens. The reactivities of samples from infected dogs were directed mainly to proteinaceous antigens, as evidenced by the fact that they were greatly reduced after treatment of CYT or MA antigens with proteinase K (Fig. 2). The differential reactivity of control and brucellosis sera was also reflected in Western blotting results. As exemplified for the O. anthropi CYT antigens (Fig. 3), some antigens were recognized by sera from both healthy and infected dogs, but many proteins with molecular masses between 35 and 65 kDa were strongly and specifically recognized by brucellosis sera.
FIG. 1.
Reactivities of human and animal brucellosis sera against CYT antigens of O. anthropi, S. meliloti, and A. tumefaciens. I, infected hosts; N, normal (uninfected) controls. Reactivity was assessed by an indirect ELISA.
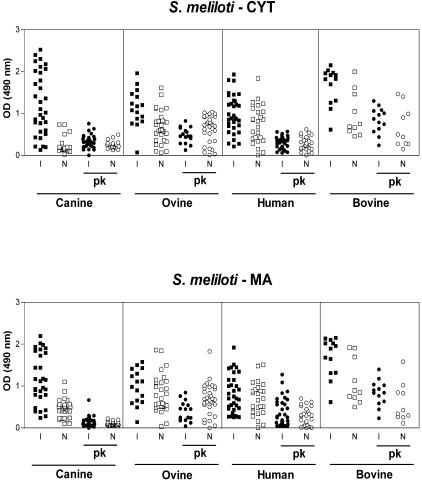
FIG. 2.
ELISA reactivities of brucellosis (I) and control (N) sera against CYT and MA antigens from S. meliloti before and after treatment with proteinase K.
FIG. 3.
Western blot reactivities of sera from dogs with brucellosis (lanes 1 to 6) and control dogs (lanes 7 and 8) against CYT antigens from O. anthropi.
Cutoff values were calculated by using ODs from healthy dogs, and the sensitivity and specificity of each ELISA for canine brucellosis were determined (Table 1). All three ELISAs using CYT antigens were highly specific, and two of them (O. anthropi and S. meliloti) also showed acceptable sensitivity. Similar results were obtained when MA antigens were used (Table 1). In addition, ELISAs with CYT or MA antigens allowed diagnosis of canine brucellosis shortly after exposure to the pathogen (15 days). Initial samples from the seven recently infected dogs were positive against O. anthropi and A. tumefaciens antigens, and five were positive against S. meliloti antigens. The remaining two of these seven dogs became positive for S. meliloti antigens later during follow-up (data not shown).
TABLE 1.
Diagnostic performance of ELISA using CYT and MA antigens from alpha-proteobacteria for the diagnosis of human and animal brucellosis
| Antigen
|
Valuea for the following serum samples:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canine
|
Human
|
Bovine
|
Ovine
|
||||||||||
| Bacterium | Fraction | Cutoff | Se | Sp | Cutoff | Se | Sp | Cutoff | Se | Sp | Cutoff | Se | Sp |
| O. anthropi | CYT | 0.560 | 84 | 91 | 1.820 | 0 | 100 | 1.040 | 6.8 | 97.2 | 0.860 | 63.6 | 95 |
| MA | 1.080 | 80 | 94 | 2.280 | 0 | 100 | 2.300 | 0 | 100 | 1.230 | 9.52 | 96 | |
| A. tumefaciens | CYT | 0.670 | 58 | 96 | 2.550 | 0 | 100 | 1.390 | 55 | 100 | 1.820 | 9 | 95 |
| MA | 1.130 | 50 | 99 | 0.420 | 41 | 100 | 1.140 | 0 | 1 | 1.280 | 100 | ||
| S. meliloti | CYT | 0.400 | 88 | 96 | 1.500 | 0 | 100 | 1.010 | 1.7 | 100 | 1.260 | 4.8 | 95 |
| MA | 0.280 | 95 | 100 | 2.220 | 0 | 100 | 1.570 | 0 | 97 | 1.100 | 4.8 | 100 | |
Se, sensitivity; Sp, specificity. Both sensitivity and specificity are expressed as percentages.
