Abstract
Human immunodeficiency virus (HIV) infection is characterized by a depletion of T cells. This depletion is caused both by the virus-induced death of infected T cells and by the death of uninfected cells (bystander depletion) by a mechanism which is largely uncharacterized. Regeneration and tolerance factor (RTF) is a subunit of the vacuolar ATPase and a protein that is involved with activation and apoptosis. Anti-RTF antibodies mediate apoptosis in T lymphocytes. When anti-RTF was added to lymphocytes from an HIV-positive individual, they underwent larger amounts of apoptosis than cells taken from healthy controls. When lymphocytes were examined by Western blotting, those from HIV-positive individuals exhibited increased levels of expression of the 50-kDa protein (P < 0.001). A 70-kDa protein was the predominant form of RTF in uninfected control lymphocytes, being expressed in 100% of individuals studied. The expression of the 50-kDa protein in HIV-positive individuals correlated with decreased absolute CD4 counts with a sensitivity of 92% and a positive predictive value of 86%. When uninfected lymphocytes were stimulated with anti-CD3 and anti-CD28, no RTF was detected during early stimulation but a 50-kDa protein was expressed during late stimulation. When the susceptibilities of the lymphocytes to anti-RTF-induced apoptosis were measured, they correlated with the size of the RTF protein expressed. The cells were not susceptible to apoptosis when the 70-kDa RTF was present but were susceptible when the 50-kDa RTF was present. We propose that the increase in the levels of the 50-kDa RTF on cells from HIV-positive individuals is important in preventing the cell from undergoing apoptosis.
Human immunodeficiency virus (HIV) infection is characterized by a steady depletion of lymphocytes, leading to an immunosuppressed state and eventually to AIDS. Both CD4+ and CD8+ T cells are the targets of this reduction of T-cell numbers (14). At least two different methods of depletion are involved (12, 17, 19). In the first, T cells are depleted by direct infection and killing by HIV itself; however, the actual number of infected cells is low compared to the amount of cell death seen (14). Several investigators have shown that cell death by direct infection cannot account for all T-cell deaths and that a second mechanism must be operating by which the uninfected T cells die, commonly known as bystander depletion (11, 12, 14, 19). One proposed mechanism for the bystander depletion of T cells is apoptosis of noninfected lymphocytes (11, 14). The state of the immune system during HIV infection is one of prolonged activation and stimulation (19). It is also reported that during the course of HIV infection T cells are anergic in an unresponsive state and undergo apoptosis at a greater frequency than T cells from uninfected individuals (20). This prolonged activation and anergy can lead to the rapid depletion of uninfected T cells.
Structurally, regeneration and tolerance factor (RTF), as it is expressed on lymphocytes, has 100% homology at all 856 amino acid residues to the α-2 isoform of the α subunit of the vacuolar ATPase (Genpept accession number P15920), which has been described by Toyomura et al. (22). RTF consists of a 50-kDa transmembrane sequence and a 20-kDa extracellular portion, which generates a 70-kDa protein in total (16, 21). The 20-kDa portion can be cleaved to leave a 50-kDa form of RTF on the membrane. RTF is a protein that has previously been associated with apoptosis (1, 2). It is known that anti-RTF antibody induces apoptosis in both peripheral blood lymphocytes and Jurkat T cells (2). Unpublished experiments have shown that anti-RTF monoclonal antibody 2C1 is a blocking antibody, which generates apoptosis in cells by abrogating its function, which is to hydrolyze ATP and prevent its interaction with the apoptosis-inducing receptor P2X7. When cells that express RTF are incubated with anti-RTF, their ability to hydrolyze ATP is abrogated and apoptosis results. The addition of ATPase inhibits this resulting apoptosis. We show that when lymphocytes from HIV-positive individuals are stimulated with anti-RTF, they undergo apoptosis at higher rates than cells taken from healthy individuals. Previously, it has been shown (6, 9) that the amount of RTF is increased on the surfaces of lymphocytes during HIV infection.
The purpose of this study was to characterize the possible role of RTF in the activation and apoptosis of T cells seen during HIV infection.
Our study characterizes RTF as being expressed in an alternate 50-kDa form on lymphocytes from HIV-positive individuals and that lymphocytes from HIV-positive individuals are more susceptible to anti-RTF-induced apoptosis than lymphocytes from healthy controls. The presence of the 50-kDa protein correlates with decreased absolute CD4 and CD8 counts. We also show that the presence of the 50-kDa protein makes the T cell more susceptible to anti-RTF-induced apoptosis.
We therefore propose that the 50-kDa RTF is protective of lymphocytes in HIV infection, with its levels increasing as lymphocyte counts begin to decrease to compensate for activation-induced apoptosis.
MATERIALS AND METHODS
Generation of monoclonal antibody 2C1.
