Abstract
Severe respiratory distress is a serious complication common to the three major causes of neonatal mortality and morbidity (prematurity, intra-partum-related hypoxia and infections). In low- and middle-income countries (LMICs), 20% of babies presenting with severe respiratory distress die.
Continuous positive airway pressure (CPAP), is an effective intervention for respiratory distress in newborns and widely used in high-income countries. Following the development of simple, safe and relatively inexpensive CPAP devices, there is potential for large-scale implementation in the developing world.
In this article, we describe existing CPAP systems and present a review of the current literature examining the effectiveness of CPAP compared to standard care (oxygen) in newborns with respiratory distress. We also discuss the evidence gap which needs to be addressed prior to its integration into health systems in LMICs.
Keywords: Continuous positive airway pressure (CPAP), respiratory distress, newborns, low- and middle-income countries (LMICs)
Introduction
An estimated 2.9 million neonatal deaths occur each year, the majority of which happen in developing countries. Three main causes account for the majority of deaths: prematurity (34%), intra-partum-related conditions (25%) and infections (including pneumonia, 22%).1
Severe respiratory distress is a serious complication which is common to these three main causes of neonatal death. In preterm newborns, respiratory distress is predominantly secondary to a deficiency in surfactant, a condition known as hyaline membrane disease or respiratory distress syndrome (RDS). Other causes are pneumonia, sepsis and pulmonary haemorrhage. In term newborns, RDS, pneumonia, intra-partum-related hypoxia and meconium aspiration syndrome are the main causes of respiratory distress.2 The case fatality rate for neonatal respiratory distress in LMICs can be as high as 20%.3
Respiratory support to treat this condition is provided by CPAP or mechanical ventilation in high-income countries.4 Surfactant is also used in newborns presenting with RDS. However, the high cost and the need for endotracheal intubation for its administration makes surfactant unsuitable for low-resource settings and in settings lacking medical staff trained in endotracheal intubation. Mechanical ventilation is expensive and requires a high level of expertise. CPAP is the only intervention which has the potential to be implemented on a large scale in LMICs as simple, safe and relatively inexpensive CPAP devices have been developed recently.3
Continuous positive airway pressure (CPAP)
CPAP is a non-invasive type of respiratory support which can be delivered without endotracheal intubation although classical mechanical ventilators can also provide CPAP. It works by providing a continuous level of positive pressure to the airways which distends the lungs, overcomes collapse and improves ventilation. CPAP can be generated in different ways: (1) by using a variable flow of air and oxygen, toward the patient during inhalation and away from the patient during exhalation (variable flow CPAP); (2) by blowing a high flow of air and oxygen (high flow nasal cannula); or (3) by immersing the end of a respiratory circuit and making the patient exhale against a column of water, generating bubbles (bubble CPAP)
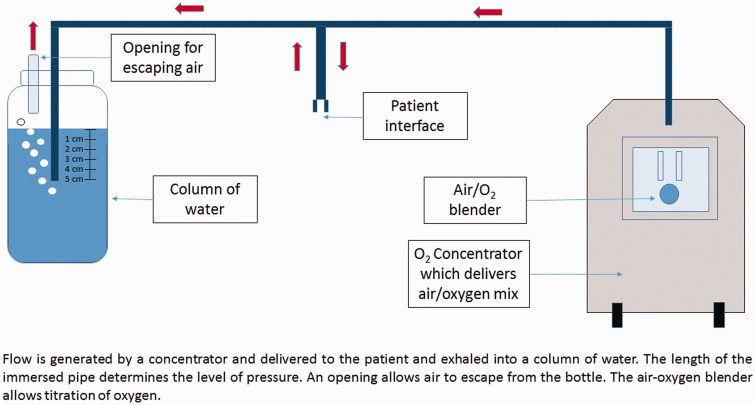
Bubble CPAP is the method that is most adapted to low-resources settings (Figure 1). Flow is generated by a concentrator and delivered to the patient and exhaled into a column of water. The length of the immersed pipe determines the level of pressure. An opening allows air to escape from the bottle. The air-oxygen blender allows titration of oxygen. Bubble CPAP devices are cheaper than variable flow CPAP; some use air and oxygen extracted from ambient air unlike variable flow CPAP which requires medical piped gas systems or oxygen cylinders. These sources of air and oxygen are often not available in hospitals in LMICs. Finally, bubble CPAP provides a more stable level of pressure compared to high flow nasal cannula. However, bubble CPAP does require a constant source of electricity which can be problematic in remote facilities.3
Figure 1.
