Abstract
Proliferation of smooth muscle cells (SMCs) represents an important event in vascular lesion formation. Despite the common belief that growth factors contribute to the development of the atherosclerotic plaque, until now there has been no direct evidence for a role of mitogens in the development of arterial lesions. Balloon catheter injury of the rat carotid artery is accompanied by death of medial SMCs and is typically followed by proliferation of SMCs with subsequent formation of an intimal lesion. Our hypothesis is that injury causes mitogens to be released from dead cells, which then stimulate cell proliferation. One such mitogen that may be important in this process is basic fibroblast growth factor (bFGF), which can be detected immunocytochemically in SMCs and endothelial cells of adult rat carotid arteries. Systemic injection of a neutralizing antibody against bFGF prior to balloon catheterization significantly decreased the induced SMC proliferation by approximately 80%. The intimal lesion that developed within 8 days after injury, however, was not significantly reduced. The results of this study support the concept that endogenous bFGF is the major mitogen controlling the growth of vascular smooth muscle cells following injury. These data may have implications for the observed failure of endarterectomy and angioplasty procedures.
Full text
PDF

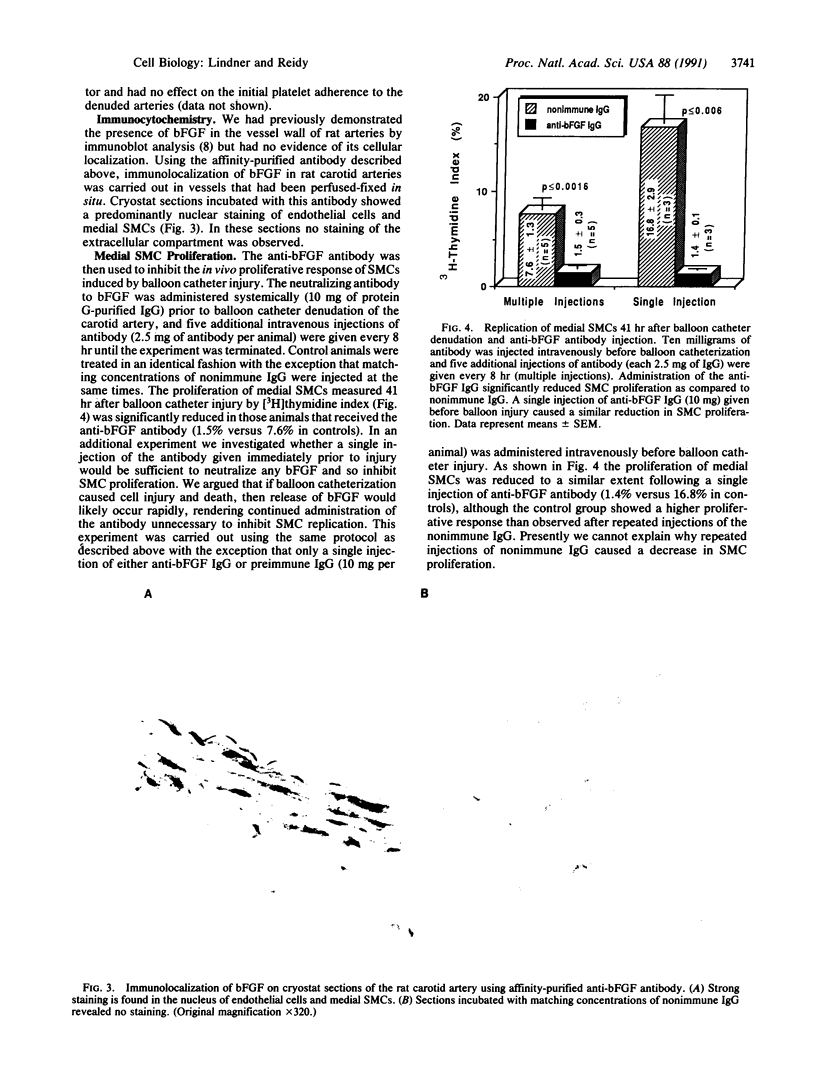
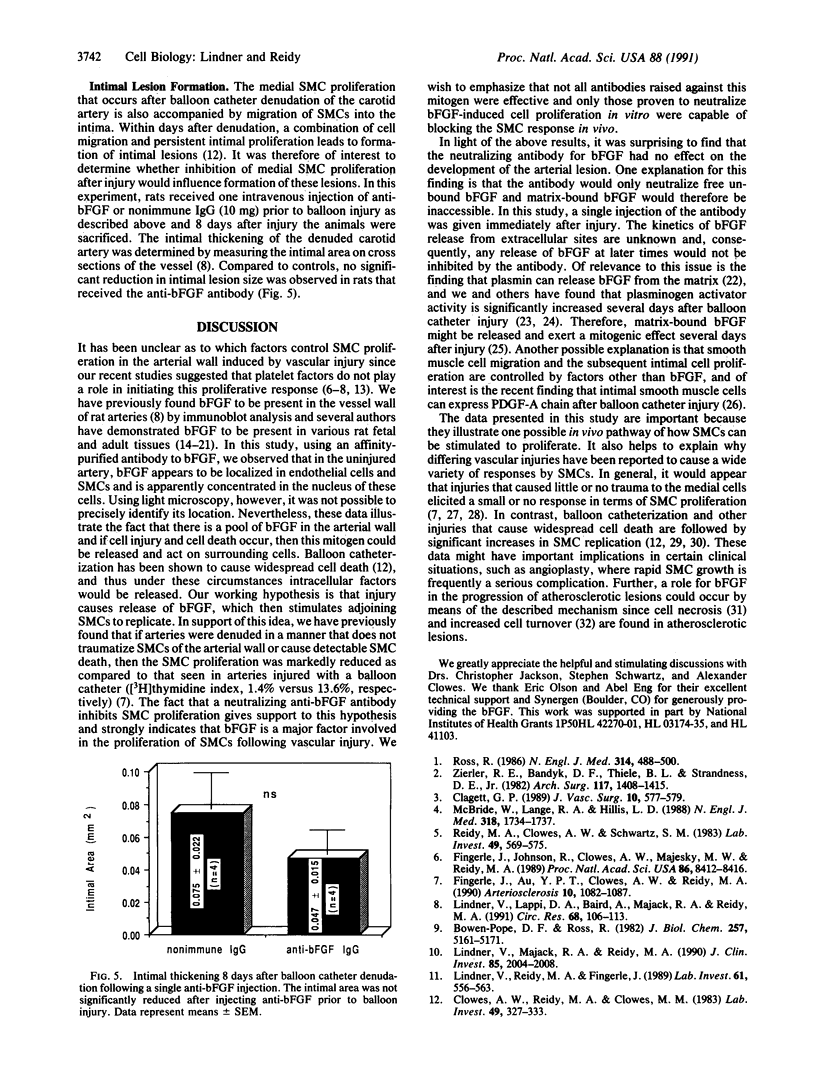

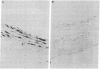
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Capron L., Bruneval P. Influence of applied stress on mitotic response of arteries to injury with a balloon catheter: quantitative study in rat thoracic aorta. Cardiovasc Res. 1989 Nov;23(11):941–948. doi: 10.1093/cvr/23.11.941. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Flaumenhaft R., Moscatelli D., Saksela O., Rifkin D. B. Role of extracellular matrix in the action of basic fibroblast growth factor: matrix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J Cell Physiol. 