Abstract
We evaluated the impact of latent toxoplasmosis (LT) on neurocognitive (NC) and neurobehavioural functioning in young adults with and without chronic HIV infection, using a standardised NC test battery, self-reported Beck Depression Inventory, Frontal System Behaviour Scale, MINI-International Neuropsychiatric Interview and risk-assessment battery. 194 young adults (median age 24 years, 48.2% males) with chronic HIV infection (HIV+) since childhood and 51 HIV seronegative (HIV-) participants were included. HIV+ individuals had good current immunological status (median CD4: 479 cells/μL) despite a low CD4 nadir (median: 93 cells/μL). LT (positive anti-Toxoplasma IgG antibodies) was present in one third of participants. The impairment rates in the HIV- with and without Toxo were not significantly different (p=0.17). However, we observed an increasing trend (p<0.001) in impairment rates with HIV and LT status: HIV-/LT- (6.1%); HIV-/LT+ (22%), HIV+/LT- (31%), HIV+/LT+ (49%). In a multivariable analysis using the entire study group there were main effects on cognition for HIV and also for LT. Within the HIV+ group LT was associated with worse performance globally (p=0.006), in memory (p=0.009), speed of information processing (p=0.01), verbal (p=0.02) and learning (p=0.02) domains. LT was not associated with depressive symptoms, frontal systems dysfunction or risk behaviors in any of the groups. HIV participants with lower Toxoplasma antibody concentration had worse NC performance, with higher GDS values (p=0.03) and worse learning (p=0.002), memory (p=0.006), speed of information processing (p=0.01) T scores.
Latent Toxoplasmosis may contribute to NC impairment in young adults, including those with and without chronic HIV infection.
Keywords: Latent toxoplasmosis, HIV, neurocognitive impairment, young adults
Graphical Abstract
1. Introduction
Latent Toxoplasma gondii (LT) infection had long been assumed to be asymptomatic, but increased evidence links its presence to increased risk for psychiatric conditions such as schizophrenia [1], bipolar disorder [2] and suicidal behavior [3, 4]. The presence of Toxoplasma gondii antibodies has also been associated with neurodegenerative syndromes such as Parkinson’s Disease [5].
Several studies reported the association between Toxoplasma gondii seropositivity and poor cognitive scores, especially in certain groups of people with low-income [6] and low education [7]. Worse performance on cognitive tests associated with Toxoplasma gondii was reported for reaction time, concentration [8], learning and memory [9]. Moreover, latent toxoplasmosis was associated with impulsive behaviors such as higher risk for work- and traffic- accidents [10–12]
Toxoplasma gondii tachyzoites are capable of invading microglia, astrocytes, and neurons [13]; the parasite thereafter forms cysts within these cells [14, 15]. Parasite strains can differ greatly in their aggressiveness during infection and in their propensity to form cysts for long-term survival [16]. Significant production of dopamine by Toxoplasma gondii induces increased production of tachyzoites and destruction of cyst walls [17] and may be responsible for behavioural changes [18]. Higher anti-Toxoplasma antibody levels have correlated with more severe psychiatric symptoms [19, 20], although the relationship between antibody levels and cognition is not always clear [8].
Almost half of the HIV-infected population has neurocognitive (NC) impairment, typically mild to moderate in severity [21, 22]. While conditions like ageing, drug abuse and chronic hepatitis C can contribute to NC impairment in HIV-infected patients [23], the influence of LT has received only limited attention [24].
We aimed to evaluate the contribution of latent infection with Toxoplasma gondii on NC performance and neuropsychiatric conditions (depression and suicidal risk, conditions associated with frontal-subcortical circuitry damage and risk taking behaviors) in a group of young adults with and without chronic HIV infection since childhood.
2. Methods
2.1. Study participants
We included in the current study all 194 HIV+ and 51 HIV-, age-matched participants recruited until 1-Oct-2014 in a cross-sectional study of the long-term effects of chronic HIV infection on the developing brain. The HIV+ participants had previously documented HIV infection, epidemiological data supporting parenteral non-IDU route of HIV transmission during the Romanian HIV Paediatric epidemic. Inclusion criteria were: at least 8 years of education, no NC impairment unrelated to HIV or psychiatric condition, no significant central nervous system opportunistic infection, or other severe medical confounds [25]. Age-matched HIV-negative participants were recruited from the siblings, partners, school- or workmates of the HIV participants with similar socioeconomic status and similar parents’ education.
The study was approved by the ethics committees of the “Dr. Victor Babes” Hospital of Infectious and Tropical Diseases, Bucharest and the University of California, San Diego. Written informed consent was provided by all participants.
2.2. Neurocognitive assessment
Participants underwent neurocognitive testing using a standardised battery that assessed seven cognitive domains: Verbal fluency (letter, animal and action fluency), Attention/Working Memory (Paced Auditory Serial Addition Test – 50 item, Wechsler Memory Scale-III Spatial Span), Speed of Information Processing (Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol, WAIS-III Symbol Search, Trailmaking Test Part A, Stroop Word, Stroop Colour), Executive Functioning (Halstead Category Test, Wisconsin Card Sorting Test –64 item, Trailmaking Test Part B, Colour Trails II, Stroop Incongruent), Learning/Memory (Hopkins Verbal Learning Test –Revised, Brief Visuospatial Memory Test –Revised), and Motor (Grooved Pegboard). These have been described previously [26]. Demographically-corrected (age, education, gender) T scores (mean of 50, standard deviation of 10) were developed using an HIV seronegative control group selected such that there were no significant comorbidities known to affect cognition (e.g., traumatic brain injury). Importantly, Toxoplasma infection was not an area of study at that time and not an exclusion. The normative cohort consisted of a) 21 individuals from a previous study –Toxoplasma status was not evaluated as part of that study, and b) the remaining 51 participants reported on in the current study. Overall neurocognitive impairment (NCI) was estimated using the global deficit score (GDS) approach, calculated as the average of deficit scores across all neuropsychological tests. Individual test deficit scores, determined via demographically-adjusted T scores generated from a healthy population of Romanian young adults, ranged from 0 (T score of ≥ 40) to 5 (T score < 20) [27]. The cut-off for NCI was a GDS score ≥ 0.5.