Diagram of a bubble CPAP system.
It is possible to assemble a ‘homemade’ bubble CPAP device by connecting nasal prongs to a source of humidified oxygen and immersing the other extremity into a column of water. However, the gas flow needed to generate CPAP (i.e. 2–10 L/min) may be too high for the diameter of regular nasal prongs, generating too much resistance and failing to produce an appropriate level of pressure. More importantly, such systems do not offer the possibility of titrating oxygen because ‘homemade’ systems are not provided with air-oxygen blenders. Therefore, there is a real risk of delivering excess oxygen and causing retinopathy of prematurity in preterm babies, a consequence of oxygen toxicity which can lead to blindness.4 As such, commercial bubble (or other) CPAP devices which can regulate the oxygen dosage are preferable when treating preterm babies, for example.
Effectiveness of CPAP
CPAP has been used for decades in high-income countries and a recent review has shown that the use of oxygen and CPAP may have resulted in a 75% reduction in RDS-related mortality over the last century in the United States.5 However, in LMICs, at the moment, oxygen alone is often the only intervention available to treat babies with respiratory distress.
A systematic review from the Cochrane collaboration on the effect of continuous distending pressure (which includes CPAP and continuous negative pressure, a technique which is no longer used in newborns) compared to oxygen has shown that CPAP in preterm infants with respiratory distress significantly reduces treatment failure (relative risk [RR], 0.61; 95% confidence interval [CI], 0.45–0.81). In addition, there was a reduction in the need for additional mechanical ventilation (RR, 0.65; 95% CI, 0.47–0.89).6 With regards to reduction in mortality, the review included two unpowered trials (199 patients in total) from the US to demonstrate the superiority of CPAP (RR, 0.52; 95% CI, 0.23–1.16). Even though the review showed that continuous distending pressure (CPAP and/or continuous negative pressure) reduces mortality in preterms with respiratory distress (RR, 0.52; 95% CI, 0.32–087), the six trials included in this analysis were all conducted in high-income countries.
Another recent review which examined the effect of CPAP in LMICs identified only one high quality randomised controlled trial (RCT) comparing CPAP to oxygen.7 The study was a multi-centre trial conducted in 12 South American centres. The study showed that the CPAP group required lower rates of additional mechanical ventilation compared to the group receiving oxygen only (29.8% vs. 50.4%, P = 0.001).8 However, both groups received a co-intervention (surfactant) which means that the results of this trial are not generalisable to settings where surfactant is not available. Moreover, some of the study sites (Chile and Uruguay) are classified as high income countries by the World Bank. Finally, the study was not sufficiently powered to show a difference in mortality rates.
Regarding adverse events, a common concern among clinicians is the occurrence of pneumothorax. None of the systematic reviews showed that CPAP significantly increases this risk. However, all these trials were conducted in controlled settings with a high level of monitoring.
Therefore, for LMICs, there is need for further research to assess whether CPAP can be implemented safely in a manner that consistently improves outcomes for newborns, including a particular effect on newborn mortality.
Summary
Interest in using CPAP for newborns with respiratory distress in LMICs has increased recently. It is a promising intervention but research to assess the feasibility of its implementation in existing health systems of LMICs is needed before implementation at scale. There is also a need to study outcomes, including case fatality rates and incidence of adverse events, in LMICs before and after the introduction of CPAP.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet 2014; 384: 189–205. [DOI] [PubMed] [Google Scholar]
- 2.Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev 2013; 14: 29–36. [DOI] [PubMed] [Google Scholar]
- 3.Duke T. CPAP: a guide for clinicians in developing countries. Paediatr Int Child Health 2014; 34: 3–11. [DOI] [PubMed] [Google Scholar]
- 4.Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology 2013; 104: 265–274. [DOI] [PubMed] [Google Scholar]
- 5.Kamath BD, MacGuire ER, McClure EM, et al. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics 2011; 127: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JJ, Subramaniam P, Henderson-Smart DJ, et al. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane DB Syst Rev 2015; 7: CD002271–CD002271. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Duke T, Davis P. Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Arch Dis Child-Fetal 2014; 99: F495–504. [DOI] [PubMed] [Google Scholar]
- 8.Tapia JL, Urzua S, Bancalari A, et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. J Pediatr 2012; 161: 75–80. [DOI] [PubMed] [Google Scholar]
- 9.World Bank. World Bank Data: High Income Countries 2014, Washington, DC: World Bank, 2015. Available at: http://data.worldbank.org/income-level/HIC (cited 24 February 2015). [Google Scholar]