Abstract
Mycoplasma pneumoniae is an important etiologic agent of primary atypical pneumonia in children and adults. The diagnosis of M. pneumoniae infection is commonly confirmed through serologic testing. In this study, we used paired sera from 51 patients (all with confirmed M. pneumoniae infection and positive complement fixation [CF] titers) to compare the results of eight enzyme immunoassays (EIAs) available commercially in the United States. We compared two single-use EIAs and six plate-type EIAs. Results from acute-phase sera ranged from only 7 (14%) positive by ImmunoWELL (GenBio) immunoglobulin M (IgM) EIA to 23 (45%) positive by Zeus IgG EIA. When both the acute-phase and convalescent-phase serum samples were examined, positive results ranged from 20 (39%) by the ImmunoWELL (GenBio) IgM assay to 45 (88%) positive by the Remel IgG-IgM EIA. In this study, the single-use EIAs by Remel and Meridian were more reliable than were the plate-type EIAs. Among the plate-type EIAs, the Zeus and DiaSorin assays (which detect antibodies to protein antigens) were more sensitive than the ImmunoWELL assay (which detects antibodies to glycolipid antigens). In general, IgG EIAs on convalescent-phase sera were more concordant with one another than were IgM EIAs with one another. Scatter plot analysis of convalescent-phase sera showed that, as the CF titer dropped, the IgM assays identified fewer positive convalescent-phase sera. In contrast, the IgG assays provided fairly consistent positive results for convalescent-phase sera with CF titers of 64 and above. Results of individual tests and overall limitations of serodiagnostics for M. pneumoniae infections are discussed.
Mycoplasma pneumoniae is an important etiologic agent of tracheobronchitis and primary atypical pneumonia in children and adults. It is responsible for 20% or more of community-acquired pneumonias overall (8) and can also be a significant cause of severe pneumonia requiring hospitalization in the elderly (12). Because they lack a cell wall, mycoplasmas do not respond to penicillins and other beta-lactams commonly used for the treatment of bacterial pneumonia. Laboratory diagnosis of M. pneumoniae infection is usually established through serological or molecular testing because the organism grows slowly and is difficult to isolate from clinical specimens (10, 11, 17). A reliable and sensitive serologic test for use in the early stages of M. pneumoniae infection is needed to confirm the clinical diagnosis and to ensure that the appropriate antibiotic therapy is used (5, 7).
The detection of specific immunoglobulin M (IgM) antibody, which appears 7 to 10 days after infection and approximately 2 weeks before IgG antibody, has been shown previously to indicate a recent or current infection with M. pneumoniae (13, 14). However, specific IgM in adults does not always indicate an acute infection because it can persist for up to a year after infection with M. pneumoniae (2, 4). In addition, an IgM response may be either minimal or undetectable when adults are reinfected (9, 15). In previous studies, approximately 20% of adults did not mount an IgM response after infection with M. pneumoniae (16, 18). Therefore, relying exclusively on the detection of specific IgM (especially in an adult population) will result in the misdiagnosis of some infections. A comprehensive review of the value of serology for the detection of M. pneumoniae in the clinical laboratory has recently been published by Waites et al. (19).
The twofold purpose of this study was to evaluate eight commercial enzyme immunoassays (EIAs) currently sold in the United States for the detection of IgM and IgG antibodies to M. pneumoniae and to determine if a more timely diagnosis of M. pneumoniae can be obtained by using these assays in the early phases of infection. This information will aid in both population-based studies and diagnostic evaluations of individual cases of suspected infections with M. pneumoniae.
MATERIALS AND METHODS
Sera.
Acute- and convalescent-phase sera were obtained from 51 adult patients (at least 18 years of age) with respiratory infections caused by M. pneumoniae. Most of the patients had chest X-rays with infiltrates compatible with atypical pneumonia and clinical signs consistent with mycoplasma infection. These signs included cough, fever, and myalgias. Etiologic diagnosis was by the demonstration of a fourfold or greater rise or a standing titer of 64 or greater in an M. pneumoniae complement fixation (CF) assay (3). The convalescent-phase sera were collected 2 to 3 weeks after the acute-phase sera, and all samples were held at −20°C before being tested with the eight EIAs. None of the specimens were linked to individual patient identifiers.