2.3. Depression, psychiatric disorders and risk taking behaviors
Depressive symptoms were assessed using the Beck II depression inventory (a 21-item self-report scale that measures depressive symptoms). A score of 0–13 represents minimal symptoms, 14–19 indicates mild depression, 20–28 is moderate, and 29–63 represents severe depression [28]. We evaluated also current and past major depression, current and past suicidal risk, alcohol abuse and dependence, substance use and dependence in study participants using the MINI-International Neuropsychiatric Interview (MINI-Plus), a structured diagnostic interview incorporating DSM-IV and ICD-10 criteria [29].
The Frontal System Behavior Scale (FrSBe) was used to quantify behaviors associated with frontal subcortical deficits (apathy, disinhibition, executive dysfunction) [30]. The self-rated version consists of 46 items rated on a 5-point Likert scale that measures behaviour before illness or injury and at the present time. As the HIV+ participants were infected in their first year of life, we used only the evaluation at present time. Since the application of U.S. FrSBe norms have not been validated in this young adult Romanian cohort, we analysed raw scores in the relatively homogeneous cohort. We used the Risk Assessment Battery (RAB), a self-administered, multiple choice questionnaire, developed to offer a quick assessment of both needle sharing practices and sexual activity associated with HIV transmission. RAB was scored by adding the values that correspond to the responses selected by the subject, where lower scores indicate fewer risk behaviors [31].
2.4. Premorbid characteristics
Data regarding CD4 nadir, HIV RNA zenith, and antiretroviral therapy history were collected.
2.5. Immunological and virological assessments
Blood samples were collected at the time of NC assessment. Lymphocyte subsets were measured immediately while other laboratory analyses were performed from samples stored at −80°C. T (CD4+ and CD8+), B and NK-lymphocyte subsets in the peripheral blood were measured by flow cytometry (4 colours MultiTest and FACSCalibur, Becton Dickinson). HIV-1 viral load was assessed using a commercial nucleic acid amplification test (COBAS TaqMan HIV-1 Test Version 2.0, Roche Molecular Systems, Branchburg, USA), with a lower detection limit of 20 copies of HIV RNA/mL and a linear range between 34 - 10000000 copies HIV RNA/mL.
Toxoplasma IgG and IgM levels were measured by a quantitative enzyme immunoassay (DiaPro, Italy). Toxoplasma IgG antibody level was dichotomised as high or low based on the median value of Toxoplasma positive levels of the entire study (1088 UI/ml).
2.6. Statistics
Statistical analyses were performed using JMP (version 11.0.0). Continuous variables were assessed for normal distribution. Log10 transformations (viral-load variables) and square root transformations (GDS, FrSBe and sex activity scores) were applied to achieve approximately normal distribution. The following variables were dichotomized: GDS (impaired/unimpaired), anti-toxoplasma IgG (positive/negative, lower than median/higher than median value).
All simple group comparisons were performed using the Chi-squared test or Fisher’s exact text (for categorical data), t test (for continuous, normally distributed variables), or Mann-Whitney U test (for continuous, non-normally distributed variables). Cochran-Armitage trend test was used to test a hypothesis of ordered proportions among the four HIV/Toxo groups. The first set of analyses focused on neurocognitive outcomes, measured by GDS, global and domain mean T scores, and NC impairment. Multivariable linear (for GDS and T scores) and logistic (for NC impairment) regressions were used to assess the main effects of HIV and toxoplasmosis status on GDS, mean T score, NC impairment, FrSBe, and risk taking behaviors, as well as to evaluate the effect of Toxoplasmosis outcomes. Interactions between HIV and toxoplasmosis were not significant, thus the final models did not include them. In addition, relevant covariates that differed between HIV/Toxo groups were included in the models as covariates. A follow-up analysis, using the same regression methods, was performed only in HIV+ cohort to investigate if inclusion of HIV-related covariates (log10 zenith viral load, duration ART, AIDS, and nadir CD4) altered the effect of toxoplasmosis on cognition. A second set of analyses focused on behavioral outcomes (FrSBe and risk taking behaviors), and their association with HIV and Toxo status. Again, linear and logistic regressions were applied to the data from the entire cohort as described above. Finally, the effect of dichotomized Toxo IgG levels on neurocognitive and behavioural outcomes was assessed among Toxo+ participants using methods described above.
3. Results
3.1. HIV+ and HIV- group characteristics
Demographic and disease characteristics are summarized in Table 1. HIV+ and HIV- participants did not differ in age, gender, or body mass index (BMI). Three participants had chronic hepatitis C infection; none had current or past Toxoplasma encephalitis.
Table 1.
