Abstract
Our understanding of cardiac fibroblast functions has moved beyond their roles in heart structure and extracellular matrix generation, and now includes contributions to paracrine, mechanical and electrical signalling during ontogenesis and normal cardiac activity. Fibroblasts have central roles in pathogenic remodelling during myocardial ischaemia, hypertension and heart failure. As key contributors to scar formation, they are crucial for tissue repair after interventions including surgery and ablation. Novel experimental approaches targeting cardiac fibroblasts are promising potential therapies for heart disease. Indeed, several existing drugs act, at least partially, through effects on cardiac connective tissue. This Review outlines the origins and roles of fibroblasts in cardiac development, homeostasis and disease; illustrates the involvement of fibroblasts in current and emerging clinical interventions; and identifies future targets for research and development.
Cardiac mesenchymal cells, often referred to simply as ‘fibroblasts’, are among the most populous cells of the heart.1, 2 Fibroblasts are also a cell type most conspicuously linked to heart disease, as they are associated with tissue remodelling involving ‘fibrosis’.1, 3–6 Fibrosis contributes to the leading causes of sickness and death in the developed world, including the pathophysiological consequences of myocardial infarction (MI), in which myocytes are replaced by a collagen-rich scar; and hypertension, diabetes, rheumatic heart disease, hypertrophic cardiomyopathy and heart failure, which are associated with the patchy or diffuse appearance of excess fibrotic tissue. Surplus collagen, generated by activated fibroblasts, increases the stiffness of the myocardial wall and is linked to myocardial remodelling, thereby impeding normal systolic and diastolic mechanical function.
Focal scarring and diffuse fibrosis are independent predictors of the likelihood of developing electrophysiological disturbances resulting in cardiac arrhythmias.2, 7, 8 Therefore, inhibiting or reversing fibrosis and its adverse consequences is an established target of many widely used clinical interventions for treating heart disease, including angiotensin converting enzyme (ACE) inhibitors, aldosterone antagonists, and statins (Table 1). These interventions may lead to a partial recovery of contractile function via a process known as ‘reverse remodelling’, and thereby delay the progression towards heart failure.
Table 1.
Selected compounds and interventions that may reduce or reverse fibrosis*
| Drug class or intervention | Target | Anti-fibrotic biological mode-of-action | Potential indication |
|---|---|---|---|
| Drugs and biologic compounds | |||
| ACE inhibitors (e.g. enalapril) | Angiotensin converting enzyme | Inhibit fibroblast activation, macrophage attracting enzymes, ECM deposition | Hypertension, ischaemic heart disease, heart failure182 |
| Aldosterone inhibitors (e.g. spirolactone) | Mineralocorticoid receptors | Reduce oedema, cardiac load | Heart failure183 |
| Statins (e.g. simvastatin) | Co enzyme A-reductase | Reduce lipid levels, inhibit fibroblast activation | Ischaemic heart disease, heart failure, atrial fibrosis179, 181 |
| Angiotensin receptorinhibitors (e.g., losartan) |
AT1 receptor | Inhibit fibroblast activation, ECM deposition | Heart failure184, 185 |
| Endothelin receptor inhibitors (e.g. bosentan) | ET receptors | Reduce fibroblast ECM deposition | Failed Phase III testing for heart failure and ischaemia reperfusion injury {Rodriguez-Pascual, 2014 #14279; Singh, 2006 #14280} |
| Beta blockers (e.g. propranolol) |
Beta adrenergic receptors | Inhibit fibroblast activation, ECM deposition | Heart failure, reverse remodelling186 |
| Acetylsalicylic acid (e.g. asprin) |
Cyclooxygenase, redox-sensitive transcription factors | Inhibit fibroblast activation | Cardiac hypertrophy, reverse remodelling187 |
| MCP-1 neutralizers (e.g., anti-CCL2 antibody) | Monocyte Chemo-attractant Protein-1 | Inhibits macrophage and fibroblast proliferation, and induction | Myocardial infarction, ischaemia reperfusion injury188 |
| PPARγ agonists (e.g. thiazolidinediones) |
Nuclear hormone receptor peroxisome proliferator activated receptor-gamma | Inhibit fibroblast activation, ECM deposition | Hypertrophic cardiomyopathy, heart failure, myocardial infarction189 |
| MMP inhibitors (e.g., batimas) | Matrix metalloprotein | Inhibit extracellular matrix remodelling | Cardiac scar re-engineering. Failed Phase III testing for heart failure190 |
| Relaxin inhibitors (e.g., serelaxin) |
Relaxin-2 | Inhibit fibroblast activation ECM expression | Heart failure, atrial fibrosis191 |
| PDGFR inhibitors and anti-PDGFR antibodies | Platelet derived growth factor receptor-α | Inhibit atrial fibrosis | Atrial fibrosis/fibrillation192 |
| Mast cell inhibitors (e.g., nedocromil) |
Mast cells | Inhibit mast cell activation and histamine release | Heart failure, hypertension, ischaemia reperfusion injury193 |
| Hyaluronan inhibitors (e.g., exogenous HA) |
Endogenous hyaluronan organization | Inhibit fibroblast activation | Myocardial infarction194 |
| Chymase inhibitors (e.g., TY51469) |
Chymase | Attenuation of inflammatory pathways, inhibition of fibroblast proliferation | Ischaemia reperfusion injury195 |
| Wnt inhibitors (e.g. sFrpl) |
Canonical Wnt signalling | Inhibit pro-fibrotic cytokine signalling, ECM remodeling | Myocardial infarction196, 197 |
| Connexin inhibitors (e.g., αCT1) |
Connexin 43 | Inhibit connexin hemichannel activity | Myocardial infarction, atrial fibrosis143, 144, 198 |
| Cardioprotective drugs (e.g., mitochondrial PTP Inhibitors) |
Mitochondrial permeability transition pore in myocytes | Prevention of muscle loss and replacement by scar during ischaemia | Myocardial infarction. {Duncker, 2000 #14300; Chen, 2008 #7585} CIRCUS trial failed to meet primary endpoints136 |
| Cell and tissue interventions | |||
| Bone marrow-derived stem cells (e.g., BMCs, CD34+ cells, MSCs) |
Multiple cells: endothelial cells, myocytes, fibroblasts | Paracrine modulation of wound healing and scarring | Myocardial infarction, ischaemic heart failure149, 150, 153 |
| Adipose-derived cells (e.g. MSCs) |
Multiple cells: endothelial cells, myocytes, fibroblasts | Paracrine modulation of wound healing and scarring | Myocardial infarction200 |
| Endogenous cardiac stem cells (e.g., cardiospheres, c-Kit+ cells) | Multiple cells: endothelial cells, myocytes, fibroblasts | Paracrine modulation, Possibly through myocyte differentiation | Myocardial infarction, ischaemic heart failure155, 156 |
| Cardioprotective procedures (e.g., remote preconditioning) | Myocytes | Prevention of muscle loss and replacement by scar during ischaemic events | Myocardial infarction201 |
| Fibroblast reprogramming (e.g. GMT) |
Cardiac fibroblasts | Convert scar-forming fibroblasts into myocytes | Myocardial infarction110–112, 122, 202 |
| Cellularized ECM Scaffolds (e.g., with iPSC-derived myocytes) | Cardiac scar | Revision/replacement of scar tissue with new myocardium | Myocardial infarction13, 15, 203 |
| miRNAs, lncRNAs etc | |||
| AntimiR-21 | Fibroblasts | Inhibition of fibrosis | Kidney fibrosis, myocardial infarction204 |
| AntimiR-92 | Endothelial cells | Improvement of angiogenesis, cardioprotection | Myocardial infarction205 |
| Other | |||
| Angioplasty | Blocked coronary arteries | Balloon catheter used to re-establish blood flow to prevent replacement of muscle by scar | Myocardial infarction194 |
| Thrombolytic therapy (e.g., tPA) |
Blocked coronary arteries | Clot-dissolving drug used to re-establish blood flow to prevent replacement of muscle by scar | Myocardial infarction182, 206 |
| Cardiac resynchronization therapy | Ventricular stress/strain patterns | Promotes reverse remodelling | Myocardial infarction207 |
| Dietary sugar reduction | p300 | Inhibit fibroblast activation, ECM expression | Diabetes-induced heart failure208 |
Substances or strategies that are or are close to clinical use are included.
The individual and societal costs of heart diseases linked to fibrosis are staggering.9 In the case of ischaemic heart disease alone, the American Heart Association reports that each year approximately 635,000 Americans are hospitalized or die from their first MI. More than half survive, but each year around 280,000 patients will go on to suffer subsequent coronary events, leading to further loss of cardiac muscle and additional deposition of non-contractile and potentially arrhythmogenic fibrotic tissue. The direct costs arising from ischaemic heart disease in the USA are in excess of $180 billion dollars per year. Heart failure after MI represents a further annual burden of $20–40 billion dollars.
There is a strong clinical and economic case, therefore, for developing new and effective approaches to targeting cardiac disease processes linked to fibroblast function. This Review will consider the origin and roles of fibroblasts, illustrate their involvement in current and emerging clinical interventions, and identify future targets for basic and applied heart research.
Fibroblasts and adult myocardial homeostasis
In the healthy heart, fibroblasts are the main cell type involved in formation and maintenance of connective tissue.1, 3, 10 Cardiac connective tissue consists of cellular and acellular components, with the acellular extracellular matrix (ECM) providing a flexible scaffold for individual myocytes and for the heart as a whole.11 The highly organized collagen-rich meshwork, laid down by fibroblasts, stabilizes the myocardial wall and supports force transmission, while allowing for intricate patterns of tissue deformation.12 This fibrous skeleton also provides regions that act as barriers and supports, for example establishing myo-fascial planes between sheetlets of muscle, reinforcing conduits for blood vessels, separating electrical activation of the atria and ventricles, or insulating bundles of the cardiac conduction system.
The fibrous skeleton of the heart also provides a substrate for tissue-engineered repair.13 In these experiments, perfused rodent hearts were decellularized, leaving the ECM scaffold, including the ECM surrounding the coronary vascular tree, intact. This ECM scaffold was then repopulated with neonatal myocytes, which – based on the available structural cues – settled to recapitulate a beating, heart-like organ. Whilst regeneration of a mature heart in toto by this approach remains a distant prospect, initial clinical applications of this method could include use of decellularized ECM patches for surgical repair.14 These cardiac ECM scaffolds can be repopulated with induced pluripotent stem cells (iPSCs) – raising the prospect of patient-specific reparative therapies.15 Conceptually, this builds on breakthroughs, over the past 10 to 15 years, in the pre-clinical and clinical application of decellularized allo- and xenografts for heart valve repair and replacement,16 including the limitations and challenges associated with the approach.17
In addition to the maintenance of connective tissue, cardiac fibroblasts express a wide array of paracrine factors and cytokines and have complex biochemical and biophysical interactions with myocytes, thereby influencing development, growth, and functional adaptation of muscle cells.1, 3, 5 Paradoxically, given the aforementioned role of fibrous tissue in insulation, there is evidence for direct electrical communication via heterocellular connexin (Cx) junctions between fibroblasts and myocytes18–20– interactions that may well have implications for the modulation of electro-mechanical activity of heart muscle in health and disease.2, 7, 21 Thus, fibroblasts form physiological signalling hubs whose relevance extends well beyond classic roles in the generation and maintenance of ‘mechanically connective, yet electrically divisive’ tissue.