Serologic assays.
A complete list of the commercial assays with a summary of principal characteristics is shown in Table 1. All assays were performed according to the manufacturer's instructions. A brief summary of each assay is provided.
TABLE 1.
EIA commercial serologic kits evaluateda
| Product name | Manufacturer or distributor | Antibody(ies) measured | Kit description and target antigen(s) | No. of tests/kit | Assay time (start to finish) |
|---|---|---|---|---|---|
| M. pneumoniae IgG and IgM antibody test system | Remel, Inc., Lenexa, Kans. | IgM and IgG, simultaneously | Qualitative membrane-based single-sample test containing inactivated M. pneumoniae protein antigen (primarily cytadhesion protein) | 10 or 40 | 10 min |
| ImmunoCard | Meridian Biosciences, Inc., Cincinnati, Ohio | IgM only | Single-sample cards consisting of a test port containing M. pneumoniae antigen and a control port containing immobilized human IgM | 30 | 12 min |
| GenBio Immuno WELL M. pneumoniae antibody IgM or IgG | Alexon-Trend, Ramsey, Minn. | IgM and IgG separately | 96-well microtiter plate format coated with purified glycolipid mycoplasma antigen (M. pneumoniae strain FH, ATCC 15531) | 96 | 2.35 h for IgG, 2.75 h for IgM |
| Mycoplasma IgG and IgM ELISA, test system | Zeus Scientific, Inc., Raritan, N.J. | IgM and IgG separately | Qualitative system for determination of IgG and IgM antibodies to M. pneumoniae with multiwell breakaway strips coated with partially purified inactivated M. pneumoniae | 96 | 50 min |
| ETI-MP IgM or IgG | Savyon; manufactured for DiaSorin, Stillwater, Minn. | IgM and IgG separately | 96-well microtiter plate format coated with a membrane preparation containing the P1 protein of M. pneumoniae | 192 | 2.5 h |
Adapted from reference 19a with permission.
Meridian IC.
The Meridian ImmunoCard (IC) mycoplasma EIA is a qualitative procedure for the detection of specific IgM antibodies to M. pneumoniae in human sera. It utilizes detergent-extracted M. pneumoniae antigens. The development of a blue color in the test well indicates a positive test result for IgM antibodies to M. pneumoniae; a blue color in the control well indicates that the test was performed properly. This assay can be run in approximately 12 min.
Remel EIA.
The Remel M. pneumoniae IgG-IgM EIA antibody test system is a qualitative detection assay for IgM and IgG antibodies to M. pneumoniae. The patient's serum or plasma is diluted 1:7 and tested with an M. pneumoniae protein antigen immobilized on a permeable membrane. Positive and negative serum control samples are included with the assay. Serum is considered positive for M. pneumoniae antibodies when the intensity of color in the serum test well is greater than that observed in the negative serum control well. This assay can be performed in approximately 10 min if the serum has not been previously frozen.
Zeus IgM and IgG EIAs.
The Zeus mycoplasma IgM EIA is qualitative and utilizes microtiter plate wells coated with a sonicated, inactivated M. pneumoniae antigen preparation. Sera are considered positive for M. pneumoniae antibodies if certain calibrated cutoff optical density (OD) levels are obtained after each microwell is read at 450 nm. In reporting results, a calibrated OD ratio of 0.90 or less indicates no current or previous infection with M. pneumoniae. A ratio of 1.10 or greater indicates an active or recent infection. Specimens with OD ratio values of 0.91 to 1.09 fall into the equivocal range and should be retested. The manufacturer recommends that, if the specimens remain equivocal after repeat testing but are positive with a second IgM EIA, they should be considered to be true positives.
The Zeus mycoplasma IgG EIA utilizes microtiter plate wells coated with partially purified, inactivated M. pneumoniae antigen. The IgG EIA is performed and interpreted in the same manner as the IgM assay except that horseradish peroxidase-conjugated goat anti-human IgG conjugate is used. This assay requires approximately 1 h to perform.