General characteristics of study participants
| Characteristics of participants | HIV+ (n = 194) | HIV- (n = 51) | |
|---|---|---|---|
| General characteristics | Male1 | 94 (48.4%) | 28 (54.9%) |
| Age (years)2 | 24.0 (1.5) | 24.2 (2.4) | |
| Education (years) 2 ** | 12.1 (2.8) | 13.3 (2.6) | |
| BMI3 | 20.8 (17.0–23.9) | 21.8 (19.5–25.2) | |
| HIV infection data | Time since estimated HIV transmission (years) 3 | 23.7 (22.8–24.4) | - |
| Time since HIV-diagnosis (years) 3 | 14.8 (9.7–17.5) | - | |
| Current CD4 T-cells/μL3 | 479 (273–713) | - | |
| Nadir CD4 T cells/μL3 | 93 (22–190) | - | |
| Time since CD4 nadir (years) 3 | 6.6 (1.6–11.5) | - | |
| AIDS defining diseases1 | 173 (89.1%) | - | |
| HIV RNA plasma current log10 copies/ml3 | 1.5 (1.5–2.7) | - | |
| HIV RNA in plasma undetectable1 | 114 (58.8%) | - | |
| HIV RNA in CSF undetectable1 | 58 (85.3%); n=68 | - | |
| HIV RNA plasma zenith log10 copies/ml3 | 5.1 (4.4–5.6); n=167 | - | |
| Currently taking cART1 | 178 (91.7%) | - | |
| Duration of current cART regimen (months) 3 | 29.3 (13.1–49.4) | - | |
| Cumulative ART exposure (months) 3 | 137 (88.6–170.7) | - | |
| Data on latent toxoplasmosis | IgG Toxoplasma antibodies positive1 | 63 (32.4%) | 18 (35.3%) |
| IgG Toxoplasma antibodies levels IU/ml (among positive participants) 3 | 1090 (482–1604); n=63 | 937 (232–1291); n=18 | |
| Neurocognitive performance | Participants with NCI1 | 71 (36.5%) | 6 (11.7%) |
| GDS levels among NCI participants3 | 0.9 (0.6–1.2); n=71 | 0.8 (0.6–0.9); n=6 | |
| GDS levels among LT+ participants3 GDS scores among LT- participants3 |
0.5 (0.2–0.8); n=63 0.3 (0.1–0.6); n=131 |
0.2 (0.1–0.5); n=18 0.2 (0–0.3); n=33 |
|
| Depression1 | BDI-II depression score >13 * | 23 (11.8%) | 1 (2.0%) |
| Current major depression (MINI-Plus) Past major depression (MINI-Plus) |
6 (3.1%) 29 (14.9%) |
1 (2.0%) 7 (13.7%) |
|
| FrSBe raw scores2 | Apathy * | 26.6 (7.4) | 24.0 (6.1) |
| Disinhibition | 25.4 (7.5) | 24.3 (6.6) | |
| Executive dysfunction * | 33.2 (8.5) | 30.2 (8.2) | |
| Total * | 85.1 (20.7) | 78.4 (18.9) | |
| Suicidal risk (MINI-Plus)1 | Current * | 14 (7.2%) | 0 (0.0%) |
| Past * | 23 (11.8%) | 1 (1.9%) | |
| Risk behaviors (RAB) 3 | Injection drug risk behaviors | 0 (0–0) | 0 (0–0) |
| Sexual risk behaviors ** | 2 (0.8–4) | 4 (2–5) | |
Note: AIDS = acquired immune deficiency syndrome, BDI = Beck depression inventory, BMI = body mass index, cART = combination antiretroviral therapy, CSF = cerebrospinal fluid, FrSBe = Frontal System Behaviour Scale, GDS = global deficit score, HIV = human immunodeficiency virus, IQR = interquartile range, LT = latent toxoplasmosis, MINI-Plus = MINI-International Neuropsychiatric Interview, NCI = neurocognitive impairment, RAB = risk assessment battery, SD = standard deviation.
n (%),
Mean (standard deviation),
Median (interquartile range),
p<0.05,
p<0.01.
The prevalence of positive Toxoplasma IgG antibodies was 35.3% in the HIV- group and 32.4% in the HIV+ group. None of the participants had IgM antibodies.
Overall, NCI was more common among HIV+ subjects, compared to HIV- participants (36.5% vs. 11.7%; p<0.001). Most HIV+ participants had mild NCI (29, 21.6%) and the rest had moderate NCI. The highest GDS score was 2.09. NCI in HIV- participants was mild.
3.2. Neurocognitive performance among HIV+/HIV− and Toxo+/− participants
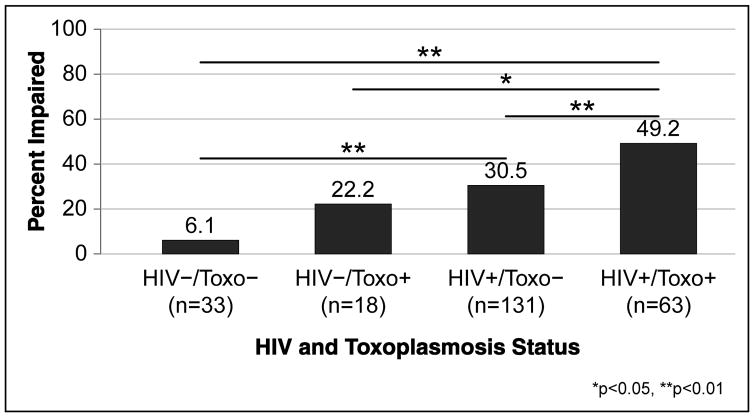
We found a significant stairstep effect for HIV and Toxoplasmosis combination on the neurocognitive impairment prevalence, so that the HIV−/Toxo - group was the least likely to be impaired, the dually infected was the most likely to be impaired, and the mono-infected groups fell between these two extremes (Cochran-Armitage trend test p<0.001; Figure 1). Pairwise comparisons showed that the dually infected participants were more likely to be impaired than each of the other three groups (all ps<0.05), while the HIV+ mono-infected group differed only from the group with neither HIV nor Toxo (p=0.004), but not the Toxo mono-infected group (p=0.47). Lastly, the two HIV- groups (22.2% vs 6.1%) did not statistically differ (p=0.17), although this may be impacted by the limited sample size.
Figure 1.
Neurocognitive impairment rates by HIV and toxoplasmosis status.
As seen in Table 2, in regression models to predict cognitive functioning across the four HIV and Toxoplasmosis groups, the main effects of HIV were seen for the GDS and global mean T, as well as across all domains except verbal fluency (executive functioning, working memory, learning, memory, motor functioning, and speed of information processing). In addition, the main effects for toxoplasmosis were seen globally (GDS, mean T), as well as for verbal fluency, learning, memory, and speed of information processing. For each outcome, a possible interaction between HIV and Toxo was investigated, but none were significant. Since the HIV-/Toxo- group had a higher education than the other three groups, education was included in the models but did not change the results.
Table 2.