Monoclonal antibody 2C1 was generated as described previously (6). This antibody is specific for amino acids 488 to 510 on the 50-kDa membrane-bound portion, which is common to both forms (the 70- and 50-kDa forms) of the RTF protein. This antibody is specific for RTF and does not cross-react with any other protein (1).
Isolation and stimulation of lymphocytes.
Blood was obtained by venous puncture from 17 healthy volunteers at the Finch University of Health Sciences/The Chicago Medical School in tubes with sodium heparin. Blood from 33 HIV-positive individuals was obtained from samples available in tubes containing sodium heparin sent from Mount Sinai Hospital, Chicago, Ill. No specific criteria other than the date that the sample was drawn (within 1 day of the experiment) were required. The approval of all appropriate local institutional review boards was given. Peripheral blood mononuclear cells (PBMCs) from both types of samples were obtained by separation of Ficoll-Hypaque gradients. Samples either were used immediately for the apoptosis assay or were lysed at time zero to be used for Western blotting. Samples to be used for the stimulation time course studies were washed two times in RPMI 1640 (Gibco) and plated at a density of 2.5 × 105 cells per well in 50 μl of RPMI 1640 in a 96-well flat-bottom plate that had previously been coated with anti-CD3ɛ (dilution, 1:200) and 2 μg of CD28 for 1 h at 37°C. The coated wells were then washed three times in phosphate-buffered saline (PBS), and the cells were incubated in these wells for 24 to 72 h at 37°C in a 5% CO2 atmosphere and harvested after 16 to 72 h.
Induction of apoptosis.
A total of 2.5 × 105 cells (unstimulated or stimulated as described above) were plated in a round-bottom 96-well plate in a total volume of 50 μl of RPMI 1640 and incubated with either 50 μg of the anti-RTF antibody 2C1, 1 μl of Fas (Pharmingen), or isotype control mouse anti-keyhole limpet hemocyanin (Pharmingen) at the same concentration as monoclonal antibody 2C1 for 16, 48, and 72 h. The cells were harvested from the wells, were washed twice with 0.1% bovine serum albumin-PBS, and then stained with 5 μl of annexin V-fluorescein isothiocyanate (FITC) (Pharmingen) in 100 μl of 1× binding buffer (1× BB; 10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2) (23), followed by a wash in 1× BB. The cells were resuspended in 300 μl of 1× BB, and 3 μl of 1 mg of propidium iodide per ml was added. The cells were then analyzed on a Becton Dickinson Facscalibur flow cytometer for annexin V binding by gating on the lymphocytes and by using a propidium iodide exclusion gate to remove necrotic cells from the analysis.
Western blotting.
A total of 1.5 × 106 cells were washed two times in PBS and resuspended for lysis in 40 μl of running dye (25 ml of 4× Tris-sodium dodecyl sulfate [SDS; pH 6.8], 20 ml of glycerol, 4 g of SDS, and 1 mg of bromophenol blue in 100 ml of H2O) with 4 μl of β2-mercaptoethanol. These were run on an SDS-8% polyacrylamide gel at 50 A for 3 h. The samples were transferred to a nitrocellulose membrane by use of a transfer apparatus (Bio-Rad) containing transfer buffer (25 mM tris, 192 mM glycine, 20% methanol [pH 8.3]) and blocked overnight with agitation in blocking buffer (5% powdered milk, 1% polyvinylpryrrolidone, 0.1% Tween 20, 0.14 M NaCl). The membrane was then probed with 40 μl of anti-RTF monoclonal antibody 2C1 in 12 ml of blocking buffer for 1 h with agitation. The membrane was washed two times with PBS for 5 min each time and incubated with 5 μl of alkaline phosphatase-conjugated anti-mouse secondary antibody in 12 ml of blocking buffer for 1 h. This mixture was incubated with Immun-Star AP substrate (Bio-Rad) for 5 min and then placed under photographic film for 8 min and developed.
Reverse transcription-PCR (RT-PCR).
A total of 2.5 × 105 PBMCs were incubated with anti-CD3 and anti-CD28 and harvested at 0, 16, 24, 48, and 72 h. The cells were washed in PBS, and RNA was isolated by suspending the cells in 1 ml of TRIZOL guanidinium reagent (Gibco) and allowing them to incubate at room temperature for 5 min. Chloroform (0.2 ml) was added, and the mixture was vortexed vigorously. The mixture was centrifuged, and the resulting aqueous phase was washed in isopropyl alcohol and centrifuged again. The RNA pellet was washed in ethanol and resuspended in double-distilled H2O.
Equal amounts of RNA (determined by spectrophotometry) was added to a thin-walled reaction tube with 5 μl of 2× reaction mixture (Gibco), 1 μl each of sense and anti-sense RTF primers (5′-GTTGAGTTTGAGCCCACTTATGAAGAATTCCCTTCC and 3′-CTCCTCCAGTGCCCGGCGCAGGTC, respectively), 1 μl of reverse transcriptase-platinum Taq mixture (Invitrogen), and 42 μl of double-distilled H2O. cDNA was generated by incubating the resulting mixture for one cycle of 50°C for 30 min, followed by 94°C for 2 min. PCR amplification was achieved by 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min.