In contrast with canine samples, control sera from cattle, sheep, and humans yielded high reactivities against CYT antigens, and their OD distribution was sometimes indistinguishable from that of samples from infected individuals (Fig. 1). Similar results were obtained with MA antigens (data not shown). Since results were expressed as specific ODs (ODantigen − ODno antigen for each serum sample), the reactivities of control sera shown in Fig. 1 were antigen specific. Several assay modifications were tested with the aim of increasing the difference in reactivity between the two groups of samples. These included different serum dilutions (up to 1:800) and different blocking agents (0.3% gelatin, 1.0% ovalbumin, and 0.1% Tween 20, alone or combined). None of these changes resulted in an increased difference between control and pathological sera.
The high reactivities of control sera from sheep, cattle, and humans resulted in cutoff values much higher than those obtained for canine samples (Table 1). While specificities were high for all the antigens, the high cutoff values resulted in low sensitivities for the diagnosis of human, ovine, and bovine brucellosis (Table 1). In general, the lower diagnostic sensitivity for ovine and bovine brucellosis than for canine disease was not due to lower signal levels of sera from infected sheep and cattle. For O. anthropi CYT antigens, the median OD of sera from infected dogs did not differ significantly from that of infected sheep or cattle (1.590 versus 1.133 and 2.133, respectively). Moreover, for A. tumefaciens CYT antigens, the median ODs for infected sheep and cattle were significantly higher than that of canine samples (1.101 and 1.680 versus 0.373, respectively; P < 0.001 for both comparisons). In contrast, reduced reactivity of sera from brucellosis patients could have contributed, in addition to the higher cutoff values, to the lower sensitivity of CYT ELISAs for human brucellosis than for canine brucellosis. Median ODs of human sera against CYT antigens from O. anthropi and S. meliloti were significantly lower than the corresponding values of canine sera (0.673 and 0.690 versus 1.590 and 1.707, respectively; P < 0.05 and P < 0.001).
To determine whether the antigens responsible for the high reactivities of uninfected ovine, bovine, and human sera were proteins, both CYT and MA antigens were treated with proteinase K before ELISA. Proteinase K digestion reduced the reactivities of sera from both healthy controls and infected individuals to CYT antigens from S. meliloti (Fig. 2) and from O. anthropi and A. tumefaciens (data not shown). However, bovine and ovine reactivities to CYT antigens from A. tumefaciens and S. meliloti were less affected than human reactivity to these antigens. In contrast, proteinase K reduced the reactivities of sera from healthy or infected individuals of all three species against O. anthropi CYT antigens in a similar fashion. Overall, the results suggested that the high reactivity of control sera against O. anthropi CYT antigens is due mostly to proteinaceous antigens. The reactivity of human control sera to CYT antigens from A. tumefaciens and S. meliloti also seemed to be due to proteins, but those of bovine and ovine samples seemed to be directed mainly to nonproteinaceous antigens. Similar results were obtained after proteinase K treatment of MA antigens from S. meliloti (Fig. 2) and from O. anthropi and A. tumefaciens (data not shown).
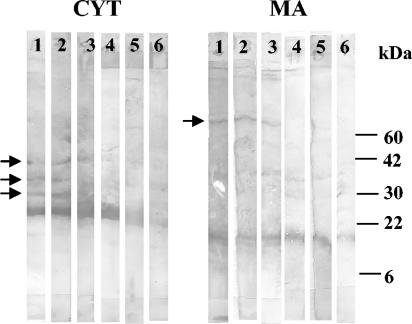
To establish whether the antigens recognized by healthy controls were different from those recognized by sera from Brucella-infected humans, cattle, and sheep, Western blot analysis was performed. While for some host species and some antigens no differential reactivity was noted between sera from healthy controls and sera from Brucella-infected individuals, some antigens present in the CYT and MA fractions of S. meliloti were recognized by infected cattle but not by normal cattle (Fig. 4).
FIG. 4.