Multivariable linear models examining association of cognitive performance (GDS and T scores) with HIV and Toxoplasmosis using 2-way ANOVA analysis. Significant effects of either HIV or Toxoplasma are shown in the last column for each cognitive outcome. Interaction between HIV and Toxoplasmosis were not significant (results are not shown).
| Cognitive domain | HIV-/Toxo- (n=33) | HIV-/Toxo+ (n=18) | HIV+/Toxo- (n=131) | HIV+/Toxo+ (n=63) | Significant Effects |
|---|---|---|---|---|---|
| GDS | 0.20 (0.21) | 0.29 (0.31) | 0.44 (0.44) | 0.59 (0.48) | HIV**, Toxo* |
| Global Mean T | 50.5 (5.1) | 48.7 (6.4) | 47.3 (6.1) | 44.8 (5.5) | HIV**, Toxo** |
| Executive | 50.8 (6.5) | 50.0 (7.1) | 47.4 (7.2) | 45.7 (7.6) | HIV** |
| Verbal | 50.1 (8.5) | 47.8 (7.3) | 50.3 (7.5) | 47.7 (6.9) | Toxo* |
| Working Memory | 50.0 (7.5) | 50.3 (11.2) | 45.9 (9.6) | 44.3 (9.2) | HIV** |
| Learning | 50.7 (81) | 49.0 (9.7) | 46.3 (9.1) | 43.2 (8.1) | HIV**, Toxo* |
| Memory | 50.5 (7.5) | 49.4 (10.5) | 46.4 (9.8) | 42.4 (10.2) | HIV**, Toxo** |
| Motor | 49.5 (9.1) | 48.8 (7.7) | 46.4 (9.5) | 44.3 (10.6) | HIV* |
| SIP | 50.9 (6.3) | 47.2 (7.4) | 47.2 (7.9) | 44.2 (7.0) | HIV**, Toxo** |
Note: GDS = global deficit score, HIV = human immunodeficiency virus, Toxo = Toxoplasma gondii IgG antibodies, SIP = speed of information processing.
Values inside the table represent mean (standard deviation).
p<0.05 ,
p<0.01.
3.3. Effects of toxoplasma on NCI within the HIV+ group
Among HIV+ participants, detectable anti-Toxoplasma antibodies were associated with a 60% increased relative risk of NCI (χ2= 6.3, RR = 1.6, p=0.012). The dually infected group had a higher zenith viral load (5.29 vs 4.90 log10 c/mL, p=0.02) and a shorter duration of ART (119 vs 144 months, p=0.008) than the HIV mono-infected group. This group also had higher BMI 21.83 vs 20.24 (p=0.001) than the HIV mono-infected group, but when the group was split by gender, only HIV+/Toxo+ males had significantly higher BMI (p<0.001).
We next analyzed the effects of Toxoplasmosis on cognition in the HIV-positive subgroup using multivariable models that controlled for zenith viral load and duration of ART. Toxo remained significantly associated with higher odds of impairment, higher GDS (p = 0.028) and lower Global Mean T score (p = 0.005), as well as lower mean T scores in Verbal (p = 0.019), Learning (p = 0.047), Memory (p = 0.018), and SIP domains (p = 0.012). The HIV and ART covariates were not statistically significant in any of these models.
In the subgroup of 118 participants with undetectable plasma HIV load, the presence of anti-Toxoplasma antibodies remained associated with worse GDS values (p=0.029) specifically with worse motor (p=0.03), verbal (p=0.04) and learning (p=0.04) performance.
3.4. Analysis of outcomes of frontal systems dysfunction, risk behaviors, depression among HIV+/HIV− and Toxo+/− participants
We did not find any significant differences in FrSBe mean raw scores in participants with and without toxoplasmosis (Table 3). In regression models, the interactions between HIV and Toxo were not statistically significant (all p>0.41). In multivariable models, HIV effect is significant in 3 of the 4 FrSBe scores (apathy, executive and total scores). There was no Toxo+ effect in these models (p>0.15). While there was no association between the disinhibition score and HIV (p=0.36), the association between Toxo+ and lower disinhibition scores approached significance (p=0.064).
Table 3.
Multivariable linear and logistic models examined association of frontal subcortical dysfunction and risk behaviors with HIV and Toxoplasma IgG antibodies status. Significant effects of either HIV or Toxoplasma are shown in the last column for each outcome. Interaction between HIV and Toxoplasmosis were not significant (results are not shown).
| HIV-/Toxo- (n=33) | HIV-/Toxo+ (n=18) | HIV+/Toxo- (n=131) | HIV+/Toxo+ (n=63) | Significant Effects | ||
|---|---|---|---|---|---|---|
| FrSBe raw scores1 | Apathy | 24.1 (6.2) | 23.8 (6.3) | 26.8 (7.1) | 26.1 (8.1) | HIV* |
| Disinhibition | 24.4 (6.7) | 24.1 (6.6) | 26.1 (7.6) | 23.9 (7.1) | ||
| Executive | 30.8 (7.9) | 29.1 (8.9) | 33.6 (8.7) | 32.3 (8.0) | HIV* | |
| Total | 79.2 (18.5) | 76.9 (20.2) | 86.4 (20.7) | 82.3 (20.5) | HIV* | |
| RAB | Drug use2 | 3.3% | 5.6% | 3.8% | 3.2% | |
| Sexual activity3 | 4 (2.5–5) | 4 (2–5) | 2 (2–4) | 2 (0–3) | HIV** | |
| MINI-Plus Alcohol2 | Alcohol abuse lifetime | 3.0% | 5.6% | 5.3% | 1.6% | |
| Alcohol abuse past 12 mths | 3.0% | 5.6% | 3.1% | 1.6% | ||
| Alcohol dependence lifetime | 6.1% | 0% | 0.8% | 3.2% | ||
| Alcohol dependence past 12 mths | 0% | 0% | 0% | 1.6% | ||
| MINI-Plus Substance2 | Substance abuse past 12 mths | 0% | 0% | 0.8% | 0% | |
| Substance dependence lifetime | 9.1% | 0% | 4.6% | 1.6% | ||
| Substance dependence past 12 mths | 3.0% | 0% | 3.8% | 0% | ||
Note: HIV = human immunodeficiency virus, Toxo = Toxoplama gondii IgG antibodies, FrSBe = Frontal System Behavior Scale, MINI-Plus = MINI-International Neuropsychiatric Interview, RAB = risk assessment battery.