Developmental origins of fibroblasts
One of the impediments to developing new mechanistically based strategies to target fibroblast function in disease has been an incomplete understanding of their multi-lineage derivation and potential regulatory interactions between mesenchymal cells of differing origin. A characteristic feature of fibroblasts is the ability to generate ECM; however, this functional capacity is frequently left unconfirmed when classifying non-myocytes in vitro or in vivo. Nonetheless, considerable progress has been made over the past 25 years, and the associated new insight is beginning to affect our understanding of the normal and diseased heart (Fig. 1).
Figure 1. Key developments and changing focus of cardiac fibroblast research.
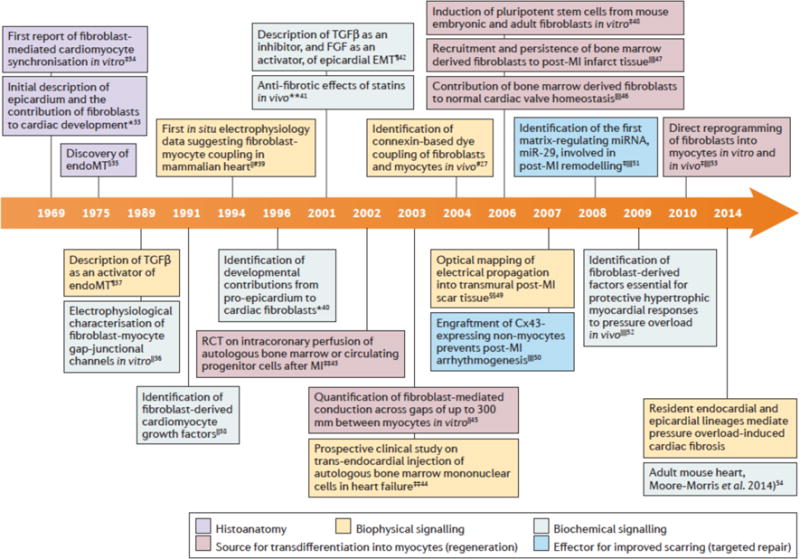
This timeline shows key articles on cardiac fibroblasts. Early research in this area was predominantly descriptive of histoanatomical structures, then changed to also describing biophysical and biochemical signalling. Since the early 2000s cardiac fibroblast research has also included the roles of fibroblasts in myocyte transdifferentiation as a potential source for regeneration as well as their potential to be utilized to improve scarring. *Embryonic chick heart; ‡cultured cells from embryonic mouse heart, §embryonic rat heart, ║cultured cells from neonatal rat heart, ¶cultured cells from embryonic chick heart, #adult rat heart, **transgenic rabbit model of hypertrophic cardiomyopathy, ‡‡humans, §§adult rabbit heart, ║║adult mouse heart. Cx43, connexin 43; EndoMT, endothelial to mesenchymal transition; FGF, fibroblastgrowth factor; MI, myocardial infarction; miRNA, microRNA; RCT, randomized clinical trial; TGFβ, transforming growth factor β.
a) Manasek 1969 J Embryol Exp Morphol. 1969,(3):333–4822
b) Goshima & Tonomura, Exp Cell Res 1969,56:387–39223
c) Markwald et al. Dev Biol. 1975 Jan;42(1): 160–8024
d) Rook et al. Pflugers Arch. 1989/414:95–825
e) Potts and Runyan Develop Biol 134, 392, 198926
f) Long et al. Cell Regul 1991,2(12): 1081–9527
g) Kohl et al., Exp Physiol 1994,79:943–5628
h) Mikawa and Gourdie Develop Biol. 174, 221–23229
i) Morabito et al., Develp Biol., 234, 204, 200130
j) Patel et al., Circulation. 2001 Jul 17;104:317–2431
k) Assmus et al., Circulation: 2002, 106, 3009–1732
l) Perin et al., Circulation: 2003, 107, 2294–30233
m) Gaudesius et al. Circ Res 2003, 93:421–834
n) Camelliti et al. Circ Res 2004, 94:828–83519
o) Visconti et al., Circ Res 2006, 98:690–635
p) Möllman et al., Cardiovasc Res 2006, 71:661–7136
q) Takahashi and Yamanaka, Cell, 126, 2006 663–7637
r) Walker et al. J Cardiovasc Electrophysiol. 2007 Aug;18(8):862–838
s) Roell W. et al. Nature 2007, 450, 819–82439,
t) van Rooij et al., Proc Natl Acad Sci U S A, 2007 105, 13027–3240
u) Takeda et al. J Clin Invest, 2009, 120, 254–6541
v) Ieda et al, Cell 2010/142: 375–38642
w) Moore-Morris et al. J Clin Invest. 2014 Jul;124(7):2921–3443
The pro-epicardium – a sprout-like cluster of extra-cardiac cells that first comes to prominence at the looped-tube stage of cardiac development29, 44 – provides the source for a thin mantle of epicardial epithelium that progressively envelopes the embryonic heart.22, 44 Epithelial-mesenchymal transition (EMT) at the epicardium is the largest initial source of resident interstitial cells (Fig. 2).29, 45, 46
Figure 2. Cardiac fibroblast and myofibroblast origins in health and disease.
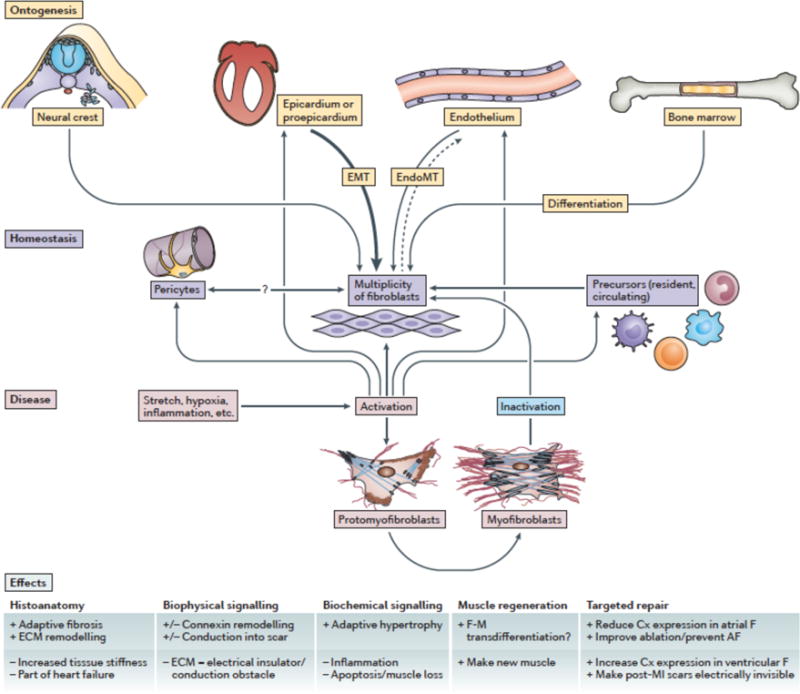
Cardiac fibroblasts originate from various sources, including the (pro-)epicardium, cardiac endothelium, neural creast and bone marrow. They form amultiplicity of cells, with hitherto ill-defined precursurs (both resident and circulating), subtypes, and relations to pericytes. Fibroblast activation by pathological stimuli, including stretch, hypoxia, and inflammation, lead to phenotype-conversion via proto-myofibrobalsts to myofibroblasts. For detailed explanations, see text.
Recent data suggest that the pro-epicardium itself is an evolutionary derivative of the primordium of an ancient pro-nephric glomerulus,47 suggestive of interesting phylogenetic links between fibrotic processes in kidneys and heart. Paracrine signalling by fibroblast growth factors (FGFs), bone morphogenetic proteins, retinoic acid, various members of the transforming growth factor β (TGFβ) family,4, 48–50 as well as transcriptional activators including Wilms tumor 1 (WT1) protein,51 and T-box proteins-18 and 20,52, 53 have been identified as early regulators of epicardial EMT. These proteins affect pathways including canonical and non-canonical Wnt signalling and hedgehog and retinoic acid signalling, which have downstream effects on gene programmes that regulate intercellular interactions, actin dynamics and cell motility. Following invagination of mesenchyme from the embryonic epicardium into the myocardium, various signal transduction and regulatory molecules, including TGFβ,54, 55 FGF,56 platelet derived growth factor (PDGF),57 proto-oncogene tyrosine-protein kinase,55 rho kinase,58 integrins,59 popeye domain-containing genes such as Bves,60, 61 serum response factor,62 transcription factor 21 (Tcf21),63, 64 and myocardin-related factors65 govern the transition of epicardium derived cells (EPDCs) into cells with more mature fates, including fibroblasts.5, 6 There is also evidence from studies in Xenopus laevis that Tcf21 functions as a transcriptional repressor to regulate proepicardial cell specification and the correct formation of a mature epithelial epicardium.64
Pericytes form a distinct cohort of mesenchymal cells that reside at perivascular locations.4 Pericytes can express stem cell markers (e.g., Oct4, Sox2)66 and may be fibroblast progenitor cells that are capable of self-renewal and differentiation into more mature mesenchymal phenotypes. There are unresolved questions as to whether pericytes contribute to cardiac fibrotic remodelling.67 Prominent fibrosis around blood vessels is a feature of many diseases of the heart, and there is evidence for phenotypic variance between quiescent and activated fibroblasts at perivascular and interstitial loci.3, 4
Mesenchymal cells can also arise in the embryonic myocardium through endothelial-mesenchymal transition (EndoMT) of endocardial endothelial cells,68 as well as from migratory populations of neural crest cells (Fig. 2).69 Cells of endocardial origin primarily contribute to the developing inlet valves, whereas neural crestderived cells appear to be largely confined to the connective tissue of the great vessels and, possibly, the cardiac outflow tract.70 Minor populations of fibroblast-like cells that originate from these sources are also resident in homeostatic myocardial tissues. Notable in this respect are neural crest-derived mesenchymal cells in the atria71 and around proximal components of the cardiac conduction system.72
The tissues of the embryonic heart are thus subject to waves of immigration of mesenchymal progenitors from heterogeneous sources, with each of these different populations demonstrating some specificity in their spatiotemporal and, possibly, functional assignments in the developing organ.