DiaSorin ETI-MP IgM and IgG EIAs.
The DiaSorin IgM and IgG EIAs utilize microtiter plate wells coated with a purified fraction of M. pneumoniae membrane proteins (containing the P1 protein) for the qualitative detection of IgM or IgG specific antibodies to M. pneumoniae. Both EIAs are performed and interpreted using the same methodology, except that, for IgM detection, the sera are diluted to 1:105 in serum diluent and absorbed with anti-human IgG antibodies (both provided by the manufacturer) in order to remove IgG antibodies which could interfere with the detection of IgM. Sera are considered positive if their absorbance values at 450 to 620 nm are interpolated (via standard curve) to contain 10 or more binding units (BU) of IgM or IgG antibodies to M. pneumoniae. Sera with values of less than 10 BU are considered negative. This test can be performed in approximately 2.5 h.
GenBio ImmunoWELL IgM and IgG EIAs.
The ImmunoWELL M. pneumoniae antibody IgM and IgG EIAs utilize a purified M. pneumoniae glycolipid antigen for the qualitative detection of IgM- or IgG-specific antibodies to M. pneumoniae. Both EIAs are performed in the same manner except that, in the IgM assay, the controls, calibrator, and patient specimens are absorbed with anti-human IgG antibodies before testing to prevent interference due to rheumatoid factor or competing IgG in patients' sera. Sera are considered positive for M. pneumoniae antibodies if certain normalized activity levels are obtained after each well is read at 405 nm. According to the manufacturer, these activity levels are determined by a two-step process. First, the absorbance value of the serum at 405 nm is multiplied by the assigned value in units per milliliter of the calibrator given in the supplement that comes with each kit. This value is then divided by the mean absorbance of the calibrator (run in triplicate) obtained in each assay to give the “normalized activity of the specimen,” expressed as a number in units per milliliter. Serum IgM values are interpreted as follows: less than 770 U/ml is negative, 770 to 950 U/ml is low positive, and greater than 950 U/ml is positive. Paired serum IgG values are interpreted by two other methods, either a seroconversion method or a serum ratio method. In the seroconversion method, a significant change is indicated if one sample is greater than 320 U and the other is less than 200 U. With the serum ratio method, a ratio is calculated by dividing the convalescent-phase serum result by the acute-phase serum result. A ratio of 2.0 or greater indicates a recent or current infection, a ratio of 0.5 or less indicates a past infection, and a ratio between 0.5 and 2.0 indicates no significant antibody change. Additional details on interpretation are provided by the manufacturer. These tests can be run in 2.5 h for IgG and 2.75 h for IgM.
Statistical methods.
SPSS for Windows (version 11) was used for data management and statistical analysis. The McNemar test for paired proportions was used to compare the EIAs with respect to the percentage with positive results. To evaluate the degree of agreement between EIAs, the kappa statistic was obtained (Table 2). A 0.05 significance level was used for all statistical tests. No one-sided statistical tests were done. IgM assays were compared with each other, as were IgG assays. The Remel assay was evaluated in both the IgM and IgG groups because it detects both antibody classes. Analyses were performed for the use of only acute-phase sera and for the use of acute- or convalescent-phase sera.
TABLE 2.