Mean (standard deviation),
%,
Median (interquartile range),
p<0.05,
p<0.01.
The HIV+ group reported a significantly lower at risk sexual activity (p=0.003) (Table 1). However, the analysis of sex activity score showed no significant interaction between HIV and Toxo (p=0.12), and no significant effect of Toxo (p=0.13).
Logistic regression was used to test the association between self-reported drug use from the RAB (none versus some) and HIV and Toxo. Neither the interaction (p=0.624), nor the individual effects of HIV (p=0.916) and Toxo (p=0.988) were significant.
There were no differences in proportions of current or lifetime alcohol and drug abuse between the HIV and Toxo status groups (Table 3). Small percent of participants met criteria for abuse and dependence in both HIV+ and HIV- group.
As noted in Table 1, very few participants met criteria for major depression or depressive symptoms. There was no difference between participants with and without latent toxoplasmosis related to past depression, current or past suicidal risk.
3.5. Comparison between the groups with higher and lower Toxo IgG levels
We aimed to investigate the magnitude of the immune response against Toxoplasma gondii, hypothesising that a high immune response, might stimulate an inflammatory response that can contribute to the neurocognitive impairment.
Among HIV participants, the group with higher levels (anti-Toxoplasma IgG ≥ 1088 IU/mL, n = 33) more frequently had AIDS-defining diseases (p=0.047), lower nadir CD4 (median 90 vs 168, p=0.009), shorter estimated duration of HIV disease since diagnosis (12.4 vs 15.3 years, p=0.03), and higher BMI (22.84 vs 21.85, p=0.04) than the group with lower levels (anti-Toxoplasma IgG < 1088 IU/ml, n = 30). Interestingly, the HIV group with lower levels had worse NC performance, with higher GDS values (p=0.03) and worse learning (p=0.002), memory (p=0.006), speed of information processing (p=0.01) T scores. These associations remained significant after controlling for medical covariates (AIDS diagnosis, nadir CD4, estimated duration of HIV disease, and BMI) in multivariable models (all p<0.03). The HIV group with lower levels also had worse scores on the disinhibition (25.9 [7.8] vs. 21.7 [5.5], p=0.016) and dysexecutive (34.3 [9.0] vs. 30.1 [6.1], p=0.036) subtests of the FrSBe, and was more likely to have at least mild depression on the BDI (24.2% vs. 3.3%, p=0.028). Among HIV+ participants, the level of anti-Toxoplasma antibodies was not associated with apathy (p=0.25), drug use risk behaviors (p=0.57), sex (p=0.27) or suicide risk (p=0.36) between the HIV+ participants with higher and lower anti-Toxoplasma antibodies. Among HIV negative participants, anti-Toxoplasma antibodies levels were not associated with any of the cognitive or behavioral tests mentioned above.
4. Discussion
In this analysis of nearly 250 HIV+ and HIV- adults, we found latent Toxoplasmosis was associated with worse overall cognitive performance. While HIV+ participants as a group performed more poorly than the HIV- participants, the presence of anti-Toxoplasma IgG antibodies was also associated with a significantly higher rate of overall impairment in HIV+ individuals, supporting the hypothesis that latent Toxoplasma infection contributes to the brain injury that can occur in adults with chronic HIV disease. Raising the clinical relevance of this finding, anti-Toxoplasma antibodies were also associated with worse global NC performance in the subgroup of subjects who had undetectable HIV plasma viral loads. This observation may help explain why NCI continues to be common among otherwise effectively treated HIV+ adults. Besides Toxoplasma gondii, other latent infections, like CMV, have also been shown to contribute to NCI [32].
Importantly, we had the advantage of evaluating the possible impact of latent infection with Toxoplasma gondii in a young population and therefore could exclude the effect of ageing on NCI in HIV-infected patients [33] as well as on the ability to respond to certain antigens [34, 35]. Toxoplasma gondii has been found to alter the cognitive performances in elderly HIV-negative persons by affecting mainly working memory [9] and immediate memory [36]. In our study, which used perhaps the most comprehensive neuropsychological test batteries in Toxoplasmosis research, we found worse performance in the domains of verbal fluency, learning and memory, and speed of information processing, consistent with recent observations from seniors [37], as well as from some of the earlier reports indicating slowed processing speed and reduced concentration in anti-Toxoplasma IgG antibody positive individuals [8]. We found a trend for higher overall impairment rates in our young HIV negative, Toxoplasma positive adults, compared to HIV negative toxoplasma negative adults, as well as a main effect for Toxoplasma across the entire sample. This is consistent with previous reports looking at nonelderly and nonpsychiatric adults [2, 6] and children [38]. The potential impact of latent toxoplasmosis infection on NCI remains controversial, as not all studies have detected cognitive differences in antibody positive and negative individuals, particularly after adjusting for potential confounding factors [6, 39]. The current study adds to the literature in that we examined young adults without such confounding conditions.