Fibroblasts in the adult heart
Myofibroblasts
Fibroblasts are changeable in character. A pertinent aspect of this phenotypic variability is the transition of quiescent fibroblasts, via a proto-myofibroblast intermediary stage, into activated fibroblasts or myofibroblasts (Fig. 2).1, 3, 5, 6 Pathways involved in the conversion of fibroblasts into myofibroblasts are thought to include TGF73 endothelin-1,6, 74 angiotensin II (Ang-II),75 and Wingless-related integration site (Wnt)/β-catenin signalling.3, 6 The gap junction protein connexin 43 (Cx43) has been found to regulate TGFβ signalling in a non-channel-dependent manner,76 and knockdown or over-expression of Cx43 inhibits or potentiates TGFβ-induced myofibroblastic phenoconversion, respectively.77 Other signal transduction pathways implicated in regulating phenotypic conversion of quiescent fibroblasts into myofibroblasts include the peroxisome proliferator-activated receptor γ, JNK and Akt signalling pathways; some effectors of these pathways are also downstream of TGFβ signalling.1, 5 The cell membrane protein caveolin-178 and a number of proteins mediating interactions between cell membrane receptors and the ECM (so-called matricellular proteins), including thrombospodins,79 SPARC,80 periostin,81 tenascin-C,82 and extracellular matrix-associated proteins (named CCN as an acronym derived from the first three proteins discovered in this family)83 are also involved in regulating myofibroblast transdifferentiation. Whilst their contribution to heart function is evident, the specific roles of each of the matricellular proteins remain poorly characterized, but probably include the generation of a conducive mechano-environment, effects on directed migration, stress fiber formation and the production and/or activation of signalling factors, such as TGFβ1, that promote the conversion of fibroblasts to myofibroblasts.84
Many of the pathways governing the transition of fibroblasts to myofibroblasts are mechano-sensitive85–87 and potentially involve stretch-activated ion channels and changes in cellular calcium handling.88–90 Myofibroblasts are able to generate mechanical force, which is important for scar contraction and remodelling.91 This is a consequence of upregulation of proteins involved in coordinated force- and tension-generation, including α smooth muscle actin (αSMA), myosins and junctional molecules such as cadherins and Cx43. These cells also have important roles in the active secretion and turnover of collagen and other ECM molecules10 and are responsive to hypoxia, cytokines and signalling molecules associated with the inflammatory milieu, such as TGFβ, tumor necrosis factor α, interleukin-6 (IL-6), and IL-2.1, 3, 5, 6, 92 Activated fibroblasts are a common component of granulation tissue throughout the body, typically peaking in density at 4–6 days post-injury. An interesting difference between wound healing in skin vs. heart is that myofibroblasts appear to persist (for up to 17 years!) in cardiac scars.93 The mechanism for their preservation in healed post-MI scars may be related to the interplay between endothelin, angiotensin and TGFβ signalling. Maintenance of myofibroblasts in the scar post-MI is thought to confer mechanical benefits: murine strains with high densities of myofibroblasts in healed infarcts showed improved ventricular function and reduced propensity for scar expansion and ventricular dilation.94
Resident and non-resident mesenchymal cell populations
The heterogeneous origin and phenotypic variability of fibroblasts pose technical challenges for experimental cell identification. Expressed proteins, including discoidin domain receptor tyrosine kinase-2 (DDR2), periostin, vimentin, fibronectin ED-A, WT1, PDGF receptor, TCF21, αSMA and CD90/Thy1, are commonly used to identify fibroblasts and myofibroblasts.4 However, these markers (including some whose naming wrongly implies fibroblast-specificity, e.g. so-called fibroblast-specific proteins such as FSP-1)95 may not be uniquely or continuously expressed and they do not, therefore, provide a means for reliable tracking of the differentiation history of mesenchymal cells.
Basic studies of cell fate in chick29 and mouse4, 96 embryos using genome integrating virus and transgenic lineage markers, respectively, were key to the demonstration that early populations of cardiac fibroblasts are derived from the pro-epicardial organ. More recently, genetic fate-mapping tools have contributed to new insights into fibroblasts in adult heart and disease. Using Cre-expressing lines and a collagen1α1-green fluorescent protein (GFP) fusion reporter, the majority of fibroblasts in a mouse model of fibrosis, induced by pressure overload, were shown to be derived from cells resident in the heart, mainly from epicardial sources.43 Concurrently, a study in which mice expressing Tbx18-Cre, Tie2-Cre and Pax-3-Cre were used to determine the origin of fibroblast lineages came to a similar conclusion – although a minor contribution by neural crest-derived cells was also noted.71
A twist was recently added to this story. Cre-LoxP technology was used to track epicardial- and bone marrow-derived mesenchymal cells,97 and although pressure overload did indeed trigger fibroblastic differentiation predominantly from resident EPDCs in these experiments, collagen deposition and scar formation following MI was determined by interactions between cells originating from both the bone marrow and the pro-epicardium. This study further suggested that regulatory interactions between the two different mesenchymal populations determined the transition to pathological levels of fibrosis – though it did not provide data that directly supported this interesting hypothesis. Notably, this report used WT1-Cre, which, because of its activation at the earliest stages of pro-epicardial differentiation, tags of a range of cardiac cells, largely with non-myocyte phenotypes.
The conclusions of this work,97 however, support earlier studies that detected hematopoietic contributions to mesenchymal cell populations in the heart, including to post-MI scar formation.36, 98 These reports were based primarily on chimeric mouse models, wherein genetically tagged (e.g., GFP) bone marrow cells were transplanted into irradiated (bone-marrow incompetent) animals. The tag was then used to trace the fate of marked cells. Using this method, it was estimated that 28–57% of fibroblast-like cells in post-MI scar tissue were of bone marrow origin, with a third of these cells adopting a myofibroblastic phenotype.
In a noteworthy extension of this experimental approach, hematopoietic stem cells were evaluated in a clonogenic manner in vivo by repopulating lethally irradiated murine recipients with a single GFP-marked bone marrow-derived stem cell clone.98 Following surgically induced MI, clonally derived GFP-expressing cells were found to densely populate post-MI scars. These cells were of fibroblastic morphology and persisted in scar tissue 30 days post-MI, a time point at which the inflammatory response was already largely resolved.
Bone marrow-derived, or myeloid fibroblasts (also known as fibrocytes) are recruited to injured loci as monocytes from the blood via the stromal cell derived Factor 1α/chemokine receptor type 4 signalling axis. They undergo a further transition to CD45+ mesenchymal cells under the influence of cytokines, including IL-6 and monocyte chemoattractant protein-1 (MCP-1).99 Myeloid fibroblasts contribute to non-adaptive cardiac fibrosis and adverse remodelling, particularly post-MI and during ischaemic cardiomyopathy, heart failure and aging.99, 100 In this context, TNF signalling, which regulates the transition of macrophages from an M2 phenotype (immunosuppressive, pro-angiogenic macrophages) to fibroblast-like cells, could be particularly relevant, as has been shown in models of heart disease.101
Finally, endothelial cells in the adult heart may undergo EndoMT and contribute to fibrosis after myocardial injury.102 In this process, endothelial cells lose their endothelial identity and acquire mesenchymal or myofibroblastic markers, such as αSMA expression and type I collagen secretion. Similar to EMT, EndoMT can be regulated by TGFβ.102 Interestingly, recent studies suggest that this process can also be reversed, and mesenchymal cells can generate coronary vascular endothelial cells by mesenchymal-endothelial transition in a p53-dependent manner.103
Thus, available evidence suggests that three main cell populations contribute to fibrosis after injury or in pathologic conditions in the adult heart: resident fibroblasts derived from EPDCs, mesenchymal populations originating from circulating precursors and cells that have undergone EndoMT (Fig. 2). There may also be a fourth minor contribution from neuroectoderm.71 EPDCs seem to be the major contributor to fibroblast cell numbers overall, but ongoing studies may well uncover disease-specific responses by different lineages, including regulatory interactions between the different mesenchymal populations that vary according to pathology.
Work on targeting or using mesenchymal cells for treating cardiac disease is broad and intensive.104 Key foci of current translational research in this context are on regenerating heart muscle lost to disease by reprogramming fibroblasts to trans-differentiate into cardiomyocytes, and on reducing fibrosis or the extent of pathologic scarring that occurs in response to injury and/or disease (Box 1)
Box 1. Effects of fibroblasts on cardiac physiology.
Fibroblasts affect numerous aspects of cardiac form and function.
Altered histoanatomy:
-
-
adaptive fibrosis
-
-
remodelling of the extracellular matrix (ECM)
-
-
increased tissue stiffness
-
-
contribute to heart failure
Changes in biophysical signalling:
-
-
alterations in connexin localisation and function
-
-
altered conduction into the scar
-
-
increased ECM can act as an electrical insulator, thereby reducing electrical connectivity
Changes in biochemical signalling:
-
-
adaptive hypertrophy
-
-
increased inflammation
-
-
apoptosis, resulting in muscle loss
Cell differentiation:
-
-
Fibroblast to myocyte transdifferentiation
-
-
Endothelial- or epithelial-mesenchymal transition
-
-
Fibroblasts may affect the microenvironment by paracrine signaling
These fibroblast-induced changes could be harnessed to regenerate or repair muscle. For example, fibroblasts could be targeted in patients with atrial or ventricular fibrillation to prevent the disease or improve the efficacy of catheter ablation, or to make scars in patients who have had myocardial infarctions electrically invisible.