Pairwise comparisons with McNemar's test
| Assay, serum, and EIA pair | Kappa | Kappa P value |
|---|---|---|
| IgM, acute phase | ||
| GenBio-DiaSorin | 0.79 | <0.0005 |
| GenBio-Zeus | 0.63 | <0.0005 |
| GenBio-Meridian | 0.48 | <0.0005 |
| GenBio-Remel | 0.60 | <0.0005 |
| DiaSorin-Zeus | 0.72 | <0.0005 |
| DiaSorin-Meridian | 0.57 | <0.0005 |
| DiaSorin-Remel | 0.70 | <0.0005 |
| Zeus-Meridian | 0.35 | 0.013 |
| Zeus-Remel | 0.57 | <0.0005 |
| Meridian-Remel | 0.31 | 0.023 |
| IgG, acute phase | ||
| Remel-DiaSorin | 0.15 | 0.24 |
| Remel-Zeus | 0.09 | 0.48 |
| DiaSorin-Zeus | 0.56 | <0.0005 |
| IgM, convalescent phase | ||
| GenBio-DiaSorin | 0.45 | <0.0005 |
| GenBio-Zeus | 0.44 | 0.001 |
| GenBio-Meridian | 0.31 | 0.008 |
| GenBio-Remel | 0.09 | 0.23 |
| DiaSorin-Zeus | 0.67 | <0.0005 |
| DiaSorin-Meridian | 0.56 | <0.0005 |
| DiaSorin-Remel | 0.29 | 0.009 |
| Zeus-Meridian | 0.40 | 0.003 |
| Zeus-Remel | 0.16 | 0.14 |
| Meridian-Remel | 0.23 | 0.047 |
| IgG, convalescent phase | ||
| GenBio-DiaSorin | 0.61 | <0.0005 |
| GenBio-Zeus | 0.39 | 0.004 |
| GenBio-Remel | 0.52 | <0.0005 |
| DiaSorin-Zeus | 0.60 | <0.0005 |
| DiaSorin-Remel | 0.77 | <0.0005 |
| Zeus-Remel | 0.77 | <0.0005 |
RESULTS
The pairwise comparisons of positive assay results for acute-phase sera and for convalescent-phase sera are found in Table 2. The percentages of positive results by assay and antibody class are given in Table 3 for each of four separate diagnostic serologic criteria: patients with positive acute-phase serum, patients with either positive acute-phase or positive convalescent-phase sera, patients with positive convalescent-phase serum, and patients with a rising titer in convalescent- versus acute-phase sera.
TABLE 3.
Assay results with paired sera from 51 patients with serologically confirmed M. pneumoniae infection
| Patient type | No. of patients whose sera would have been positive by assay results (% of total [n = 51])
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CF test (IgG-IgM) | Meridian IgMb | Zeus IgMc | DiaSorin IgMd | GenBio IgMe | Remel IgG-IgMf | GenBio IgGg | DiaSorin IgG | Zeus IgG | |
| Positive acute-phase serum | 11 (22) | 14 (27) | 13 (25) | 10 (20) | 7 (14) | 11 (22) | Not done | 20 (39) | 23 (45) |
| Either positive acute-phase or positive convalescent-phase sera | 49a (94) | 36 (71) | 31 (61) | 33 (65) | 20 (39) | 45 (88) | 37 (73) | 44 (86) | 43 (84) |
| Positive convalescent-phase serum | 49a (94) | 35 (69) | 31 (61) | 33 (65) | 20 (39) | 45 (88) | 37 (73) | 42 (82) | 42 (82) |
| Rising titer in paired sera | 51 (100) | 22 (43) | 18 (35) | 23 (45) | 13 (25) | 34 (67) | 24 (47) | 20 (39) | |
Two of the serum pairs had acute-phase CF titers of 8 and convalescent-phase CF titers of 32. Although interpreted as positive because of a fourfold rise in titer, titers of 32 or less would not be considered positive for single samples.
A single serum dilution was tested. A visible change in the membrane color in both the control and test reaction ports to blue was considered positive.
A single serum dilution was tested. A calibrated OD ratio of >1.10 indicated an active or recent infection, and a ratio of 0.91 to 1.09 was in the equivocal range and the sample was retested.
A single serum dilution was tested. A serum sample was considered positive if it contained 10 or more BU of IgM or IgG antibodies.
A single serum dilution was tested. A normalized OD reading of >770 was considered positive.
A single serum dilution was tested. A visible change in the membrane color in the test well to purple was considered positive.
Single serum dilutions of paired sera were tested. With the seroconversion method, a positive result was indicated if one sample was above 320 U and the other was below 200 U. With the ratio method, a ratio of >2.0 indicated a recent or current infection, a ratio of <0.5 indicated a past infection, and a ratio between >0.5 and <2.0 indicated no significant antibody change.
IgM antibodies in acute-phase sera.