ATPase assay.
PBMCs from healthy individuals were suspended in 50 μl of RPMI 1640 at a density of 2.5 × 105 cells per well in a 96-well flat-bottom plate and incubated with 10 μg of either monoclonal antibody 2C1 (anti-RTF) or an isotype-matched control antibody for 45 min at 37°C in a 5% CO2 atmosphere. The cells were washed with PBS and were resuspended in 100 μl of assay buffer (5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 1.5 μCi of [γ-32P]ATP) in 1.5-ml Eppendorf tubes. The ATPase reaction was allowed to continue for 10 min at room temperature, after which the cells were assayed for viability by trypan blue exclusion. Viability was found to be above 90%. A total of 500 μl of 20% (wt/vol) activated charcoal (in distilled water) was added to scavenge any unused [γ-32P]ATP, and the mixture was allowed to sit for 10 min on ice. The cells were centrifuged at 3,200 × g for 5 min to pellet the charcoal. A total of 500 μl of supernatant containing the γ-32P released was removed and added to scintillation vials with 3 ml of scintillation fluid, and the amount of radioactivity released was read in a scintillation counter for 1 min/tube (4, 5, 7).
ATP-induced apoptosis.
Approximately 2.5 × 105 J774A1 cells were suspended in 100 μl of RPMI 1640 (Gibco) in a 96-well round-bottom plate; and 3 mM ATP (Sigma), 0.75 mM ATP, 10 μg of anti-RTF, or 0.75 mM ATP and 10 μg anti-RTF together (final concentrations) was added. The cells were incubated under these conditions at 37°C in 5% CO2 for 6 h. The cells were then washed twice in 0.01% bovine serum albumin-PBS and resuspended in 100 μl of binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2) with 5 μl of annexin V-FITC (BD/Pharmingen) for 15 min. The cells were washed in binding buffer and resuspended in 300 μl of binding buffer with 3 μl of 1 mg of propidium iodide (Sigma) per ml. Apoptosis was measured by quantifying the annexin V binding by flow cytometry by gating on propidium iodide-negative, annexin V-positive cells.
RESULTS
Lymphocytes from HIV-positive individuals are susceptible to anti-RTF-induced apoptosis.
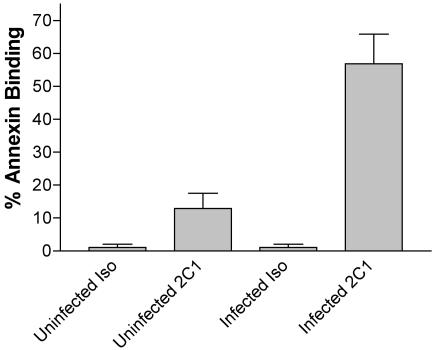
Anti-RTF antibody induces apoptosis in RTF-expressing cells (1, 2), such as CD4+ and CD8+ T cells (6). Therefore, we decided to investigate whether RTF had an effect on the apoptosis of lymphocytes from HIV-positive individuals. Freshly isolated, unstimulated lymphocytes from seven HIV-positive individuals and seven healthy controls were incubated with monoclonal antibody 2C1 for 24 h and examined for apoptosis by annexin V binding by flow cytometry. While the lymphocytes from the healthy controls exhibited apoptosis (rate of change, 12.91% ± 4.6%) under these conditions, as expected, the cells from HIV-positive individuals exhibited a much greater percentage of apoptosis and susceptibility to anti-RTF, with a change of 56.93% ± 9.0% (Fig. 1). The difference in annexin V binding between the two populations was found to be highly significant by Student's t test (P < 0.001).
FIG. 1.
Anti-RTF antibody-induced increased apoptosis in lymphocytes from HIV-positive individuals compared to those from healthy control individuals. Lymphocytes from seven HIV-positive individuals and seven healthy donors (uninfected) were incubated for 24 h either with the isotype control (Iso) or with monoclonal (anti-RTF) antibody 2C1. These were then analyzed for apoptosis by incubating them with annexin V-FITC and determination of the level of annexin V binding by flow cytometry on a Facscalibur (BD) flow cytometer. All values at the top indicate the percentage of annexin V binding for each population. The difference between the healthy and HIV-positive populations was judged to be significant if the P value was <0.001 by Student's t test.
The 50-kDa RTF is expressed in association with HIV infection.