Fibroblast reprogramming in cardiac repair
Fibroblast reprogramming falls into the burgeoning field of regenerative medicine. Whereas the adult mammalian heart repairs by forming a scar, amphibians and fish can regenerate injured myocardial tissue.105, 106 There have been numerous tantalizing hints that therapies could be developed which recapitulate or simulate regenerative processes similar to those occurring in lower vertebrates, most recently from studies of reparative responses in newborn mice.107, 108
The rationale for exploring whether fibroblasts can be reprogrammed into myocytes as a cardiac regeneration strategy can be traced back to the Nobel prize-winning discoveries of Yamanaka and colleagues, who reported the generation of iPSC from somatic cells by retrovirus-mediated overexpression of a combination of four transcription factors – Oct4, Sox2, Klf4 and c-Myc.37 Subsequently, it was shown that forced expression of lineage-specific factors could reprogramme cells into defined phenotypes, without reverting to an intervening stem cell.109 In cardiac biology, Gata4, Mef2c, and Tbx5 (collectively referred to as GMT), are sufficient to prompt the trans-differentiation of murine cultured fibroblasts into cardiomyocytes with early ontogenetic traits.42 The GMT cocktail –subsequently extended by adding a fourth transcription factor, the heart and neural crest derivatives gene 2 (Hand2) – improved cardiac function in a murine model of MI.110, 111
In parallel, micro-ribonucleic acid (miRNA) was shown to reprogramme cardiac fibroblasts into cardiomyocytes in vitro and in vivo.112 Priming of EPDCs with the actin-binding peptide thymosin-β4 also triggers trans-differentiation of these cells into myocytes – albeit at very low frequency113 and potentially not in clinically relevant settings.114
The path to cardiac reprogramming has not been smooth. In a replication study, the GMT-induced conversion was relatively inefficient.115 This work also indicated that the electrophysiological phenotype of converted fibroblasts might not align with that of cardiomyocytes. Methodological differences between studies may underlie variable observations. For example, vector-dependent imbalances in protein expression of the G, M, and T factors have been proposed as one reason underlying study-to-study variation.116 A further impediment to translation may come from the finding that human cardiac fibroblasts appear to be resistant to reprogramming by GMT, though may convert at low efficiency to immature beating myocytes if GMT is combined with Hand2, myocardin and two muscle-specific miRNAs: miR-1 and miR-133.117
Ongoing work on fibroblast reprogramming has focused on improving its reliability, safety and efficiency. These efforts include the optimization of the stoichiometry of factors in the reprogramming cocktail;116 identification of combinatorial miRNA treatments;118 development of delivery methods other than genome-integrating retroviruses;119 and attempts to generate specific cardiac phenotypes, including atrial, ventricular and pacemaking cells,120 with the long-term aim of producing cells with adult-like structural and functional properties. In addition, manipulation of cell transduction pathways including those associated with TGFβ121 and Akt1 signalling122 could increase the efficiency of cell reprogramming. Enhancing Akt1 activation notably increased the efficiency of fibroblast to myocyte conversion in vitro.122 Interestingly, thymosin β4, which activates Akt, has been reported to enhance function post-MI in the presence of GMT reprogramming.110
Novel drugs and biologics
Partial recovery of disease-induced changes in structural and functional tissue properties (reverse remodelling) is a target of clinical interventions,123 in part because fibrosis is an independent predictor of arrhythmogenesis and sudden death in patients.124, 125 Several established pharmacological therapies that are known to reduce arrhythmias or progression of heart disease affect connective tissue, including ACE inhibitors, aldosterone antagonists and statins.1, 3, 6 Novel cardioprotective therapies are being developed that preserve myocardial muscle, thereby inhibiting its replacement by scar tissue in the acute phase following cardiac injury. In addition, interventions, including cell therapies, may provide both cardioprotection and enhance the ongoing wound healing response of damaged hearts. Third, pharmacological approaches are also being developed to target chronic pathologic processes that lead to the accumulation of fibrotic tissue and heart failure. These are discussed in detail below.
Cardioprotection
The single most important contribution to reducing the fibrotic burden in ischaemic heart disease in the last 25 years has come from the advent of reperfusion procedures such as angioplasty and antiplatelet and antithrombotic medications, which restore blood flow to ischaemic myocardium in the immediate aftermath of MI.126 Myocyte viability is critically dependent on the availability of sufficient oxygen and thus swift re-establishment of coronary vascular flow following MI increases the survival of myocardial cells, thereby reducing post-infarction fibrosis. Whilst revolutionizing the critical care of MI patients, the generation of reactive oxygen species associated with reperfusion can itself cause damage to the heart, known as ischaemia-reperfusion injury, resulting in diminished contractile function and increased risk of arrhythmia.127
Ischaemic pre-conditioning, in which short episodes of hypoxia reduce infarct size upon subsequent MI,128 has inspired the search for therapeutic approaches that take advantage of, or mimic, this cardioprotective effect, in particular in the critical 1–3 hour window following coronary arterial occlusion.129 An important step in the search for clinically useful cardioprotection strategies has been the recent agreement on standardized animal models for preclinical screening.130 In addition to classic preconditioning, cardioprotection can also be induced by temporary hypoxia at body locations other than the heart (for example, a limb) prior to MI (remote preconditioning),131 acutely following MI (post-conditioning),132 or by subjecting the heart to mechanical stretch.133 All these may contribute to the beneficial cardiovascular effects of regular exercise.
Novel approaches to pharmacologically induce cardioprotection include targeting signalling through mitochondrial-associated protein kinase C and its substrates, such as aldehyde dehydrogenase 2, which reduces ischemic damage by reactive aldehydesCompounds inhibiting the mitochondrial permeability transition pore (mPTP) may also mitigate changes that initiate mitochondrial-driven cardiomyocyte death.129, 135 This being said, the mPTP blocker cyclosporine A recently failed to meet endpoints in the CIRCUS trial – a study designed to determine whether this inhibitor protected hearts from ischaemia/reperfusion injury.136 Other experimental and clinical-stage therapeutics that may mimic pathways activated in ischemic preconditioning include phosphodiesterase 5 inhibitors (such as Viagra),137 chloride channel and transporter modulators,138 hydrogen sulfide,139 rapamycin,140 and nitric oxide (NO) donors.141 Overexpression of extracellular superoxide dismutase has been shown to increase NO bioavailability and provide cardioprotective benefit in transgenic mouse models.142
Connexin hemichannels (connexons) are single membrane channels in transit to docking within gap junctions. Hemichannels have recently come into focus as another novel determinant of acute injury severity following MI.143, 144 The majority of hemichannels in the cardiomyocyte cell membrane reside in a zone surrounding the gap junction, called the perinexus.145 In response to an ischaemic insult, hemichannels activate, opening non-selective pores that discharge high concentrations of pro-inflammatory, pro-fibrotic molecules such as adenosine triphosphate (ATP), while admitting cytotoxic levels of Na2+ and Ca2+ into cells.144 There is also evidence that Cx43 hemichannels act in concordance with mPTP channels in response to ischaemic injury.146 The short peptides Gap26/27, αCT1 and Gap19 mimic key functional domains of Cx43, and have all been shown to inhibit hemichannel activity (Gap26/27 blocks gap junction channels as well).147 Gap19 has cardioprotective effects, significantly decreasing infarct size in a mouse MI model.144 Gap26 confers cardioprotection post-injury in a rat MI model in vivo: intra-arterial injection of the peptide 30 minutes after coronary artery ligation reduced infarct size by 61%.143 Interestingly, the same experimental hemichannel blockers have proved effective in reducing glial scarring, providing neuroprotection following ischaemic stroke in animal models.148
Cell therapies
Mesenchymal stromal cells (MSCs), haematopoietic and endothelial progenitor cells, as well as mononuclear cells that are derived from bone marrow and other sources are at the core of a concerted effort in experimental reparative therapy development.149, 150 Supported by preclinical studies in animal models, several groups have tested cell therapies in patients with MI. Many, but not all, trials reported modest increases in ejection fraction or other signs of functional improvement, and some meta-analyses suggest that cell therapy with bone marrow mononuclear cells or mesenchymal cells may assist in the recovery of function of injured or diseased hearts.149, 151
However, thus far analyses have been based on small or moderately sized clinical investigations. Given the heterogeneity of both the cell preparations used and the patient populations studied, such analyses should be interpreted with caution.152 The US clinicaltrials.gov website lists more than 300 trials involving cardiac cell therapy, the majority of which are early Phase I and II safety and efficacy studies. Outcomes from Phase III clinical trials involving thousands of patients are still missing but are necessary to establish whether or not largely bone marrow or adipose tissue-derived mesenchymal populations reliably provide clinically meaningful improvements, on top of current standard-of-care. A further note of caution on these therapies came from meta-analyses, such the ACCRUE review of 12 randomized clinical trials, of the effects of intracoronary cell therapy on ischaemic heart disease, which failed to identify patient benefit, in terms of either clinical events or improved left ventricular function.153
Cardiac stem cells have been suggested to be, or at least include, resident progenitor cell populations in the heart, with potential roles in regenerating myocardium.154 Various surface antigens such as tyrosine-protein kinase Kit (c-Kit) or Stem cell antigen-1 (Sca-1), or spheroid cultivation assays, have been used to isolate cardiac stem cells. Engraftment of different cell populations improves cardiac function and reduces infarct area in mouse models, and two first-in-human clinical trials assessing c-Kit+ (SCIPIO)155 and cardiosphere-derived cells (CADUCEUS)156 reported promising results. However, the contribution of cardiac stem cells to regeneration of the heart is still a matter of debate, as is the potential for heart muscle cells to contribute to self-repair by proliferation.157 Whereas mouse heart failure models have suggested that resident c-Kit+ cells may affect cardiac repair by direct cardiomyogenic contributions,158 Cre-reporter-based experiments have demonstrated that c-Kit expression is largely confined to non-myocyte cardiac lineages.159 The latter finding is consistent with other reports, which found that the cardiogenic potential of c-Kit expressing cells in the adult heart is limited,160 and that c-Kit predominantly labelled cardiac endothelial cells in developing and adult hearts.161 Another study suggested that the large majority of c-Kit-derived muscle cells were pre-existing c-Kit-expressing cardiomyocytes, rather than cells formed de novo from CSCs.162 Similarly, lineage tracing of Sca-1-positive stromal cells revealed only a modest contribution of these cells to cardiovascular lineages after injury and during aging.163
Unfortunately, the debate on cardiac cell therapy has, in a few high-profile cases, moved beyond scientific controversy.164 The emphasis of the field has also shifted from regeneration to the potential cardioprotective effects of cell therapies – effects that, in large part, are now thought to be mediated by paracrine mechanisms. This latter view is consistent with the fact that many of the paracrine factors released by the applied cells are known to influence fibrosis, angiogenesis and inflammation, and by these means, affect cardiac healing responses and scar properties.165 Matrix remodelling can be influenced by the release of matrix-modulating enzymes, such as matrix metalloproteases,166 or by modulating expression of tenascins.167 The secretion of HGF,168 adrenomedullin,169 and thymosin-β4170 may also directly affect cardiac fibrosis. Interleukins may act to inhibit cardiac fibroblast proliferation and collagen production as well as myofibroblast differentiation,171 while MSC-derived leptin was shown to inhibit fibroblast activation by blocking pathways involving TGFβ/‘small mothers against decapentaplegic’ (Smad) and Myocardin-Related Transcription Factor-A.172
Detailed analysis of bone marrow mononuclear cell secretomes has led to the identification of novel cardioprotective proteins, such as myeloid-derived growth factor.173 In addition to secreted proteins and growth factors, cells of different origins can release extracellular vesicles, such as exosomes, which contain various bioactive molecules, including microRNA.174 Cell therapies, thus, may profoundly alter the paracrine microenvironment in the healing heart, thereby not only affecting mesenchymal cell activity and trans-differentiation, but also enhancing regeneration, potentially by inducing cardiomyocyte proliferation,175 albeit at rates as low as 1% in 25 years.157
The low homing and survival rates of externally applied cells in scar tissue, however, are likely to limit the duration of paracrine protective actions.176 Augmenting homing and survival, either by pre-treating the engrafted cells with pro-survival factors, genes or miRNA, or by preconditioning the target tissue, may enhance the efficacy of cell therapy.176 With ongoing development of the field, it will be interesting to see whether cell-free cocktails of bioactive molecules can be formulated that supersede cell-injection based therapies.177
Antifibrotic therapies
Congestive heart failure, a progressive and ultimately incurable disease, is marked by the increasing inability of the ventricle to pump blood sufficiently to meet the demands of the body. Replacement of cardiac muscle by fibrotic tissue is thought to be at the pathogenic nidus of heart failure.1, 3–6 The acute loss of muscle, such as sparked by MI, viral infection, or drug toxicity, could be the precipitating cause of heart failure, and is followed by disease progression. Fibrosis at interstitial and perivascular locations in the failing ventricle can also occur as part of longer term processes associated with aging or diseases such as hypertension, aortic stenosis, metabolic disease and obesity. In the atria, connective tissue accumulation is an underlying cause of atrial fibrillation. Preventing, or at least inhibiting, the accumulation of pathologic levels of fibrotic tissue in both ventricular and atrial myocardium is thus a major therapeutic goal.