When the 51 acute-phase serum samples were tested with the IgM-specific EIAs and the results were evaluated with McNemar's test, all assay results were found to be statistically significantly different from each other because of discordant identification of which sera were positive (Table 2). The Meridian and Zeus IgM EIAs identified the most positive sera with 25 and 27% of samples being positive, respectively (Table 3).
IgG antibodies in acute-phase sera.
When the 51 acute-phase serum samples were tested with the IgG-specific EIAs and the results were evaluated with McNemar's test, the Remel EIA did not give results significantly different from those of the DiaSorin or Zeus IgG EIAs (P ≥ 0.24), but the DiaSorin and Zeus EIAs identified discordant subsets of positive sera (P < 0.05, Table 2). GenBio does not recommend testing single serum specimens with the ImmunoWELL IgG EIA. Although the subsets of positive sera identified were statistically significantly different, the DiaSorin and Zeus IgG EIAs identified the highest percentage of positive acute-phase samples of any assays at 39 and 45%, respectively (Table 3).
IgM antibodies in convalescent-phase sera and diagnosis with both acute- and convalescent-phase sera.
Whether the serologic criteria for diagnosis were (i) a positive convalescent-phase serum sample alone or (ii) a positive acute- or convalescent-phase sample, the results were virtually identical. This is because only one patient had a positive acute-phase and negative convalescent-phase assay result, and then only with the Meridian IgM EIA. For this reason, statistics were evaluated only for convalescent-phase sera alone. When the 51 convalescent-phase sera were tested with the IgM-specific EIAs, only the positive subsets identified by the GenBio-Remel and Zeus-Remel comparisons were not statistically different (P ≥ 0.14, Table 2). Of the IgM assays, the Remel and Meridian IgM EIAs identified the most positive samples with 88 and 69% positives, respectively (Table 3).
IgG antibodies in convalescent-phase sera and diagnosis with both acute- and convalescent-phase sera.
Again, whether the serologic criteria for diagnosis were (i) a positive convalescent-phase serum sample alone or (ii) a positive acute- or convalescent-phase sample, the results were virtually identical. Although the subsets of positive sera identified were statistically significantly different, the Remel EIA identified the highest percentage of positive samples (88%) (Table 3).
Use of rising titer in paired sera as the diagnostic criterion.
Because a rising or falling titer of M. pneumoniae-specific IgM or IgG antibodies indicates recent infection, the use of paired sera can increase the accuracy of detection of M. pneumoniae infections. Of all assays, the Remel assay identified the most positive rising pairs with 67%. The rest of the assays identified from 25% (GenBio IgG) to 47% (DiaSorin IgG) positive rising pairs (Table 3). When interpreting the Remel and Meridian IC EIAs with paired sera, an increase in antibody titer was defined as a negative result for acute-phase serum combined with a positive result for convalescent-phase serum.
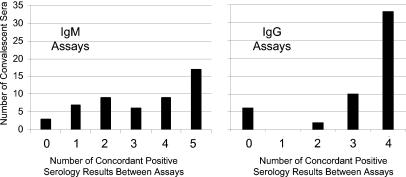
Concordance between assays on convalescent-phase sera.
Although McNemar's test provides a statistical basis for judging concordance of results, a graphical analysis of concordance was also conducted. Based upon the premise that all 51 convalescent-phase sera should test positive (all were positive by CF), concordance between the IgM EIAs was evaluated graphically by determining the frequency with which the five IgM and four IgG assays gave the same (concordant) results (Fig. 1). Data for the Remel IgG-IgM assay were included in both graphs. Sera that tested positive by all assays (score of 5 on the IgM graph and score of 4 on the IgG graph) or negative by all assays (score of 0 on both graphs) were considered to have concordant results. The IgM concordance graph shows no clear pattern, and in only 39% of the samples (20 of 51) were assay results fully concordant (all assays gave positive results or all assays gave negative results). In contrast, the IgG graph shows a preponderance of sera with all assays positive. Compared to 39% of sera with IgM assay results fully concordant, 76% (39 of 51) of IgG kit results were fully concordant.
FIG. 1.
Concordance of assay results for convalescent-phase sera.
Relationship of convalescent-phase CF titer with commercial assay results.