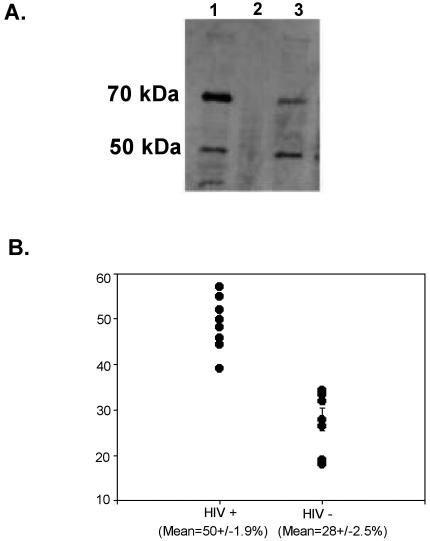
RTF is expressed predominantly as a 70-kDa form in resting lymphocytes (21). RTF expression was examined in the PBMCs of 12 HIV-positive individuals (Table 1) and 7 HIV-negative individuals by Western blotting (Fig. 2A), in which the blots were probed with monoclonal antibody 2C1. It was found that freshly isolated lymphocytes from HIV-positive individuals expressed both the 70- and the 50-kDa RTF forms or none at all. The percentage of the 70-kDa RTF protein compared with that of the 50-kDa RTF protein was determined by desitometry (Fig. 2B). It was found that the lymphocytes from HIV-positive individuals expressed the 50-kDa RTF protein at higher levels (mean proportions of the 50-kDa form, 50% ± 0.9% for HIV-positive individuals and 28% ± 0.5% for HIV-negative individuals) (Fig. 2B).
TABLE 1.
Absolute counts of cells expressing CD3, CD4, and CD8 as determined by flow cytometry and their corresponding amounts of 50-kDa RTF as determined by densitometry
| RTF expression and % 50-kDa form | No. of cells/μl of blood
|
|
|---|---|---|
| CD4 | CD8 | |
| No detectable 50-kDa RTF | ||
| 0 | 499 | 358 |
| 0 | 437 | 1,613 |
| 0 | 411 | 826 |
| 0 | 454 | 1,204 |
| 0 | 664 | 1,610 |
| 0 | 843 | 1,120 |
| 0 | 1,125 | 1,080 |
| 0 | 759 | 1,577 |
| 0 | 129 | 196 |
| 0 | 107 | 759 |
| Detectable 50-kDa RTF | ||
| 54 | 129 | 196 |
| 47 | 14 | 837 |
| 57 | 338 | 342 |
| 43 | 368 | 292 |
| 55 | 66 | 412 |
| 46 | 119 | 1,264 |
| 39 | 206 | 878 |
| 48 | 339 | 846 |
| 45 | 6 | 332 |
| 53 | 360 | 586 |
| 60 | 46 | 16 |
| 50 | 135 | 837 |
| 47 | 454 | 1,204 |
| 38 | 338 | 342 |
| 56 | 1,003 | 936 |
FIG. 2.
(A) Western blot. RTF is expressed in two different forms in HIV-positive and HIV-negative individuals. Lymphocytes from HIV-negative individuals (lane 1) and HIV-positive individuals (lanes 2 and 3) were tested by Western blotting and probed with monoclonal antibody 2C1. Top row, 70-kDa RTF protein; bottom row, 50-kDa RTF protein. The results are representative of those for lymphocytes from HIV-negative individuals and HIV-positive individuals that express RTF (lane 3) and that do not express RTF at detectable levels (lane 2). (B) The percentage of the 50-kDa RTF protein is increased in lymphocytes from HIV-positive individuals. The results are the percentages of the 50-kDa RTF protein expressed by nine HIV-positive individuals and seven HIV-negative individuals, as measured by desitometry. Means are presented with standard errors. The results were deemed to be significant if the P value was <0.001 by Student's t test.
The lymphocytes from each healthy control analyzed had both the 50- and the 70-kDa RTF proteins, but the 70-kDa protein was predominant (mean proportions, 72% ± 2.5% of the 70-kDa form versus 28% ± 2.5% of the 50-kDa form, as measured by densitometry). A statistical examination of the difference between the forms of RTF seen in healthy and HIV-positive individuals was shown to be significant, with a P value of <0.001, as measured by Student's t test.
Expression of the 50-kDa RTF protein correlates with decreased absolute lymphocyte counts.
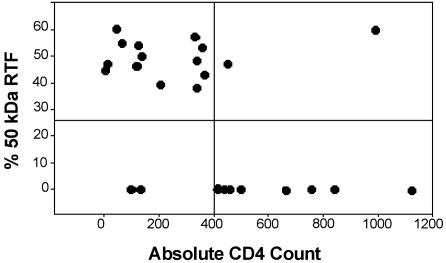
The percentage of the 50-kDa RTF protein was plotted against the absolute CD4 count (Fig. 3) and the absolute CD8 count (data not shown). It was found that the percentage of the 50-kDa RTF protein increased as the absolute CD4 counts decreased. When the level of expression of the 50-kDa RTF protein was compared to CD4 counts by using a two-by-two contingency table, the positive predictive value for an increased level of RTF expression and a decreased CD4 count was greater than 86%, and the sensitivity for the decrease in the CD4 count was 92% (Table 2). No correlation with CD8 counts was found.