Exploration in this area is focused on anti-fibrotic activities of established drugs, and on novel compounds. Examples of the former category include statins, which are anything but new and are among the most widely prescribed drugs of the last quarter century. Their primary mode-of-action is to lower cholesterol levels via inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase.178 It has emerged from preclinical and clinical studies that certain statins also inhibit scarring, fibrosis and adverse myocardial remodelling.31, 179, 180 The mechanism through which statins inhibit fibrosis is not entirely clear, though simvastatin inhibits trans-differentiation of cardiac fibroblasts into myofibroblasts, an effect that is overcome by application of TGFβ.181 Other established drugs that have anti-fibrotic actions include aldosterone antagonists and angiotensin receptor and ACE inhibitors.3
A number of recent reviews have summarized preclinical and clinical progress on novel therapeutics, which are only briefly covered here (Table 1).1, 3–6 Experimental or clinical stage antifibrotic compounds include TGFβ pathway inhibitors,209–214 matrix metalloproteinase inhibitors,10 inhibitors of mast cell function,195, 215 monoclonal antibodies neutralizing the monocyte chemo-attractant MCP-1,188 chemokine interferon-gamma-inducible protein IP-10/CXCL10,216 endothelin pathway inhibitors,217 Ang-II pathway inhibitors,184, 185 and modulators of Wnt-signalling.196, 197
Other new anti-fibrotic targets include the proto-oncogene ski, the basic helix-loop-helix transcription factor scleraxis, and the cell membrane proteins β3 integrin and caveolin-1. Ski inhibits TGFβ signalling by binding to downstream transcriptional regulatory proteins in the TGFβ/Smad pathway.218 Overexpression of ski in isolated cardiac myofibroblasts was found to result in phenotypic reversion, marked by reduced collagen expression and myofibroblast contractility.219 Scleraxis is a key regulator of collagen synthesis in cardiac fibroblasts, with potential regulatory roles in fibroblast to myofibroblast differentiation.220
Mice deficient for β3 integrin showed substantial decreases in pressure overload-induced fibrosis, suggesting the involvement of this integrin in maladaptive ECM accumulation.221 By contrast, loss of caveolin-1 function in mice following MI222 and in isolated human atrial fibroblasts223 resulted in TGFβ1 associated fibrosis and upregulation of pro-fibrotic genes. Whilst the path to clinical translation of ski, scleraxis, and β3 Integrin to druggable targets is still in exploratory phases,224, 225 peptidergic drugs targeting caveolin-1 function have demonstrated promise as anti-fibrotic therapeutics in studies of lung fibroblasts from scleroderma patients.226
Relaxin-2, a neurohormone historically linked to pregnancy and parturition, is another novel biologic compound that has made the translational jump from basic research to the clinic (under the generic name serelaxin), and had efficacy in clinical testing of patients with acute heart failure.227, 228 The large placebo-controlled Phase III trial of 1,161 patients with heart failure, RELAX-AHF, reported significant improvements in patient symptoms, as well as decreases in their length of hospital stays and increased survival rates.191 It is presently unclear what molecular mechanism underlies the effect of relaxin on fibrosis. However, experiments in cell culture models indicate that relaxin inhibits and/or reverses myofibroblast differentiation induced by angiotensin and TGFβ, as assessed by changes in gene expression and markers of cellular electrophysiology.229, 230
A number of miRNA-based strategies have also shown potential recently for inhibiting cardiac fibrotic processes.231, 232 The first matrix-regulating miRNA to be identified in the heart, miR-29, targets several matrix proteins, including collagens.40 Over-expression of miR-29 in cardiac fibroblasts reduces collagen expression, albeit modestly.40 Antagomir-mediated silencing of miR-21 in vivo inhibits interstitial fibrosis and attenuates dysfunction in a murine ventricular pressure-overload model.204 Similarly, knockdown of miR-21 by anti-sense RNA in the atria was found to suppress atrial fibrosis in a rat model of MI-induced heart failure.233 However, in other studies, locked-nucleic acid anti-miR-based inhibition or genetic deletion of miR-21 failed to block the adverse ventricular remodelling response of mouse hearts subjected to pressure overload.234 Differences in the anti-fibrotic response may in part rely on the chemistry that was used to inhibit miR-21.233 Although the anti-fibrotic activity of anti-miR-21 in the heart is debated, a modified miR-21 antisense (RG-012) is currently being clinically tested in patients with kidney fibrosis (the ATHENA trial).235 Many additional miRNAs control myocyte survival after MI, thereby indirectly or directly affecting fibrosis.236 Inhibition of age-induced miR-34 expression can also decrease age-related cardiac fibrosis.237 Interestingly, miRNA may not only directly or indirectly target fibroblasts, but it is also released by cardiac fibroblasts. For example, fibroblast-derived exosomal miR-21-3p (miR-21*) acts as a potent paracrine-acting RNA molecule that induces cardiomyocyte hypertrophy.238
All in all, present conceptual approaches for therapeutic interventions are focused on avoiding or inhibiting fibrosis and/or on turning non-myocytes into working cardiac muscle. An alternative idea, providing a lower-hanging fruit of potentially high value, will be discussed next: the making of better scars.2
Re-engineering scars
Utilizing the natural reparative processes of fibroblasts to modify properties of the forming cardiac scar is quietly emerging as an exciting therapeutic avenue. Part of the rationale here comes from the recognition that, whilst fibrotic responses are common in the repair processes in the human heart, not all fibroblast-mediated cardiac remodelling is detrimental.41, 53, 94
Fibroblast lineage studies already hint at differences in regulatory interactions that determine fibrosis in response to ischaemic versus hypertrophic diseases.97, 99, 100 Furthermore, fibroblasts in the atrium and ventricle show differences in gene expression,239 electrophysiological characteristics,88, 240 and propensity for pro-fibrotic activity.241, 242
Cardiac scar formation is not always the result of disease. Interventions such as catheter ablation therapy intentionally generate scars in the heart to obliterate reentrant activation pathways, for example to alleviate atrial rhythm disturbances.243 On the other hand, pediatric cardiologists face a growing (and in some ways welcome) problem of managing adult patients with morbidities that are linked to the accumulation of scar tissue resulting from childhood surgeries to correct heart birth defects.244
It is important in this context to recall that not all cardiac scars are created equal. Post-surgery, de-novo generation of both ECM and cellular content are involved in scar generation, while post-ablation scars are re-cellularized on the background of pre-existing ECM. Post-MI scars, in contrast, contain not only native ECM but also surviving heart cells of different types, albeit with reduced myocyte content, caused by the pronounced sensitivity of these cells to oxygen starvation. These pose three potentially quite different pathophysiological settings.
The ability to personalize the properties of cardiac scar tissue to meet the needs of different types of scar and patient is thus an interesting and currently unmet clinical need.2, 245
Therapeutic potential of modifying scar mechanics
Notable aspects of the effort to “engineer a better scar” include insights into biomechanical inputs in fibrotic tissue differentiation, as well as the role of scar structure in determining the efficiency of cardiac output. Ventricular function has recently been recognized to be affected more by the alignment of collagen fibers within post-MI scars than by scar stiffness or collagen density.246 Collagen alignment, in turn, is a function of mechanical stress patterns that cardiac scars are subjected to during the contraction cycle.
Scar mechanical properties are also critical factors for the genesis of complications arising from ischaemic heart disease — including infarct rupture and scar expansion — that can occur after MI. Experimental therapies that attempt to modify mechanics and inhibit pathological remodelling of the infarct include mechanical restraints that limit or direct the motion of scars during the heartbeat,247, 248 injection of polymers to modify scar rigidity,249–251 MMP9 loss-of-function,252 and hydrogels containing tissue MMP inhibitors or viruses over-expressing them.253, 254 It is also of note that cardiac resynchronization therapy, an approved treatment for dilated heart failure, can promote reverse remodelling by normalizing regional stress/strain patterns across the infarcted ventricle.255
The risk of post-MI scar dilation and rupture is an important safety consideration for therapies targeting scar mechanical properties. The ventricle of the adult human heart generates systolic pressures of 120 to 180 mmHg, similar to mice and rats post-partum, but nearly one order of magnitude higher than pressures in utero (~30 mmHg) and about two orders above that of animals in whom myocardial regeneration is maintained into adulthood, such as the zebrafish (~2 mmHg).256 It seems a reasonable conjecture that the evolutionary emergence of higher blood pressure in adult mammals may have had an impact on the risks and benefits of healing the heart by scarring versus regeneration. In an illustration of this potential trade-off, adult mice in whom the gene encoding the ECM protein periostin has been knocked out are prone to ventricular rupture following MI.81, 257 However, those periostin knockout mice that survived exhibited reduced levels of scarring and improved cardiac performance.81
One might expect that therapies based on cardiomyogenic stem cells also pose increased risk of rupture and ventricular dilation, wherein infarcts could be more likely to show mechanical failure when healing is shifted towards regeneration and away from expeditious formation of a mechanically strong scar. Bone marrow-derived cells, which have limited cardiomyogenic potential, may be beneficial in this respect. Pharmacologically induced increases in recruitment of bone marrow-derived mesenchymal progenitor cells to MI in a mouse model was reported to be associated with reduced rates of ventricular rupture and death.258 These effects may provide a further explanation for how bone marrow-derived cell infusion contributes to clinical improvements following MI, and also suggest novel strategies to manipulate these cells, which to home-in on infarcts, to generate better scars. In an example of this approach, injection into femur bone marrow of a lentivirus expressing a small hairpin RNA targeting periostin prior to ventricular injury was reported to reduce scar size and improve cardiac function – without ventricular rupture that was found to occur following MI in mice with a global knockout of periostin.259 In this experiment, periostin knockdown was limited to bone marrow cells migrating to the MI, suggesting that selectively decreasing periostin expression in this extracardiac cell population may provide a therapeutic path to reduce scarring, and possibly promoting regenerative healing, following cardiac injury.