Western blot reactivities of sera from cattle with brucellosis (lanes 1 to 3) and healthy cattle (lanes 4 to 6) against CYT and MA antigens from S. meliloti. Arrows indicate antigens recognized by infected cattle but not by healthy controls.
DISCUSSION
Serological cross-reactivities can constitute a drawback when cross-reacting species are common pathogens, since they make the differential diagnosis of infections difficult. In contrast, if one of the species is not pathogenic and/or is unlikely to enter the host, antibodies to cross-reacting antigens should be found only in individuals infected with the pathogenic species. Therefore, cross-reacting antigens from the nonpathogenic species could be used to diagnose infections caused by the pathogenic species While most Brucella species are pathogenic, many related alpha-proteobacteria are not pathogenic for immunocompetent individuals. The present study reveals the existence of extensive cross-reactivities, especially evident in infected dogs, between CYT and MA antigens from Brucella and those of A. tumefaciens and S. meliloti.
These serological cross-reactivities allowed the sensitive diagnosis of canine brucellosis by ELISAs performed with CYT and MA antigens from S. meliloti and O. anthropi (but less-sensitive diagnosis with A. tumefaciens). The background reactivity of canine control sera was generally low and resulted in high specificities for all the tests.
In contrast, control sera from humans, cattle, and sheep produced rather high reactivities with most antigens, yielding high cutoff values and low diagnostic sensitivities. The reasons for the higher reactivities of these sera are unclear. One possibility is that cattle, sheep, and humans are more exposed than dogs to these alpha-proteobacteria because of their diets. Alternatively, CYT and MA components may cross-react with antigens from bacteria not belonging to the alpha-proteobacteria division that may have more frequent contact with humans or cattle than with dogs. In any case, CYT and MA antigens should be further fractionated to improve their diagnostic performance. As shown in Fig. 4, some proteins of the S. meliloti CYT and MA fractions were preferentially recognized by sera from infected cattle.
To our knowledge, this study is the first to assess the usefulness of antigens from nonpathogenic bacteria of related genera for the diagnosis of an infectious disease. In the few cases in which heterologous antigens have been used, they were obtained from a different species of the same genus (e.g., Taenia crassiceps instead of Taenia solium [9]) or from a different stage of the same species (e.g., Trypanosoma epimastigotes instead of trypomastigotes). The use of antigens from related nonpathogenic organisms could constitute a useful strategy to be applied when the preparation of antigens from the etiological agent is complicated by biosafety and/or bacterial growth issues. In the specific case of Brucella, pathogenicity is the most important issue. Expert committees, including those of the Centers for Disease Control and Prevention and the National Institutes of Health, have recommended the use of BSL-3 facilities for the large-scale culture of Brucella species (6). Such facilities are seldom available in the developing countries where brucellosis is more prevalent. Only B. ovis can be regarded as safe, since no human infections by this species have been reported. However, this species requires a controlled CO2 atmosphere for growth, which can complicate production procedures. In addition, the use of antigens from nonpathogenic alpha-proteobacteria could circumvent the undesirable false-positive reactions due to smooth or rough Brucella LPS (4, 7). By reducing the biosafety requirements, the use of antigens from nonpathogenic alpha-proteobacteria would simplify the production of diagnostic kits for brucellosis. While the use of recombinant antigens is also a valuable strategy for avoiding the large-scale culture of pathogenic species, the recombinant Brucella antigens tested to date have shown insufficient sensitivity for the diagnosis of brucellosis (5, 11). This suggests that a mixture of several proteins may be necessary to achieve high diagnostic sensitivity for brucellosis.
In summary, this study shows that CYT and MA antigens from nonpathogenic alpha-proteobacteria are recognized by sera from humans and animals infected with Brucella. It also shows that these antigens can be used for the specific and sensitive diagnosis of canine brucellosis. Because of the high reactivities of sera from healthy individuals, the whole CYT and MA fractions do not seem to have diagnostic usefulness for ovine, bovine, and human brucellosis. However, selected components of these fractions seemed to be preferentially recognized by infected individuals and could constitute the basis of a diagnostic assay for human and animal brucellosis.