Much of the literature describes behavioural changes in humans due to the presence of latent toxoplasmosis. Overall, we did not identify among our groups associations between having latent Toxoplasmosis and alcohol and substance dependence or abuse, suicidal thoughts, the presence of sexual risk-taking behaviors or significant frontal dysfunction as assessed by the FrSBe. The power of these statistical comparisons is limited by the relatively small proportion of our sample who had experienced suicidal thoughts or had engaged in substantial drug or alcohol use. This may reduce the generalizability of our findings to clinical populations who may be more likely to have substance use disorders, more severe depression, or risky sex practices. It is also possible; that these behavioural indicators require prolonged exposure in order to manifest, compared to the focused cognitive challenge presented by the neuropsychological tests in our study. Of note, substance use and risky sex could alter the effects of Toxoplasmosis on the brain via dysregulated dopamine production and metabolism (among other mechanisms). Dopamine can upregulate Toxoplasma tachyzoite replication, which could either have a) detrimental effects if it caused dissemination of infection to additional neurons and glia or b) beneficial effects if it improved the immune response to Toxoplasma with killing of infected cells and clearance of parasites. Although our study group included few subjects with depression, other studies have also found no association between latent toxoplasmosis and depression [40]. These HIV+ participants, infected since early childhood, also engaged in very little sexual activity. One study of adult (mean age ~45 years) HIV+ patients in the United States with or without toxoplasmosis antibodies did not find an association between latent toxoplasmosis and psychiatric disorders, although the determination of psychiatric status was limited to a review of electronic medical records. The authors speculated that the failure to find a significant difference could be due to the high prevalence of psychiatric disorders among their group of HIV-infected patients [41]. We found an effect of HIV, but not Toxo, on behaviors associated with disruption in frontal-subcortical circuitry.
Of note, a comparison of the concentration of Toxoplasma antibodies and NCI in our cohort (based on binary split as a clinical approach) showed that HIV-infected participants with lower levels of anti-Toxoplasma antibodies had higher NCI rates and indicators of frontal systems dysfunction. Our findings are in contrast to several studies reporting higher levels of Toxoplasma gondii to be associated with worse outcomes, such as higher depression rates [42], suicide attempts [43–45], first episode of schizophrenia [20], or more severe psychotic symptoms [46]. On the other hand, a previous double-blind study found that lower Toxoplasma levels were associated with significantly longer reaction times in 60 subjects with latent toxoplasmosis compared with those of 56 controls[8]. Since Toxoplasma levels decrease as infection time increases, it is possible that Toxoplasma gondii may exert its negative effects on cognition as a result of the slow and cumulative effects of infection, rather than the acute affects (which would be associated with higher levels) [8, 47].
None of these studies were performed in an HIV+ population. Among our HIV+ group, participants with higher anti-Toxoplasma antibody levels were more ill in the past (had more AIDS diseases, a lower CD4 nadir, and were diagnosed more recently [late-presenters]). However, most had been treated with cART and the group had a mean CD4 level approaching 500 at the moment of testing. It is possible that they may have rapidly restored their cellular immunity, and subsequently mounted a strong immune response to Toxoplasma gondii [48]. The higher antibody titre in this case might reflect a more robust response to Toxoplasma gondii as reported previously [49], or a seroconversion closer to the time of NC testing [50] as opposed to a subclinical reactivation of Toxoplasma infection in the brain [51].
Regardless, the finding of a relationship between Toxoplasma levels and neurobehavioural outcomes in the HIV positive group, but not the HIV negative group, raises the possibility that exposure to HIV in the developing brain may alter the relationship between toxoplasmosis and behavioural outcomes, at least when compared to a relatively healthy young adult sample with and without toxoplasmosis.
An interesting observation is related to the higher BMI (although within normal ranges) of men from the group of HIV-participants with latent toxoplasmosis. The explanation could be related to metabolic or hormonal disturbances induced by Toxoplasma infection. We did not find an association between latent toxoplasmosis and cholesterol, triglyceride or glucose levels (data not shown).
Of note, certain characteristics of the Romanian cohort support to the specificity of this observation: our cohort consists entirely of young adults (eliminating the confounding effect of ageing) who mostly do not have chronic hepatitis C infection, depression or history of drug abuse. Anti-Toxoplasma IgG positive and negative individuals also had similar duration of HIV disease and ART use.
There are a number of limitations to the current study. The sample for the HIV negative group was small. Due to the cross-sectional design, we could not establish the time of Toxoplasma seroconversion, and therefore could not fully interpret the role of higher vs lower Toxoplasma antibodies in relationship to NCI. It is possible that the presence of toxoplasmosis infection is related to some factor that we did not account for, although the similar infection rates (approximately one-third) in the HIV positive and negative groups suggests that at least those two groups had likely similar exposure risks. The selection of participants for the norming process excluded those with known significant comorbidities, such as severe medical, psychiatric or neurologic conditions; at the time latent Toxoplasmosis was not considered such a comorbidity. Excluding all individuals with unknown or positive status would have resulted in a normative group of only 33. Given that Toxo is highly prevalent in Romania, we opted to keep such individuals in the normative group. Thus, the prevalence of Toxo-related cognitive impairment is likely somewhat underestimated in the current study. We did not have specific measures of socioeconomic status (SES), although extreme poverty was excluded since HIV infected people in Romania receive rent assistance from the government, and the control group included siblings and partners of the HIV participants, suggesting a similar SES background as the HIV+ group. Data regarding the prevalence of Toxoplasma antibodies in the Romanian population are sparse and vary by region of the country, study group and age [52, 53]. Since the prevalence of latent Toxoplasma infection may increase with age [54], particularly in young-to-middle aged adults [55], an important concern in this young population is that the seroprevalence will rise as this population ages with even greater impact of the group’s NC functioning. In addition, a longitudinal follow-up is warranted to determine the long-term impact of latent toxoplasmosis on NCI and also in behavioural changes and psychiatric conditions of HIV-infected patients.
In conclusion, we describe worse cognitive performance in our group of young patients with latent toxoplasmosis, both with and without chronic HIV-infection, and in particular emphasize the role of latent Toxoplasmosis as a potential confounder in attributing the cause of NCI to HIV.
Highlights.
We found a significant main effect for Toxoplasmosis and HIV on cognitive performance, as well as a stairstep effect for HIV, Toxoplasmosis, and the combination on impairment rates, such that the lowest impairment rate was seen in the HIV-/Toxo-, the highest in the dually- infected
In HIV-infected participants lower T. gondii IgG levels were associated with worse cognitive performance
Latent toxoplasmosis was not associated with behavioral changes and risk-taking behaviors in HIV positive nor negative participants.