FIG. 3.
The amount of the 50-kDa RTF protein correlates with decreased absolute CD4 counts. The amount of the 50-kDa RTF protein was measured from Western blots with monoclonal antibody 2C1 by densitometry to generate the percentage of the 50-kDa protein expressed, and the result was plotted against the absolute CD4 counts. The graph is divided to represent counts of >400 and <400 cells and RTF positive and RTF negative (above and below the horizontal line, respectively).
TABLE 2.
Contingency table for individuals expressing RTF or not expressing RTF and their CD4-cell countsa
| RTF expression | No. of individuals with CD4-cell counts of:
|
||
|---|---|---|---|
| >400 | <400 | Total | |
| Positive | 2 | 12 | 14 |
| Negative | 10 | 1 | 11 |
| Total | 12 | 13 | 25 |
A positive predictive value of 86% and a sensitivity of 92% were determined.
RTF is expressed in two forms, depending on the time and activation signal received by the lymphocyte.
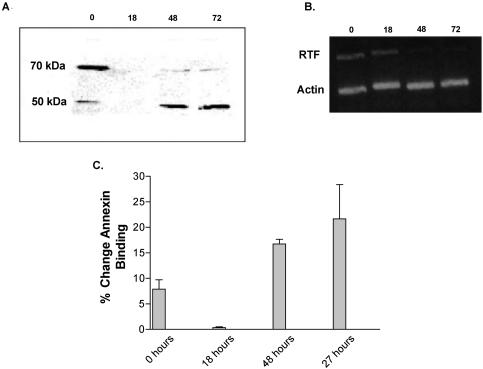
RTF is expressed as a 70-kDa band by resting cells and a 50-kDa band by stimulated cells (CD3 and CD28). Lymphocytes from healthy controls were stimulated with anti-CD3 and anti-CD28 antibodies for 24 to 72 h (Fig. 4A). Unstimulated, resting cells taken at the time of isolation expressed a 70-kDa RTF protein as their major form of RTF. Upon stimulation with anti-CD3 and anti-CD28 antibodies, the level of expression of RTF fell to below detectable levels at 18 h. After 24 h the level of RTF expression returned to detectable levels; however, the predominant form was the 50-kDa protein. This 50-kDa protein remained the major form throughout the stimulation time course.
FIG. 4.
(A) Western blotting. RTF is expressed in two different forms in unactivated and activated (CD3 and CD28) lymphocytes. Lymphocytes from a healthy individual were stimulated with plate-bound CD3 and CD28 and harvested at the indicated times (in hours), tested by Western blotting, and probed with monoclonal antibody 2C1. Top row, proteins of 70 kDa; bottom row, proteins of 50 kDa. Each column represents a stimulation time of 0, 18, 48, and 72 h, respectively. (B) RT-PCR. RTF mRNA expression precedes 50-kDa protein expression. The RT-PCR products of PBMCs were stimulated with anti-CD3 antibody. Top row, RTF message; bottom row, β-actin message. Stimulation times of 0, 18, 48, and 72 h are indicated at the top. (C) Susceptibility to anti-RTF-induced apoptosis during stimulation. Lymphocytes were stimulated (CD3 and CD28) for 18 to 72 h and incubated with monoclonal antibody 2C1. Apoptosis, by way of annexin V binding, was then measured by flow cytometry. The x axis represents the times that the lymphocytes were stimulated before the addition of 2C1. The y axis represents the changes in the levels of annexin V binding between the populations incubated with 2C1 and those incubated with the isotype control.
Next we examined the RTF mRNA by RT-PCR during the time course of stimulation described above (Fig. 4B). In normal resting cells, when the level of RTF expression is highest, the amount of mRNA is maximal. mRNA can still be detected when the expression of the protein is not detectable. As protein expression returns and expression of the 50-kDa protein becomes predominant, the mRNA levels begin to decrease. As shown in Fig. 4B, the levels of β-actin RNA remained constant throughout.
Susceptibility to apoptosis during different patterns of RTF expression.
To determine if the three different patterns of RTF expression alter cell susceptibility to anti-RTF-induced apoptosis, we stimulated (with anti-CD3 and anti-CD28 antibodies) healthy PBMCs for 1 to 3 days by incubation with either monoclonal antibody 2C1 or isotype control antibody and examined them for annexin V binding (Fig. 4C). As nonspecific apoptosis due to activation-induced cell death is expected, the amount of apoptosis in the population incubated with the isotype control antibody was measured and subtracted from the amount of apoptosis seen with the population incubated with 2C1 to compensate for the background apoptosis. The increased apoptosis seen in the population incubated with 2C1 can be attributed to the effects of the antibody. It was found that at the unstimulated time, when the cell expresses only the 70-kDa protein, the cells were not as susceptible to apoptosis by anti-RTF antibody (which induced 7.82% ± 1.9% more apoptosis than the isotype control antibody did). At 18 h, when RTF could not be detected by Western blotting, the amount of apoptosis detected for the population incubated with 2C1 showed no difference from that detected for the controls. At 48 and 72 h, when the 50-kDa band was predominant, the levels of susceptibility to anti-RTF-induced apoptosis increased 2.1- and 2.7-fold, respectively.