Therapeutic potential of modifying scar electrical properties
Fibrous, collagen-rich tissues in the heart have traditionally been viewed solely as non-conducting and electrically insulating structures. Undoubtedly, that is one of the most relevant functional effects of fibrosis, as observed more than a century ago.260 However, newer data has led to a more nuanced understanding of the electrical properties of cardiac scars and the implications of the electrical interaction between myocytes and non-myocytes.7, 21, 261 Since the 1960s, it has been known that fibroblasts and myocytes can couple electrically in vitro,23 and more recently it was confirmed that cultured fibroblasts can bridge the transmission of action potentials (APs) between otherwise separated clusters of myocytes across gaps of up to 300 μm.34 In an analogous example from the clinical literature, 10–20% of heart transplant recipients have been reported to develop electrical coupling across scar tissue at suture lines separating donor and recipient heart tissue.262 Similarly, electrical conduction across fully transmural scars can be seen after surgical correction of cardiac birth defects.263
Myocyte-fibroblast coupling may occur via connexin-based gap junctions,20, 264–266 which are far from uncommon in native myocardium.267 However, functionality of connexin-based fibroblast-myocyte coupling in native cardiac tissue has been corroborated only in rabbit sino-atrial node tissue.19 Underlying structures may also involve filamentous projections made by mesenchymal cells, called tunnelling nanotubes, through which ions and small molecules can be directly exchanged.268 Ephaptic conduction, a non-gap junction coupling-based method to transfer currents between cells, has been reported between myocytes and could also have a role in myocyte-fibroblast coupling.269, 270
In situ, cardiac fibroblasts exhibit complex and extended flat shapes, with irregular folds and elongated cell processes.18 To give a sense of their extended spatial scale in vivo, cardiac fibroblasts are typically an order of magnitude larger in the intact heart than when freshly isolated.2 Fibroblasts have membrane potentials of between −10 and −50 mV and a relatively high membrane resistance.2 Their extended morphology and elevated membrane resistance enhances the ability of fibroblasts to act as passive, long-distance conductors of electrical signals in vivo, including APs, when coupled to electrically active cells such as myocytes.264, 271 In quantitative mathematical simulations of heterocellular electrical cross-talk in the 1990s, using ‘passive’ fibroblast models, fibroblasts that were electrically coupled to muscle cells could affect cardiomyocyte excitability, altering heart rate and rhythm.272 More recently, based on emerging experimental insight into their electrophysiology, detailed ionic models of cardiac fibroblasts have been developed.273 Modelling studies have since provided indications that activated fibroblasts (myofibroblasts) may have specific electrophysiological relevance for arrhythmogenesis,274 a phenomenon that may relate to fibroblast-myocyte coupling and include changes in fibroblast membrane ion currents that occur following MI.2, 5, 7, 274–277 Both passive and active fibroblast models have been productive for theoretical assessment and hypothesis testing, at times preceding experimental validation.278–281 A major step towards more realistic computer simulations occurred with the advent of detailed three-dimensional scar reconstructions,282 paving the way towards clinically relevant prediction of scar effects on heart function, though thus far these studies have focussed largely on the classic view of scars as electrical insulators.283–285
In an influential study, ventricular scars were shown to be capable of conducting electrical signals;38 optically mapped AP waveforms were observed to conduct into scar tissue in a rabbit transmural MI model. Infarct tissue continued to support conduction even after sub-endocardial muscle layers were chemically ablated, thus excluding the possibility of electrical contributions from surviving contiguous myocardium. This observation was followed by reports from other teams,286, 287 indicating the possibility that nonmyocytes may serve as conduction pathways that link surviving myocyte islands inside post-MI scar tissue, thus allowing trans-scar conduction. Further support for this theory was provided at a recent Keystone conference, where data were presented demonstrating Cx43-dependent conduction into scar tissue in a fully transmural ventricular injury in a transgenic mouse model;288 another group reported direct evidence of conduction into non-myocytes of the scar border zone, using a fluorescent voltage reporter genetically targeted to non-myocytes by the WT1 promoter.289
Non-myocyte based trans-scar conduction could be used in novel therapeutic approaches. If, in a clinical setting, scar tissue could be rendered electrically “invisible” with respect to the propagating impulse, then the potential for infarcts to cause life-threatening arrhythmias might be reduced. For example, engraftment of Cx43-expressing cells into murine scar tissue prevents post-MI arrhythmogenesis.39 A peptide mimetic of the Cx43 carboxyl-terminus (αCT1) has been reported to reduce hemichannel activity and increase intercellular coupling by enhancing the recruitment of undocked connexin hemichannels into gap junctions.145 Similar to the engraftment of Cx43-expressing cells post-infarction,39 treatment of mouse ventricular injuries with αCT1 reduced the incidence of arrhythmia and increased conduction velocity, including through the infarcted section of the ventricle.198
Tissue-engineered constructs containing cardiac myocytes and fibroblasts have also been used successfully to conduct excitation from atria to ventricles in cases of experimental atrio-ventricular conduction block.290 This is another potential therapeutic application that depends on efficient heterocellular coupling, both among tissue graft components and at the graft-myocardial interface, both of which involve propagation of electrical signals across non-myocytes.261
The goal of engineering more conductive scars in post-MI patients is contrasted by the need for certain types of scars to remain impenetrable to electrical inputs from surrounding myocardial tissues, such as in the context of atrial ablation.243 For this procedure, focal energy delivery, e.g. from a radiofrequency catheter, is used to generate lesions that interrupt conduction of spurious excitation waves, thereby terminating atrial arrhythmias. Unfortunately, a large proportion of patients show re-emergence of functional conduction across intra-procedurally established ablation lines.291 Extrapolating from (and ‘inverting’) the electrophysiological effects of Cx43 over-expression in post-MI scar tissue, a decrease in Cx expression in atrial fibroblasts could potentially provide an approach to improving outcomes of atrial ablation procedures. Alternatively, one could build on an engineering approach in which Cx43-expressing fibroblasts were first transfected to express voltage-sensitive potassium channels, such as Kv1.3, and then engrafted into rat hearts.292 These grafts of clustered mesenchymal cells were found to alter local electrophysiological properties and, owing to their enhanced potassium current, reduced automaticity and prolonged refractoriness of surrounding electrically coupled cardiac myocytes. This concept, pioneered in ventricular tissue, could be adapted to counter trans-scar conduction across atrial ablation lines.
Outlook
Research over the past 25 years has identified the cardiac fibroblast as a cell with an unexpected range of effects, integrating and organizing myocardial structure and function at various levels, from local signalling to global electro-mechanical function. There has been further insight into the well-established roles of fibroblasts in cardiac structure and ECM generation, together with an expanding appreciation of their paracrine effects, functions as mechanical and electrical signalling hubs, and roles in myocardial repair. There can be little doubt that there will be new surprising roles for fibroblasts in homeostasis and disease, but this is a suitable moment to take stock of this protean cell. By analogy to the glia of the central nervous system, which was once regarded as a trophic glue but is now recognized as a structure whose relevance for tissue function is on par with that of the neural network itself, we know too little about the relevance of non-excitable cells in homeostasis of excitable tissues and organs. What we do know is that the replacement of excitable cells, whether by gliosis or fibrosis, is a chief element of pathogenic processes in diseases of the brain and heart.
It is time, therefore, to shift our appreciation of cardiac mesenchymal cells away from viewing them as a passive component in an integrated and dynamic tissue system – diverse in ontogeny and inseparable from the myocardial syncytium but otherwise secondary in relevance – to one where they are credited with active roles in complex, multifunctional regulatory processes crucial not only for development but also for structural maintenance and functional operation of the heart.
The concept that fibroblasts link together to form a cardiac sub-system of co-equal importance to the syncytial network of myocytes should inform our approach to targeting or utilizing mesenchymal cells in therapies for heart disease. In this respect there are a number of exciting opportunities and important questions. The implications of complex sets of intercellular interactions, both homocellular and heterocellular, are poorly understood, and modulating cell-to-cell communication in the heart via biochemical and biophysical factors may provide new therapeutic targets. Novel paracrine factors could be identified by analyzing the secretome of cells – as has been done for cells used for cell therapy.173 Recent studies suggest that among the many long non-coding RNA molecules, some may code for small peptides with potent biological activity.293 Similarly, well-known cardiac proteins such as Cx43 can generate alternative bioactive peptides in response to ischaemia by cap-independent translation,294, 295 peptidase processing,296 or as a docking motif for exosome-cell communication,297 further suggesting the possibility of new regulatory molecules and mechanisms that have been overlooked so far. In addition to proteins and peptides,298 metabolites (e.g. lipids, metabolic intermediates etc.) may be identified by new “omics” tools as structural and functional regulators of the heterocellular heart. Development of pharmaceutically tractable compositions and delivery systems, including cell-specific targeting, are among the hurdles that must be overcome if these newly identified factors are to be harnessed as therapies for improving cardiac health.
Better markers and lineage tracing tools are needed for cardiac mesenchymal cells. Such tools would enable the exploration of the regulation of cell plasticity, allowing insight into the heterogeneous origins of fibroblasts, processes such as EMT, EndoMT and (reverse) mesenchymal-endothelial transition, and their various response patterns to physiological and pathophysiological challenges. Improved lineage tools may also provide us with the knowledge necessary for co-opting fibroblast behaviours, such as homing to injured loci, as a treatment modality. One might envisage genetic modifications of these migratory cells to carry payloads to damaged myocardial tissues in personalized medicine-type approaches. Further, the ability to more reliably trace cell fate would enable a more thorough understanding of mechanisms underlying fibroblast reprogramming and cell therapies, thus moving us closer to being able to comprehensively repair and/or regenerate the damaged heart.
In the meantime, the more modest goal of modifying scar tissue for patient benefit seems to be a useful tangible objective. Humans and other mammals heal damaged hearts by scarring and fibrosis. We should take a cue from Mother Nature, and determine how to therapeutically nudge or re-engineer this process, to improve clinical outcomes for patients with heart disease.
Acknowledgments
RGG acknowledges support by the National Institute of Health, the Center Innovative Technology and the Virginia Biosciences Health Research Corporation. SD is supported by the LOEWE Center for Cell- and Gene Therapy (State of Hesse), the Excellence Cluster ECCPS (Exc147-2, DFG) and the Leducq Network “MIRVAD”. PK acknowledges support by a European Research Council Advanced Grant (CardioNECT), the British Heart Foundation (Senior Research Fellowship and New Horizon support) and the BBSRC. The authors express deep gratitude to their colleagues Drs. Roger Markwald, Robert Dettman, Chip Norris, Greg Morley, Steve Poelzing, Tom Borg and Jeffrey Holmes for productive discussions and thoughtful suggestions. This review was inspired by a Keystone Symposium – “Cell Biology of the Heart: Beyond the Myocyte-Centric View (X2)” organized by the authors in 2015. (http://www.keystonesymposia.org/15X2).