To determine if any relationship between CF titer and kit results could be found, scatter plot analyses of the CF titer versus number of positive IgM or IgG assays were performed (Fig. 2). Again, the Remel IgG-IgM EIA was included in both analyses. As the CF titer dropped, the IgM assays identified fewer and fewer positive convalescent-phase sera. In contrast, the IgG assays provided fairly consistent positive results for convalescent-phase sera with CF titers of 64 and above.
FIG. 2.
CF titer in convalescent-phase serum versus number of positive assays.
DISCUSSION
Reliable and sensitive tests are needed for the serologic diagnosis of M. pneumoniae infection. In particular, assays are needed for commonly occurring agents of respiratory infections to quickly rule out etiologic causes of unknown respiratory illnesses. Several commercial M. pneumoniae antibody detection kits, which utilize passive (particle) agglutination, indirect immunofluorescence, or EIA formats, are available for the detection of antibodies to M. pneumoniae in human sera. Some laboratory settings continue to use the CF test (3) for the serological diagnosis of M. pneumoniae infection. However, CF has been reported by some investigators to be insensitive and nonspecific (6, 9), and with the wide variability in performing and interpreting CF assays between laboratories, CF is no longer widely accepted. Most laboratories have replaced the CF assay with EIAs due to their ease of use, time to run, and low variability. Of the various test formats, EIA is the most widely used and the most adaptable to clinical laboratory settings. Accordingly, we performed the present study to evaluate currently available commercial kits sold in the United States utilizing the EIA format.
EIA is a valid methodology for detecting IgM antibodies to M. pneumoniae by using acute-phase serum from some patients with suspected cases of M. pneumoniae infections (1, 13, 20). This approach of testing for IgM alone has a theoretical advantage because only one specimen is required, and it can be detected approximately 7 to 10 days after the onset of infection. However, a major limitation of an IgM-specific test is that detectable levels of IgM antibodies may not be present if the serum sample is obtained too early in the infection, and only 14 to 27% of acute-phase sera tested positive by the various IgM kits. This may have been because the sera were collected very early in the course of infection before an antibody response was detectable. The number of IgM-positive sera rose to 39 to 88% when convalescent-phase sera were tested (sample taken approximately 2 weeks after the acute-phase sample was taken).
Specific IgM antibodies to M. pneumoniae are detectable in most pediatric patients with a recent infection of at least a week's duration (20). However, in the diagnosis of infection in adults and in cases of adult reinfections where IgM is not always produced, the detection of specific IgM antibodies alone may be problematic. Adults may produce only IgG antibodies, particularly to protein antigens, which would be detected only with IgG-IgM combined EIAs or IgG-specific EIAs. In our earlier studies, the combined detection of IgM and IgG antibodies and the use of paired sera greatly increased detection of M. pneumoniae infections (16). Regardless, the lack of IgM may also be explained by repeated exposures among these adult patients.
The percentage of sera positive for IgG in acute-phase sera from infected individuals ranged from 39 to 45% in pure IgG assays (Remel IgM-IgG not included), or about 1.5 times that of the pure IgM assays. This may have been caused by preexisting IgG antibodies from previous infections. The number of IgG-positive sera rose to 73 to 82% in pure IgG assays (Remel IgM-IgG not included) on convalescent-phase sera.
Obviously, in some adults IgG is produced more quickly as an anamnestic response to reinfection, but the variability in results among these assays illustrates their limitations in detecting current infections. Moreover, the low positive titers may represent remote infections; therefore, there is still the challenge of differentiating between remote and current infections by serologic means.
Kenny found seroconversions in CF antibody titers in 53% of culture-positive patients; therefore, it has been documented that an infection may result in a seroconversion in approximately one of two patients tested (11). Furthermore, if a patient is successfully treated with antibiotics early in the course of illness, the serologic response may not reach detectable levels.
It has been reported elsewhere that the detection of IgA-specific antibody seems to be a good indicator of recent M. pneumoniae infection in both children and adults (21). We planned to include a commercial IgA EIA in our evaluations, but the test was removed from the market before our study was completed. Also, we did not include the Wampole mycoplasma IgG enzyme-linked immunosorbent assay (ELISA; Wampole Laboratories, Cranbury, N.J.). In control studies carried out by Wampole, their EIA gave a 79.6% prevalence rate when tested with 367 healthy sera from donors of various geographic locations and ages (data provided in the product insert).