In HIV-infected patients, latent toxoplasmosis is a risk factor for neurocognitive impairment
Acknowledgments
We also want to thank to all the participants in the study, Mr. Terence Hendrix from the HIV Neurobehavioural Research Center, Drs., Roxana Radoi, Ruxandra Burlacu, Simona Tetradov and Psych. Anca Luca, Psych Adina Bulacu-Talnariu, Psych. Adrian Luca for neurocognitive and neuropsychological testing
This work was presented in part at the 13th International Symposium on NeuroVirology, June 2–6, 2015 San Diego, California, USA, abstract P40
Funding This work was supported by 1R01MH094159 and P30 MH62512 from National Institute of Mental Health
Footnotes
Authors declaration
I, Luminita Ene certify herby that this manuscript contains original work and that it has not been published or submitted for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nimgaonkar VL, Yolken RH. Neurotropic infectious agents and cognitive impairment in schizophrenia. Schizophrenia bulletin. 2012;38:1135–6. doi: 10.1093/schbul/sbs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickerson F, Stallings C, Origoni A, Katsafanas E, Schweinfurth L, Savage C, et al. Antibodies to Toxoplasma gondii and cognitive functioning in schizophrenia, bipolar disorder, and nonpsychiatric controls. The Journal of nervous and mental disease. 2014;202:589–93. doi: 10.1097/NMD.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 3.Okusaga O, Postolache TT. Toxoplasma gondii, the Immune System, and Suicidal Behavior. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- 4.Zhang Y, Traskman-Bendz L, Janelidze S, Langenberg P, Saleh A, Constantine N, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. The Journal of clinical psychiatry. 2012;73:1069–76. doi: 10.4088/JCP.11m07532. [DOI] [PubMed] [Google Scholar]
- 5.Miman O, Kusbeci OY, Aktepe OC, Cetinkaya Z. The probable relation between Toxoplasma gondii and Parkinson's disease. Neuroscience letters. 2010;475:129–31. doi: 10.1016/j.neulet.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Pearce BD, Kruszon-Moran D, Jones JL. The association of Toxoplasma gondii infection with neurocognitive deficits in a population-based analysis. Social psychiatry and psychiatric epidemiology. 2014;49:1001–10. doi: 10.1007/s00127-014-0820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale SD, Brown BL, Erickson LD, Berrett A, Hedges DW. Association between latent toxoplasmosis and cognition in adults: a cross-sectional study. Parasitology. 2014:1–9. doi: 10.1017/S0031182014001577. [DOI] [PubMed] [Google Scholar]
- 8.Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent 'asymptomatic' toxoplasmosis. Parasitology. 2001;122:515–20. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski PD, Falkenstein M, Hengstler JG, Golka K. Toxoplasma gondii impairs memory in infected seniors. Brain, behavior, and immunity. 2014;36:193–9. doi: 10.1016/j.bbi.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Alvarado-Esquivel C, Torres-Castorena A, Liesenfeld O, Estrada-Martinez S, Urbina-Alvarez JD. High seroprevalence of Toxoplasma gondii infection in a subset of Mexican patients with work accidents and low socioeconomic status. Parasites & vectors. 2012;5:13. doi: 10.1186/1756-3305-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegr J. How and why Toxoplasma makes us crazy. Trends in parasitology. 2013;29:156–63. doi: 10.1016/j.pt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Yereli K, Balcioglu IC, Ozbilgin A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic science international. 2006;163:34–7. doi: 10.1016/j.forsciint.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Koshy AA, Cabral CM. 3-D imaging and analysis of neurons infected in vivo with Toxoplasma gondii. J Vis Exp. 2014;9:52237. doi: 10.3791/52237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luder CG, Giraldo-Velasquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Experimental parasitology. 1999;93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 15.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophrenia bulletin. 2007;33:745–51. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song C, Chiasson MA, Nursimulu N, Hung SS, Wasmuth J, Grigg ME, et al. Metabolic reconstruction identifies strain-specific regulation of virulence in Toxoplasma gondii. Molecular systems biology. 2013;9:708. doi: 10.1038/msb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobl JS, Goodwin DG, Rzigalinski BA, Lindsay DS. Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. The Journal of parasitology. 2012;98:1296–9. doi: 10.1645/GE-2760.1. [DOI] [PubMed] [Google Scholar]
- 18.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PloS one. 2011;6:e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinze-Selch D, Daubener W, Erdag S, Wilms S. The diagnosis of a personality disorder increases the likelihood for seropositivity to Toxoplasma gondii in psychiatric patients. Folia parasitologica. 2010;57:129–35. doi: 10.14411/fp.2010.016. [DOI] [PubMed] [Google Scholar]
- 20.Hinze-Selch D, Daubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophrenia bulletin. 2007;33:782–8. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Current HIV/AIDS reports. 2014;11:317–24. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- 23.Schuster RM, Gonzalez R. Substance Abuse, Hepatitis C, and Aging in HIV: Common Cofactors that Contribute to Neurobehavioral Disturbances. Neurobehavioral HIV medicine. 2012;2012:15–34. doi: 10.2147/NBHIV.S17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharti A, Smith D, Ellis R, Cherner M, Woods S, Heaton R, McCutchan AJ, Grant I, Letendre S. Latent Toxoplasmosis may affect neurocognitive functioning in HIV-positive individuals. 19th International AIDS Conference; Washington DC, USA. 2012. [Google Scholar]
- 25.Patrascu IV, Dumitrescu O. The epidemic of human immunodeficiency virus infection in Romanian children. AIDS research and human retroviruses. 1993;9:99–104. doi: 10.1089/aid.1993.9.99. [DOI] [PubMed] [Google Scholar]