Footnotes
Disclosures
RGG is a member of the scientific advisory board of FirstString Research Inc and holds modest ownership in this company (<5%). SD is founder of t2Cure GmbH and scientific advisor of miRagen.
Contributor Information
Robert G. Gourdie, Center for Heart and Regenerative Medicine Research, Virginia Tech Carilion Research Institute, Roanoke, 2 Riverside Circle, VA 24016, United States Virginia Tech-Wake Forest University School of Biomedical Engineering and Sciences, Kelly Hall, 325 Stanger St, Blacksburg, Blacksburg, VA 24061, United States; Department of Emergency Medicine, Carilion Clinic, 1906 Belleview Ave, VA 24016, United States.
Stefanie Dimmeler, Institute for Cardiovascular Regeneration, Centre for Molecular Medicine, Frankfurt, University Frankfurt, Theodor Stern Kai 7, 60590 Frankfurt, Germany; German Center of Cardiovascular Research, DZHK, RheinMain, Theodor Stern Kai 7, 60590 Frankfurt, Germany.
Peter Kohl, Institute for Experimental Cardiovascular Medicine, University Heart Centre Freiburg · Bad Krozingen, Medical School of the Albert‐Ludwigs-Universität Freiburg, Elsässer Str. 4, 79110 Freiburg, Germany; Cardiac Biophysics and Systems Biology, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield UB9 6JH, UK.
References
- 1.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore-Morris T, Guimaraes-Camboa N, Yutzey KE, Puceat M, Evans SM. Cardiac fibroblasts: from development to heart failure. J Mol Med (Berl) 2015;93:823–30. doi: 10.1007/s00109-015-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–76. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez C, Morley GE. The origin and arrhythmogenic potential of fibroblasts in cardiac disease. J Cardiovasc Transl Res. 2012;5:760–7. doi: 10.1007/s12265-012-9408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ongstad EL, Gourdie RG. Myocyte-fibroblast electrical coupling: the basis of a stable relationship? Cardiovascular Research. 2012;93:215–217. doi: 10.1093/cvr/cvr338. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 10.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield JB, Borg TK. The collagen network of the heart. Laboratory Investigation. 1979;40:364–371. [PubMed] [Google Scholar]
- 12.Hales PW, et al. Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI. Prog Biophys Mol Biol. 2012;110:319–30. doi: 10.1016/j.pbiomolbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008 doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez PL, et al. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials. 2015;61:279–89. doi: 10.1016/j.biomaterials.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 15.Lu TY, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 16.Tedder ME, et al. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A. 2009;15:1257–68. doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yacoub MH. In Search of Living Valve Substitutes. J Am Coll Cardiol. 2015;66:889–91. doi: 10.1016/j.jacc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 18.De Maziere AM, van Ginneken AC, Wilders R, Jongsma HJ, Bouman LN. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. Journal of Molecular & Cellular Cardiology. 1992;24:567–78. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 19.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–35. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez C, et al. Enhanced Fibroblast-Myocyte Interactions in Response to Cardiac Injury. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TP, Xie Y, Garfinkel A, Qu Z, Weiss JN. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc Res. 2012;93:242–51. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manasek FJ. Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol. 1969;22:333–48. [PubMed] [Google Scholar]
- 23.Goshima K. Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in monolayer culture. Experimental Cell Research. 1969;58:420–426. doi: 10.1016/0014-4827(69)90523-0. [DOI] [PubMed] [Google Scholar]
- 24.Markwald RR, Fitzharris TP, Smith WN. Sturctural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42:160–80. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- 25.Rook MB, Jongsma HJ, de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989;414:95–8. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- 26.Runyan RB, Potts JD, Weeks DL. TGF-·3-mediated tissue interaction during embryonic heart development. Molecular Reproduction and Development. 1992;32:152–159. doi: 10.1002/mrd.1080320211. [DOI] [PubMed] [Google Scholar]
- 27.Long CS, Henrich CJ, Simpson PC. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regulation. 1991;2:1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–56. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 29.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental Biology. 1996;174:221–32. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 30.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–15. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 31.Patel R, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–24. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 33.Perin EC, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 34.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–8. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 35.Visconti RP, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–6. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 36.Mollmann H, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–71. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Walker NL, Burton FL, Kettlewell S, Smith GL, Cobbe SM. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction. J Cardiovasc Electrophysiol. 2007;18:862–8. doi: 10.1111/j.1540-8167.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 39.Roell W, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–24. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 40.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda N, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–65. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore-Morris T, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–34. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiruma T, Hirakow R. Epicardial formation in embryonic chick heart: computer-aided reconstruction, scanning, and transmission electron microscopic studies. American Journal of Anatomy. 1989;184:129–38. doi: 10.1002/aja.1001840204. [DOI] [PubMed] [Google Scholar]
- 45.Dettman Rw, Denetclaw W, Ordahl Cp, Bristow J. Common Epicardial Origin Of Coronary Vascular Smooth Muscle, Perivascular Fibroblasts, and Intermyocardial Fibroblasts In the Avian Heart. Developmental Biology. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 46.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation Research. 1998;82:1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 47.Cano E, Carmona R, Velecela V, Martinez-Estrada O, Munoz-Chapuli R. The proepicardium keeps a potential for glomerular marker expression which supports its evolutionary origin from the pronephros. Evol Dev. 2015;17:224–30. doi: 10.1111/ede.12130. [DOI] [PubMed] [Google Scholar]
- 48.Schlueter J, Manner J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol. 2006;295:546–58. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Merki E, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–60. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev Dyn. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Estrada OM, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu SP, Dong XR, Regan JN, Su C, Majesky MW. Tbx18 regulates development of the epicardium and coronary vessels. Dev Biol. 2013;383:307–20. doi: 10.1016/j.ydbio.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furtado MB, et al. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res. 2014;114:1422–34. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez NS, Barnett JV. TGFbeta and BMP-2 regulate epicardial cell invasion via TGFbetaR3 activation of the Par6/Smurf1/RhoA pathway. Cell Signal. 2012;24:539–48. doi: 10.1016/j.cellsig.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allison P, Espiritu D, Barnett JV, Camenisch TD. Type III TGFbeta receptor and Src direct hyaluronan-mediated invasive cell motility. Cell Signal. 2015;27:453–9. doi: 10.1016/j.cellsig.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vega-Hernandez M, Kovacs A, De Langhe S, Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011;138:3331–40. doi: 10.1242/dev.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artamonov MV, et al. Signaling pathways that control rho kinase activity maintain the embryonic epicardial progenitor state. J Biol Chem. 2015;290:10353–67. doi: 10.1074/jbc.M114.613190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dettman RW, Pae SH, Morabito C, Bristow J. Inhibition of alpha4-integrin stimulates epicardial-mesenchymal transformation and alters migration and cell fate of epicardially derived mesenchyme. Dev Biol. 2003;257:315–28. doi: 10.1016/s0012-1606(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 60.Benesh EC, et al. Bves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix deposition. Mol Biol Cell. 2013;24:3496–510. doi: 10.1091/mbc.E12-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brand T, Simrick SL, Poon KL, Schindler RF. The cAMP-binding Popdc proteins have a redundant function in the heart. Biochem Soc Trans. 2014;42:295–301. doi: 10.1042/BST20130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landerholm TE, et al. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–2062. doi: 10.1242/dev.126.10.2053. [DOI] [PubMed] [Google Scholar]
- 63.Acharya A, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–49. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tandon P, Miteva YV, Kuchenbrod LM, Cristea IM, Conlon FL. Tcf21 regulates the specification and maturation of proepicardial cells. Development. 2013;140:2409–21. doi: 10.1242/dev.093385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trembley MA, Velasquez LS, de Mesy Bentley KL, Small EM. Myocardin-related transcription factors control the motility of epicardium-derived cells and the maturation of coronary vessels. Development. 2015;142:21–30. doi: 10.1242/dev.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouacida A, et al. Pericyte-like progenitors show high immaturity and engraftment potential as compared with mesenchymal stem cells. PLoS One. 2012;7:e48648. doi: 10.1371/journal.pone.0048648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birbrair A, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014;5:122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circulation Research. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 69.Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today. 2003;69:2–13. doi: 10.1002/bdrc.10002. [DOI] [PubMed] [Google Scholar]
- 70.Rentschler S, Jain R, Epstein JA. Tissue-tissue interactions during morphogenesis of the outflow tract. Pediatr Cardiol. 2010;31:408–13. doi: 10.1007/s00246-009-9611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali SR, et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–35. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 72.Cheng G, et al. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–9. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- 73.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 74.Martin P, Tzanidis A, Stein-Oakley A, Krum H. Effect of a highly selective endothelin-converting enzyme inhibitor on cardiac remodeling in rats after myocardial infarction. J Cardiovasc Pharmacol. 2000;36:S367–70. doi: 10.1097/00005344-200036051-00106. [DOI] [PubMed] [Google Scholar]
- 75.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–58. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 76.Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell. 2007;18:2264–73. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asazuma-Nakamura Y, et al. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009 doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 78.Sanders YY, et al. SMAD-independent down-regulation of caveolin-1 by TGF-beta: effects on proliferation and survival of myofibroblasts. PLoS One. 2015;10:e0116995. doi: 10.1371/journal.pone.0116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia Y, et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–11. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC) J Mol Cell Cardiol. 2010;48:544–9. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oka T, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishioka T, et al. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol. 2007;49:261–8. doi: 10.1097/FJC.0b013e318033dfd4. [DOI] [PubMed] [Google Scholar]
- 83.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–38. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 84.Dubash AD, et al. Plakophilin-2 loss promotes TGF-beta1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol. 2016;212:425–38. doi: 10.1083/jcb.201507018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blaauw E, et al. Stretch-induced upregulation of connective tissue growth factor in rabbit cardiomyocytes. J Cardiovasc Transl Res. 2013;6:861–9. doi: 10.1007/s12265-013-9489-5. [DOI] [PubMed] [Google Scholar]
- 86.Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol. 2012;52:1083–90. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalla Costa AP, et al. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–31. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 88.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res. 2003;57:793–803. doi: 10.1016/s0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 89.Kiseleva I, Kamkin A, Kohl P, Lab MJ. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc Res. 1996;32:98–111. [PubMed] [Google Scholar]
- 90.Peyronnet R, Nerbonne JM, Kohl P. Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ Res. 2016;118:311–29. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van den Borne SW, et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–7. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 92.Roche PL, Filomeno KL, Bagchi RA, Czubryt MP. Intracellular signaling of cardiac fibroblasts. Compr Physiol. 2015;5:721–60. doi: 10.1002/cphy.c140044. [DOI] [PubMed] [Google Scholar]