Serodiagnosis with single serum specimens, even in adult patients, is better suited as a diagnostic tool when several days to a week have passed before specimens are collected. However, when testing paired sera, serology is most reliable when a true acute-phase specimen is obtained early in the course of infection and a convalescent-phase specimen is collected 2 to 3 weeks later. The results in this study (Table 3) reveal the need for improving the specificity of several of the EIAs and the variability in sensitivity and specificity among these assays. Serologic diagnosis of M. pneumoniae respiratory infections will continue to remain extremely important despite the recent development of more-sensitive molecular diagnostic assays. However, in the future as they become more commercially available, tests such as PCR may be better suited for directly diagnosing the M. pneumoniae agent in the very early stages of illness.
The choice of a commercial EIA will depend on the age of the patient, the timing of the serum collection, whether paired sera can be obtained, and the equipment available to the laboratory. The Zeus, DiaSorin, and GenBio test results are available within hours, although an ELISA plate washer and reader are necessary in order to perform the assays. In our study, the single-use EIAs by Remel and Meridian were better able to identify seropositive samples than were the plate-type EIAs. Among the plate-type EIAs (which may be more practical and cost-efficient for some lab settings) the Zeus and DiaSorin assays were more sensitive than the GenBio assay was.
Because concordance of individual sample results is a component of McNemar's test for pairwise comparisons between assays, few pairs of tests could be considered to provide statistically concordant results. However, when sample results for convalescent-phase sera alone were graphed for concordant results (Fig. 1), the IgG assays gave more concordant results. When evaluating the relationship between CF titer of convalescent-phase sera and positive results by either IgM or IgG EIAs, scatter plot analysis shows that the number of positive IgM kit results is more dependent on high CF titer than is the number of IgG kit results.
In this study, all necessary reagents and controls were included in each commercial EIA kit. Other factors such as vendor customer service may be an important factor when choosing between tests with similar performance characteristics. Both the Remel EIA and Meridian IC assays were performed in minutes and did not require any specialized or expensive equipment. The Remel EIA and Meridian IC tests were cost-effective when performed on single sera or small batches of sera. Even in the early phases of infection with use of single acute-phase sera, approximately 25% of infections may be diagnosed serologically using an IgM-based EIA. Therefore, these tests may afford health care providers with timely information needed to diagnose and treat patients with M. pneumoniae infections.
Acknowledgments
We thank Ken B. Waites for critical review of the manuscript.
Use of trade names is for identification only and does not constitute endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
REFERENCES
- 1.Alexander, T. S., L. D. Gray, J. A. Kraft, D. S. Leland, M. T. Nikaido, and D. H. Willis. 1996. Performance of Meridian ImmunoCard Mycoplasma test in a multicenter clinical trial. J. Clin. Microbiol. 34:1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biberfield, G. 1971. Antibody responses in Mycoplasma pneumoniae infection in relation to serum immunoglobulins, especially IgM. Acta Pathol. Microbiol. Scand. Sect. B 79:620-634. [PubMed] [Google Scholar]
- 3.Casey, H. L. 1965. Part II. Adaptation of LBCF method to microtechnique, p. 31-34. In Standardized diagnostic complement fixation method and adaptation to micro test. Public Health Service Monograph no. 74. U.S. Public Health Service, Washington, D.C. [PubMed]
- 4.Chamberlin, P., and A. A. Saeed. 1983. A study of the specific IgM antibody response in Mycoplasma pneumoniae in man. J. Hyg. (Cambridge) 90:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimolai, N., and A. C. H. Cheong. 1996. An assessment of a new diagnostic indirect enzyme immunoassay for the detection of anti-Mycoplasma pneumoniae IgM. Am. J. Clin. Pathol. 105:205-209. [DOI] [PubMed] [Google Scholar]
- 6.Dussaix, E., A. Slim, and P. Tournier. 1983. Comparison of enzyme-linked immunosorbent assay (ELISA) and complement fixation test for detection of Mycoplasma pneumoniae antibodies. J. Clin. Pathol. 36:228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedorko, D. P., D. D. Emery, S. M. Franklin, and D. D. Congdon. 1995. Evaluation of a rapid enzyme immunoassay system for serologic diagnosis of Mycoplasma pneumoniae infection. Diagn. Microbiol. Infect. Dis. 23:85-88. [DOI] [PubMed] [Google Scholar]
- 8.Foy, F. M. 1993. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin. Infect. Dis. 17(Suppl. 1):S37-S46. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs, E. 1993. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin. Infect. Dis. 17(Suppl. 1):S579-S582. [DOI] [PubMed] [Google Scholar]
- 10.Karppelin, M., K. Hakkarainen, M. Kleemola, and A. Miettinen. 1993. Comparison of three serological methods for diagnosing Mycoplasma pneumoniae infection. J. Clin. Pathol. 46:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenny, G. E. 1992. Serodiagnosis, p. 505-512. In J. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, D.C.