RTF regulates surface ATPase activity in lymphocytes and macrophages.
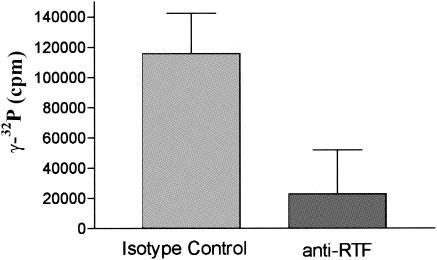
It is our hypothesis that RTF regulates surface ATPase activity and that anti-RTF antibody blocks it. To demonstrate that RTF has surface ATPase activity, PBMCs were incubated with either anti-RTF or isotype control antibody for 45 min, and their surface ATPase activities were measured by determination of the level of [γ-32P]ATP degradation (4, 5, 7). The cells were checked for viability after incubation with ATPase buffer to ensure that no cytoplasmic ATPases were being measured. The PBMCs incubated with anti-RTF had a 10-fold decrease in surface ATPase activity (11,758 ± 3,394 cpm) compared to that for the PBMCs incubated with the isotype control antibody (115,592 ± 23,891 cpm) (P < 0.001) (Fig. 5).
FIG. 5.
Surface ATPase activity is inhibited by anti-RTF. PBMCs with anti-RTF antibody or isotype control antibody were examined for surface ATPase activity by adding [γ-32P]ATP and measuring the release of 32Pi. The y axis represents surface ATPase activity (measured in counts of 32Pi per minute). Error bars represent the standard error of the mean.
RTF prevents ATP from inducing apoptosis.
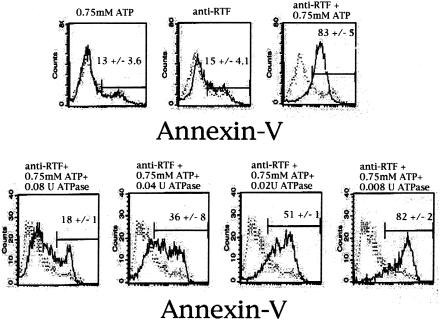
We next sought to demonstrate if RTF can prevent ATP-induced apoptosis. Our theory was that since RTF regulates surface ATPase activity, it therefore regulates ATP binding to P2X7. If this is correct, then it follows that anti-RTF antibody and ATP would work together to generate more apoptosis than either one would alone by blocking the ATPase activity of RTF. For this purpose we chose the macrophage cell line J774A1. J774A1 cells were used as a model to demonstrate the interaction of RTF with P2X7 because this cell line expresses both molecules constitutively and is known to undergo P2X7-mediated, ATP-induced apoptosis (3, 13). When anti-RTF antibody and ATP were added together at an incubation time of 1 h at the same concentration of each component used alone, a nearly maximal amount of apoptosis occurred, as measured by annexin V binding (Fig. 6). When 3 mM ATP was added, 84.14% ± 1.63% of the cells underwent apoptosis, but when the amount of ATP was decreased to 0.75 mM, only 13.26% ± 3.6% of the cells underwent apoptosis. When 10 μg of anti-RTF was added alone, 15.49% ± 4.05% of the cells were apoptotic. However, when anti-RTF and 0.75 mM ATP were added together, 83.05% ± 5.09% of the cells were apoptotic. Purified ATPase (from dog kidney; Sigma) was also added. When ATPase was added at the same incubation time, no apoptosis occurred, showing that the apoptosis-inducing ability of RTF is dependent on the presence of ATP (Fig. 5). Previously, in initial experiments we demonstrated that anti-RTF antibody had no effect on the purified ATPase used, negating the possibility of their interaction (data not shown).
FIG. 6.
Anti-RTF and ATP generate more apoptosis together than either one does alone. Top row, flow cytometry histograms of J774A1 macrophages incubated with ATP or anti-RTF, or both; bottom row, J774A1 cells incubated with ATP, anti-RTF, and different concentrations of ATPase, as indicated. The x axis represents annexin V staining. The means of four independent experiments with standard errors are given for each panel.
DISCUSSION
We have shown that PBMCs from HIV-positive individuals are more susceptible to apoptosis than PBMCs from healthy controls when the PBMCs are blocked with anti-RTF antibody. This may indicate a role of RTF in HIV infection overlooked at present, since apoptosis of HIV-infected cells occurs when RTF is prevented from functioning.