1989 Jul;140(1):75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Buscaglia M., Ong M., Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990 Mar;110(3):753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneken A., Lutty G. A., McLeod D. S., Robey F., Harvey A. K., Hjelmeland L. M. Localization of basic fibroblast growth factor to the developing capillaries of the bovine retina. J Cell Physiol. 1989 Jan;138(1):115–120. doi: 10.1002/jcp.1041380116. [DOI] [PubMed] [Google Scholar]
- Joseph-Silverstein J., Consigli S. A., Lyser K. M., Ver Pault C. Basic fibroblast growth factor in the chick embryo: immunolocalization to striated muscle cells and their precursors. J Cell Biol. 1989 Jun;108(6):2459–2466. doi: 10.1083/jcb.108.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A., Fingerle J. Regrowth of arterial endothelium. Denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest. 1989 Nov;61(5):556–563. [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W., Lange R. A., Hillis L. D. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988 Jun 30;318(26):1734–1737. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Joseph-Silverstein J., Rifkin D. B. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol. 1986 Nov;129(2):273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Risau W., Vollmer E., Sorg C. In situ detection of basic fibroblast growth factor by highly specific antibodies. Am J Pathol. 1990 Jul;137(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Reidy M. A. Endothelial regeneration. IX. Arterial injury followed by rapid endothelial repair induces smooth-muscle-cell proliferation but not intimal thickening. Am J Pathol. 1987 Dec;129(3):429–433. [PMC free article] [PubMed] [Google Scholar]
- Zierler R. E., Bandyk D. F., Thiele B. L., Strandness D. E., Jr Carotid artery stenosis following endarterectomy. Arch Surg. 1982 Nov;117(11):1408–1415. doi: 10.1001/archsurg.1982.01380350016003. [DOI] [PubMed] [Google Scholar]