Acknowledgments
This work was supported by grant PICT99-0506324 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and by a grant from the Fundación Antorchas. M.V.D. was supported by a fellowship of the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET). C.A.F. and P.C.B. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
REFERENCES
- 1.Alnor, D., N. Frimodt-Moller, F. Espersen, and W. Frederiksen. 1994. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin. Infect. Dis. 18:914-920. [DOI] [PubMed] [Google Scholar]
- 2.Amaya, R. A., and M. S. Edwards. 2003. Agrobacterium radiobacter bacteremia in pediatric patients: case report and review. Pediatr. Infect. Dis. J. 22:183-186. [DOI] [PubMed] [Google Scholar]
- 3.Baldi, P. C., S. E. Miguel, C. A. Fossati, and J. C. Wallach. 1996. Serological follow-up of human brucellosis by measuring IgG antibodies directed to LPS and cytoplasmic proteins of Brucella. Clin. Infect. Dis. 22:446-455. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael, L. E., S. J. Zoha, and R. Flores-Castro. 1984. Problems in the serodiagnosis of canine brucellosis: dog responses to cell wall and internal antigens of Brucella canis. Third International Symposium on Brucellosis, Algiers. Dev. Biol. Stand. 56:371-383. [PubMed] [Google Scholar]
- 5.Cassataro, J., M. V. Delpino, C. A. Velikovsky, L. Bruno, C. A. Fossati, and P. C. Baldi. 2002. Diagnostic usefulness of antibodies against the ribosome recycling factor from Brucella melitensis in human or canine brucellosis. Clin. Diagn. Lab. Immunol. 9:366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention and National Institutes of Health. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. U.S. Department of Health and Human Services, Washington, D.C.
- 7.Corbel, M. J., F. A. Stuart, and R. A. Brewer. 1983. Observations of serological cross-reaction between smooth Brucella species and organisms of other genera. Dev. Biol. Stand. 56:341-363. [PubMed] [Google Scholar]
- 8.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira, A. P., A. J. Vaz, P. M Nakamura, A. T. Sasaki, A. W. Ferreira, and J. A. Livramento. 1997. Hemagglutination test for the diagnosis of human neurocysticercosis: development of a stable reagent using homologous and heterologous antigens. Rev. Inst. Med. Trop. Sao Paulo 39:29-33. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, A. M., M. R. Lum, and J. A. Downie. 2001. What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127:1484-1492. [PMC free article] [PubMed] [Google Scholar]
- 11.Letesson, J. J., A. Tibor, G van Eynde, V. Wansard, V. Weynants, P. Denoel, and E. Saman. 1997. Humoral immune response of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood, M. S., A. R. Sarwari, M. A. Khan, Z. Sophie, E. Khan, and S. Sami. 2000. Infective endocarditis and septic embolization with Ochrobactrum anthropi: case report and review of literature. J. Infect. 40:287-290. [DOI] [PubMed] [Google Scholar]
- 13.Moreno, E., A. Cloeckaert, and I. Moriyón. 2002. Brucella evolution and taxonomy. Vet. Microbiol. 90:209-227. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzfira, T., and V. Citovsky. 2002. Partners-in-infection: host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell. Biol. 12:121-129. [DOI] [PubMed] [Google Scholar]
- 16.Velasco, J., R. Diaz, M. J. Grillo, M. Barberan, C. Marin, J. M. Blasco, and I. Moriyon. 1997. Antibody and delayed-type hypersensitivity responses to Ochrobactrum anthropi cytosolic and outer membrane antigens in infections by smooth and rough Brucella spp. Clin. Diagn. Lab. Immunol. 4:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanke, M. M., M. V. Delpino, and P. C. Baldi. 2002. Comparative performance of tests using cytosolic or outer membrane antigens of Brucella for the serodiagnosis of canine brucellosis. Vet. Microbiol. 88:367-375. [DOI] [PubMed] [Google Scholar]