The data show that RTF is expressed by PBMCs from HIV-positive individuals in a different form than that in which it is expressed by PBMCs from healthy individuals. This form of RTF (50 kDa) is the same form expressed by healthy cells that have been stimulated in vitro. This 50-kDa form correlates with decreased T-cell levels. Furthermore, cells in which this 50-kDa protein is expressed are more susceptible to anti-RTF blocking antibody-induced apoptosis. RTF is known to be a component of an ATPase complex, and it is probable that it binds to ATP or is necessary for its conversion to ADP (22). Recent findings that ATP induces apoptosis in macrophages and other PBMCs (15, 25) suggest that the ATPase activity of RTF may be important in regulating ATP-induced apoptosis. Anti-RTF-mediated apoptosis is known to operate through caspases 3 and 9 (the same caspases that characterize ATP-induced apoptosis) (25). Anti-RTF antibody does not interact with the Fas-Fas ligand or other tumor necrosis factor alpha receptors and is a monoclonal antibody that does not cross-react with any other molecule found in lymphocytes when it is tested by Western blotting (1, 6).
ATP is commonly released from neutrophils, macrophages, and damaged cells in general during an infection (10). This ATP can interact with ATP binding purinoceptors on lymphocytes that subsequently mediate apoptosis (3, 8, 24, 26). During the course of HIV infection, when the number of T cells begins to decrease, many secondary infections that result in the release of ATP begin to follow. This can cause apoptosis and death in T cells (whether they are HIV infected or not), further resulting in bystander T-cell death in a self-perpetuating process. We propose that during HIV infection the active 50-kDa form of RTF, a subunit of a vacuolar ATPase seen on the surface of the cell membrane, is up-regulated to combat the effects of extracellular ATP-induced cell death. We have shown that RTF regulates surface ATPase activity. Antibody to RTF will almost totally abrogate surface ATPase activity in lymphocytes. This suggests that the increase in the level of RTF during activation counteracts the effects of ATP. When cells are given anti-RTF antibody, they become susceptible to apoptosis due to the presence of minimal amounts of ATP, to which they would not normally be susceptible before antibody treatment, further strengthening the argument that RTF may be important in combating ATP-induced cell death.
T cells are chronically stimulated during the course of HIV infection (9, 18). We propose that this chronic stimulation results in an alteration of RTF. Over the course of time, when stimulation leads to activation-induced cell death and lymphocyte levels start to decrease, RTF is up-regulated in its 50-kDa form in an attempt to further prevent apoptosis. It was found that this effect was most pronounced in the case of CD4+ cells, with the level of expression of the 50-kDa form of RTF increasing as CD4 levels decreased. This is an effect specific to CD4+ cells, as it was not noticed in the CD8+ population. We believe that this demonstrates that as T-cell levels begin to fall as a result of apoptosis, RTF is up-regulated in the 50-kDa form to prevent this eventual fate.
To better understand what factors govern the change of RTF from a 70-kDa form into a 50-kDa form or no expression at all, as seen in the HIV-positive lymphocyte population, it was decided to generate an in vitro model. Since HIV infection is known to correspond with immune activation (11, 14), we decided to stimulate PBMCs from healthy individuals with anti-CD3 and anti-CD28. Interestingly, at 18 h, during early stimulation, RTF levels fall below detectable levels. RT-PCR at this time, however, showed that the mRNA was present. After 24 h, RTF returns in the 50-kDa form, with both states (expression of the 50-kDa form or no detectable expression) mimicking that seen in PBMCs from HIV-positive individuals. This shows that the signal which induces the change from the 70- to the 50-kDa form is stimulatory in nature and is independent of viral infection of the individual cell. This signal is most likely indirectly caused by changes in the immune system (activation, cytokines, etc.) and not directly by the virus.
It is interesting that during early stimulation the level of RTF expression falls below detectable levels, as is seen in some HIV-positive patients with high lymphocyte counts. It can be theorized that RTF expression is down-regulated during early activation, while the immune system is still in good health, changing to the 50-kDa form during late activation, when anergy begins to affect the cell.
Finally, the experiment whose results are presented in Fig. 4 shows that the 50-kDa protein is necessary for cell survival during late stimulation. When the 70-kDa form is expressed or no is RTF detectable at all, there is no apoptosis when RTF is blocked with antibody. However, when the 50-kDa form is present and it is blocked, apoptosis is the result. This suggests that when T-cell levels begin to decrease, expression of the 50-kDa RTF protein, which results from the ATP released from neutrophils and other lymphocytes during secondary infections, is induced to help protect the cells from further cell death. These findings suggest that the presence of the 50-kDa RTF protein is important in the continued survival of the T cell and that RTF may be important in preventing the cell death that correlates with the increased susceptibility to apoptosis in HIV infection.