- 12.Marston, B. J., J. F. Plouffe, T. M. File, B. A. Hackman, S. J. Salstrom, H. B. Lipmam, M. S. Kolczak, and R. F. Breiman. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch. Intern. Med. 157:1709-1718. [PubMed] [Google Scholar]
- 13.Matas, L., J. Dominguez, F. De Ory, N. Garcia, N. Gali, P. J. Cardona, A. Hernandez, C. Rodrigo, and V. Ausina. 1998. Evaluation of Meridian ImmunoCard Mycoplasma test for the detection of Mycoplasma pneumoniae-specific IgM in paediatric patients. Scand. J. Infect. Dis. 30:289-293. [DOI] [PubMed] [Google Scholar]
- 14.Sherman, M. J., H. A. Cubic, and J. M. Inglis. 1993. Mycoplasma pneumoniae infection: early diagnosis by detection of specific IgM by immunofluorescence. Br. J. Biomed. Sci. 50:305-308. [PubMed] [Google Scholar]
- 15.Sillis, M. 1990. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J. Med. Microbiol. 33:253-258. [DOI] [PubMed] [Google Scholar]
- 16.Thacker, W. L., and D. F. Talkington. 2000. Analysis of complement fixation and commercial enzyme immunoassays for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin. Diagn. Lab. Immunol. 7:778-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tully, J. G., D. L. Rose, R. F. Whitcomb, and R. P. Wenzel. 1979. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly modified culture medium. J. Infect. Dis. 13:478-482. [DOI] [PubMed] [Google Scholar]
- 18.Vikerfors, T., G. Brodin, M. Grandien, L. Hirschberg, A. Krook, and C. A. Petterson. 1988. Detection of specific IgM antibodies for the diagnosis of Mycoplasma pneumoniae infections: a clinical evaluation. Scand. J. Infect. Dis. 20:601-610. [DOI] [PubMed] [Google Scholar]
- 19.Waites, K. B., W. L. Thacker, and D. F. Talkington. 2001. The value of culture and serology for detection of Mycoplasma pneumoniae infections in the clinical laboratory in the age of molecular diagnostics. Clin. Microbiol. Newsl. 23:123-130. [Google Scholar]
- 19a.Waites, K. B., D. F. Talkington, and C. M. Bébéar. 2002. Mycoplasmas, p. 201-224. In A. L. Truant (ed.), Manual of commercial methods in clinical microbiology. ASM Press, Washington, D.C.
- 20.Waris, M. E., P. Toikka, T. Saarinen, S. Nikkari, O. Meurman, R. Vainionpaa, J. Mertsola, and O. Ruuskanen. 1998. Diagnosis of Mycoplasma pneumoniae in children. J. Clin. Microbiol. 36:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins-Riedel, T., G. Stanek, and F. Daxboeck. 2001. Comparison of SeroMp IgA with four other commercial assays for serodiagnosis of Mycoplasma pneumoniae infection. Diagn. Microbiol. Infect. Dis. 40:21-25. [DOI] [PubMed] [Google Scholar]