- 26.Ene L, Franklin DR, Burlacu R, Luca AE, Blaglosov AG, Ellis RJ, et al. Neurocognitive functioning in a Romanian cohort of young adults with parenterally-acquired HIV-infection during childhood. Journal of neurovirology. 2014;20:496–504. doi: 10.1007/s13365-014-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of clinical and experimental neuropsychology. 2004;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 29.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 30.Grace J, Malloy P. Frontal Systems Behavior Scale: Professional Manual. In: Lutz F, editor. Psychological Assessment Resources. 2001. [Google Scholar]
- 31.Metzger D, et al. The risk assessment battery (RAB): validity and reliability. Proceedings of the 6th Annual Meeting of the National Cooperative Vaccine Development Group for AIDS; Alexandria, Va, USA. 1993. [Google Scholar]
- 32.Letendre SBA, Perez-Valero I, et al. Higher CMV antibody concentrations are associated with older age, lower nadir CD4+ cell counts, and worse global neurocognitive functioning in people with HIV disease. 19th Conference of Retroviruses and Opportunistic Infections; Seattle, US. 2012. [Google Scholar]
- 33.Hellmuth J, Milanini B, Valcour V. Interactions between ageing and NeuroAIDS. Current opinion in HIV and AIDS. 2014;9:527–32. doi: 10.1097/COH.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing research reviews. 2013;12:1069–78. doi: 10.1016/j.arr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner ID, Remington JS. Aging and the immune response. I. Antibody formation and chronic infection in Toxoplasma gondii-infected mice. Journal of immunology. 1978;120:939–43. [PubMed] [Google Scholar]
- 36.Mendy A, Vieira ER, Albatineh AN, Gasana J. Immediate rather than delayed memory impairment in older adults with latent toxoplasmosis. Brain, behavior, and immunity. 2014 doi: 10.1016/j.bbi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Beste C, Getzmann S, Gajewski PD, Golka K, Falkenstein M. Latent Toxoplasma gondii infection leads to deficits in goal-directed behavior in healthy elderly. Neurobiology of aging. 2014;35:1037–44. doi: 10.1016/j.neurobiolaging.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Mendy A, Vieira ER, Albatineh AN, Gasana J. Toxoplasma gondii seropositivity and cognitive functions in school-aged children. Parasitology. 2015;142:1221–7. doi: 10.1017/S0031182015000505. [DOI] [PubMed] [Google Scholar]
- 39.Guenter W, Bielinski M, Deptula A, Zalas-Wiecek P, Piskunowicz M, Szwed K, et al. Does Toxoplasma gondii infection affect cognitive function? A case control study. Folia parasitologica. 2012;59:93–8. doi: 10.14411/fp.2012.014. [DOI] [PubMed] [Google Scholar]
- 40.Gale SD, Brown BL, Berrett A, Erickson LD, Hedges DW. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia parasitologica. 2014;61:285–92. [PubMed] [Google Scholar]
- 41.El Lakkis I, Di Pace BS, Cunningham TD, Troy SB. Association between latent toxoplasmosis and psychiatric disorders in HIV-infected subjects. Journal of acquired immune deficiency syndromes (1999) 2015;68:e8–9. doi: 10.1097/QAI.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 42.Groer MW, Yolken RH, Xiao JC, Beckstead JW, Fuchs D, Mohapatra SS, et al. Prenatal depression and anxiety in Toxoplasma gondii-positive women. American journal of obstetrics and gynecology. 2011;204:433e1–7. doi: 10.1016/j.ajog.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. The Journal of nervous and mental disease. 2009;197:905–8. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- 44.Okusaga O, Langenberg P, Sleemi A, Vaswani D, Giegling I, Hartmann AM, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophrenia research. 2011;133:150–5. doi: 10.1016/j.schres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Yagmur F, Yazar S, Temel HO, Cavusoglu M. May Toxoplasma gondii increase suicide attempt-preliminary results in Turkish subjects? Forensic science international. 2010;199:15–7. doi: 10.1016/j.forsciint.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Amminger GP, McGorry PD, Berger GE, Wade D, Yung AR, Phillips LJ, et al. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biological psychiatry. 2007;61:1215–7. doi: 10.1016/j.biopsych.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 47.Flegr J, Kodym P, Tolarova V. Correlation of duration of latent Toxoplasma gondii infection with personality changes in women. Biological psychology. 2000;53:57–68. doi: 10.1016/s0301-0511(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 48.Alfonzo M, Blanc D, Troadec C, Huerre M, Eliaszewicz M, Gonzalez G, et al. Temporary restoration of immune response against Toxoplasma gondii in HIV-infected individuals after HAART, as studied in the hu-PBMC-SCID mouse model. Clinical and experimental immunology. 2002;129:411–9. doi: 10.1046/j.1365-2249.2002.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furco A, Carmagnat M, Chevret S, Garin YJ, Pavie J, De Castro N, et al. Restoration of Toxoplasma gondii-specific immune responses in patients with AIDS starting HAART. Aids. 2008;22:2087–96. doi: 10.1097/QAD.0b013e3283136d68. [DOI] [PubMed] [Google Scholar]
- 50.Machala L, Maly M, Beran O, Jilich D, Kodym P. Incidence and clinical and immunological characteristics of primary Toxoplasma gondii infection in HIV-infected patients. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2013;17:e892–6. doi: 10.1016/j.ijid.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Gubareva EV, Goncharov DB, Peregudova AB, Parkhomenko Iu G, Tishkevich IM, Tishkevich OA, et al. Features of epidemiology and diagnostics of toxoplasmosis during HIV-infection. Zhurnal mikrobiologii, epidemiologii, i immunobiologii. 2010:28–32. [PubMed] [Google Scholar]
- 52.Dubey JP, Hotea I, Olariu TR, Jones JL, Darabus G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology. 2014;141:311–25. doi: 10.1017/S0031182013001509. [DOI] [PubMed] [Google Scholar]
- 53.Crucerescu E. Epidemiological data on toxoplasmosis. The aspects of congenital toxoplasmosis. Bacteriologia, virusologia, parazitologia, epidemiologia. 1998;43:147–55. [PubMed] [Google Scholar]
- 54.Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. The American journal of tropical medicine and hygiene. 2014;90:1135–9. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Veen J, Polak MF. Prevalence of toxoplasma antibodies according to age with comments on the risk of prenatal infection. J Hyg (Lond) 1980;85:165–74. doi: 10.1017/s0022172400063191. [DOI] [PMC free article] [PubMed] [Google Scholar]