REFERENCES
- 1.Boomer, J. S., R. A. Derks, G. W. Lee, B. K. DuChateau, A. Gilman-Sachs, and K. D. Beaman. 2001. Regeneration and tolerance factor is expressed during T-lymphocyte activation and plays a role in apoptosis. Hum. Immunol. 62:577-588. [DOI] [PubMed] [Google Scholar]
- 2.Boomer, J. S., G. W. Lee, T. S. Givens, A. Gilman-Sachs, and K. D. Beaman. 2000. Regeneration and tolerance factor's potential role in T-cell activation and apoptosis. Hum. Immunol. 61:959-971. [DOI] [PubMed] [Google Scholar]
- 3.Di Virgilio, F. 1995. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today 16:524-528. [DOI] [PubMed] [Google Scholar]
- 4.Dombrowski, K. E., Y. Ke, L. F. Thompson, and J. A. Kapp. 1995. Antigen recognition by CTL is dependent upon ectoATPase activity. J. Immunol. 154:6227-6237. [PubMed] [Google Scholar]
- 5.Dombrowski, K. E., J. M. Trevillyan, J. C. Cone, Y. Lu, and C. A. Phillips. 1993. Identification and partial characterization of an ectoATPase expressed by human natural killer cells. Biochemistry 32:6515-6522. [DOI] [PubMed] [Google Scholar]
- 6.DuChateau, B. K., G. W. Lee, M. P. Westerman, and K. D. Beaman. 1999. Increased expression of regeneration and tolerance factor in individuals with human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 6:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filippini, A., R. E. Taffs, T. Agui, and M. V. Sitkovsky. 1990. Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J. Biol. Chem. 265:334-340. [PubMed] [Google Scholar]
- 8.Gargett, C. E., J. E. Cornish, and J. S. Wiley. 1997. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br. J. Pharmacol. 122:911-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Givens, T. S., B. K. DuChateau, J. S. Boomer, M. P. Westerman, A. Gilman-Sachs, and K. D. Beaman. 1999. Regeneration and tolerance factor: a correlate of human immunodeficiency virus-associated T-cell activation. Clin. Diagn. Lab. Immunol. 6:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon, J. L. 1986. Extracellular ATP: effects, sources and fate. Biochem. J. 233:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gougeon, M. L., E. Ledru, H. Lecoeur, and S. Garcia. 1998. T cell apoptosis in HIV infection: mechanisms and relevance for AIDS pathogenesis. Results Probl. Cell Differ. 24:233-248. [DOI] [PubMed] [Google Scholar]
- 12.Gougeon, M. L., and L. Montagnier. 1999. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS. Molecular control and effect of highly active anti-retroviral therapy. Ann. N. Y. Acad. Sci. 887:199-212. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg, S., F. Di Virgilio, T. H. Steinberg, and S. C. Silverstein. 1988. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J. Biol. Chem. 263:10337-10343. [PubMed] [Google Scholar]
- 14.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Y., P. L. Fisette, L. C. Denlinger, A. G. Guadarrama, J. A. Sommer, R. A. Proctor, and P. J. Bertics. 1998. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J. Biol. Chem. 273:27170-27175. [DOI] [PubMed] [Google Scholar]
- 16.Lee, G. W., J. S. Boomer, A. Gilman-Sachs, A. Chedid, L. Gudelj, D. Rukavina, and K. D. Beaman. 2001. Regeneration and tolerance factor of the human placenta induces IL-10 production. Eur. J. Immunol. 31:687-691. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, D. E., D. S. Tang, A. Adu-Oppong, W. Schober, and J. R. Rodgers. 1994. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J. Immunol. 153:412-420. [PubMed] [Google Scholar]
- 18.Mahalingam, M., M. Peakman, E. T. Davies, A. Pozniak, T. J. McManus, and D. Vergani. 1993. T cell activation and disease severity in HIV infection. Clin. Exp. Immunol. 93:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 20.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 21.Nichols, T. C., J. A. Kang, V. Angkachatchai, A. E. Beer, and K. D. Beaman. 1994. Expression of a membrane form of the pregnancy-associated protein TJ6 on lymphocytes. Cell. Immunol. 155:219-229. [DOI] [PubMed] [Google Scholar]
- 22.Toyomura, T., T. Oka, C. Yamaguchi, Y. Wada, and M. Futai. 2000. Three subunit a isoforms of mouse vacuolar H+-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J. Biol. Chem. 275:8760-8765. [DOI] [PubMed] [Google Scholar]
- 23.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 24.Wiley, J. S., J. R. Chen, M. B. Snook, and G. P. Jamieson. 1994. The P2Z-purinoceptor of human lymphocytes: actions of nucleotide agonists and irreversible inhibition by oxidized ATP. Br. J. Pharmacol. 112:946-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaborina, O., N. Dhiman, M. L. Chen, J. Kostal, I. A. Holder, and A. M. Chakrabarty. 2000. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology 146:2521-2530. [DOI] [PubMed] [Google Scholar]
- 26.Zheng, L. M., A. Zychlinsky, C. C. Liu, D. M. Ojcius, and J. D. Young. 1991. Extracellular ATP as a trigger for apoptosis or programmed cell death. J. Cell Biol. 112:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]