- 93.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–75. [PMC free article] [PubMed] [Google Scholar]
- 94.van den Borne SW, et al. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–82. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 95.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–72. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–61. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruiz-Villalba A, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–66. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 98.Visconti RP, Markwald RR. Recruitment of new cells into the postnatal heart: potential modification of phenotype by periostin. Ann N Y Acad Sci. 2006;1080:19–33. doi: 10.1196/annals.1380.003. [DOI] [PubMed] [Google Scholar]
- 99.Crawford JR, Haudek SB, Cieslik KA, Trial J, Entman ML. Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J Cardiovasc Transl Res. 2012;5:749–59. doi: 10.1007/s12265-012-9402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cieslik KA, Trial J, Crawford JR, Taffet GE, Entman ML. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J Mol Cell Cardiol. 2014;70:56–63. doi: 10.1016/j.yjmcc.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duerrschmid C, Trial J, Wang Y, Entman ML, Haudek SB. Tumor necrosis factor: a mechanistic link between angiotensin-II-induced cardiac inflammation and fibrosis. Circ Heart Fail. 2015;8:352–61. doi: 10.1161/CIRCHEARTFAILURE.114.001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 103.Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ongstad E, Kohl P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J Mol Cell Cardiol. 2016;91:238–46. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–41. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerbin KA, Murry CE. The winding road to regenerating the human heart. Cardiovasc Pathol. 2015;24:133–40. doi: 10.1016/j.carpath.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakada Y, Kimura W, Sadek HA. Defining the Limit of Embryonic Heart Regeneration. Circulation. 2015;132:77–8. doi: 10.1161/CIRCULATIONAHA.115.017070. [DOI] [PubMed] [Google Scholar]
- 109.Sadahiro T, Yamanaka S, Ieda M. Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications. Circ Res. 2015;116:1378–91. doi: 10.1161/CIRCRESAHA.116.305374. [DOI] [PubMed] [Google Scholar]
- 110.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jayawardena TM, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smart N, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–4. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gajzer DC, Balbin J, Chaudhry HW. Thymosin beta4 and cardiac regeneration: are we missing a beat? Stem Cell Rev. 2013;9:303–12. doi: 10.1007/s12015-012-9378-3. [DOI] [PubMed] [Google Scholar]
- 115.Chen JX, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–5. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang L, et al. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ Res. 2015;116:237–44. doi: 10.1161/CIRCRESAHA.116.305547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nam YJ, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110:5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muraoka N, et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565–81. doi: 10.15252/embj.201387605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pratico ED, et al. RNA-mediated reprogramming of primary adult human dermal fibroblasts into c-kit<sup>+</sup> cardiac progenitor cells. Stem Cells Dev. 2015 doi: 10.1089/scd.2015.0073. [DOI] [PubMed] [Google Scholar]
- 120.Nam YJ, et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development. 2014;141:4267–78. doi: 10.1242/dev.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ifkovits JL, Addis RC, Epstein JA, Gearhart JD. Inhibition of TGFbeta signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS One. 2014;9:e89678. doi: 10.1371/journal.pone.0089678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou H, Dickson ME, Kim MS, Bassel-Duby R, Olson EN. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:11864–9. doi: 10.1073/pnas.1516237112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Towbin JA. Scarring in the heart–a reversible phenomenon? N Engl J Med. 2007;357:1767–8. doi: 10.1056/NEJMcibr075397. [DOI] [PubMed] [Google Scholar]
- 124.Gulati A, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 125.Klem I, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–20. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Esposito G, Dellegrottaglie S, Chiariello M. The extent of irreversible myocardial damage and the potential for left ventricular repair after primary percutaneous coronary intervention. Am Heart J. 2010;160:S4–10. doi: 10.1016/j.ahj.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 127.Vander Heide RS, Steenbergen C. Cardioprotection and myocardial reperfusion: pitfalls to clinical application. Circ Res. 2013;113:464–77. doi: 10.1161/CIRCRESAHA.113.300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 129.Fordyce CB, Gersh BJ, Stone GW, Granger CB. Novel therapeutics in myocardial infarction: targeting microvascular dysfunction and reperfusion injury. Trends Pharmacol Sci. 2015 doi: 10.1016/j.tips.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 130.Jones SP, et al. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–86. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–41. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 133.Duncker DJ, Verdouw PD. Role of K+ATP channels in ischemic preconditioning and cardioprotection. Cardiovasc Drugs Ther. 2000;14:7–16. doi: 10.1023/a:1007866102585. [DOI] [PubMed] [Google Scholar]
- 134.Chen CH, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kornfeld OS, et al. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116:1783–99. doi: 10.1161/CIRCRESAHA.116.305432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Monassier L, Ayme-Dietrich E, Aubertin-Kirch G, Pathak A. Targeting myocardial reperfusion injuries with cyclosporine in the CIRCUS Trial – Pharmacological reasons for failure. Fundam Clin Pharmacol. 2015 doi: 10.1111/fcp.12177. [DOI] [PubMed] [Google Scholar]
- 137.Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adkins GB, Curtis MJ. Potential role of cardiac chloride channels and transporters as novel therapeutic targets. Pharmacol Ther. 2015;145:67–75. doi: 10.1016/j.pharmthera.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 139.Shen Y, Shen Z, Luo S, Guo W, Zhu YZ. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxid Med Cell Longev. 2015;2015:925167. doi: 10.1155/2015/925167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Das A, et al. Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling. Basic Res Cardiol. 2015;110:31. doi: 10.1007/s00395-015-0486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–96. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luo J, Obal D, Dimova N, Tang XL, Rokosh G. Cardiac myocyte-specific transgenic ecSOD targets mitochondria to protect against Ca(2+) induced permeability transition. Front Physiol. 2013;4:295. doi: 10.3389/fphys.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hawat G, Helie P, Baroudi G. Single intravenous low-dose injections of connexin 43 mimetic peptides protect ischemic heart in vivo against myocardial infarction. J Mol Cell Cardiol. 2012;53:559–66. doi: 10.1016/j.yjmcc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 144.Wang N, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Molecular biology of the cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sluijter JP, et al. Novel therapeutic strategies for cardioprotection. Pharmacol Ther. 2014;144:60–70. doi: 10.1016/j.pharmthera.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 147.Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–52. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 148.Davidson JO, Green CR, Bennet L, Gunn AJ. Battle of the hemichannels – Connexins and Pannexins in ischemic brain injury. Int J Dev Neurosci. 2014 doi: 10.1016/j.ijdevneu.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 149.Afzal MR, et al. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights from Randomized Controlled Trials. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.114.304792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–30. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Delewi R, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35:989–98. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Assmus B, Dimmeler S, Zeiher AM. Cardiac cell therapy: lost in meta-analyses. Circ Res. 2015;116:1291–2. doi: 10.1161/CIRCRESAHA.115.306330. [DOI] [PubMed] [Google Scholar]
- 153.Gyongyosi M, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–60. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leri A, Rota M, Pasqualini FS, Goichberg P, Anversa P. Origin of cardiomyocytes in the adult heart. Circ Res. 2015;116:150–66. doi: 10.1161/CIRCRESAHA.116.303595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Makkar RR, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ellison GM, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 159.van Berlo JH, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sultana N, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Q, et al. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26:119–30. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uchida S, et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abbott A. Doubts over heart stem-cell therapy. Nature. 2014;509:15–6. doi: 10.1038/509015a. [DOI] [PubMed] [Google Scholar]
- 165.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kinnaird T, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 167.Pak HN, et al. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–8. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- 168.Seeger FH, et al. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–9. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 169.Li L, et al. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. 2009;36:725–31. doi: 10.1007/s11033-008-9235-2. [DOI] [PubMed] [Google Scholar]
- 170.Gnecchi M, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 171.Li Z, Wei H, Deng L, Cong X, Chen X. Expression and secretion of interleukin-1beta, tumour necrosis factor-alpha and interleukin-10 by hypoxia- and serum-deprivation-stimulated mesenchymal stem cells. FEBS J. 2010;277:3688–98. doi: 10.1111/j.1742-4658.2010.07770.x. [DOI] [PubMed] [Google Scholar]
- 172.Chen P, et al. Hypoxia preconditioned mesenchymal stem cells prevent cardiac fibroblast activation and collagen production via leptin. PLoS One. 2014;9:e103587. doi: 10.1371/journal.pone.0103587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Korf-Klingebiel M, et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med. 2015;21:140–9. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- 174.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–44. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 175.Malliaras K, et al. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med. 2014;6:760–77. doi: 10.1002/emmm.201303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20:814–21. doi: 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- 177.Marban E, Cho HC, Cingolani E. Taking the cells out of cell therapy. J Am Coll Cardiol. 2012;60:1707–8. doi: 10.1016/j.jacc.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 178.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–72. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–5. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 180.Abulhul E, et al. Long-term statin therapy in patients with systolic heart failure and normal cholesterol: effects on elevated serum markers of collagen turnover, inflammation, and B-type natriuretic peptide. Clin Ther. 2012;34:91–100. doi: 10.1016/j.clinthera.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 181.Shiroshita-Takeshita A, et al. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 182.Van de Werf F. The history of coronary reperfusion. Eur Heart J. 2014;35:2510–5. doi: 10.1093/eurheartj/ehu268. [DOI] [PubMed] [Google Scholar]
- 183.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–6. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 184.De Mello WC, Specht P. Chronic blockade of angiotensin II AT1-receptors increased cell-to-cell communication, reduced fibrosis and improved impulse propagation in the failing heart. J Renin Angiotensin Aldosterone Syst. 2006;7:201–5. doi: 10.3317/jraas.2006.038. [DOI] [PubMed] [Google Scholar]
- 185.Shibasaki Y, et al. Impact of the angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end-stage renal disease: assessment by ultrasonic integrated backscatter and biochemical markers. Hypertens Res. 2005;28:787–95. doi: 10.1291/hypres.28.787. [DOI] [PubMed] [Google Scholar]
- 186.Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc. 2009;84:718–29. doi: 10.4065/84.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Siddesha JM, et al. Acetylsalicylic acid inhibits IL-18-induced cardiac fibroblast migration through the induction of RECK. J Cell Physiol. 2014;229:845–55. doi: 10.1002/jcp.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hayashidani S, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–40. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 189.Mughal RS, et al. Peroxisome proliferator-activated receptor gamma-independent effects of thiazolidinediones on human cardiac myofibroblast function. Clin Exp Pharmacol Physiol. 2009;36:478–86. doi: 10.1111/j.1440-1681.2008.05088.x. [DOI] [PubMed] [Google Scholar]
- 190.Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc Res. 2006;69:677–87. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 191.Tietjens J, Teerlink JR. Serelaxin and acute heart failure. Heart. 2016;102:95–9. doi: 10.1136/heartjnl-2014-306786. [DOI] [PubMed] [Google Scholar]
- 192.Liao CH, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–53. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Levick SP, et al. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–7. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 194.Braunwald E. The ten advances that have defined modern cardiology. Trends Cardiovasc Med. 2014;24:179–83. doi: 10.1016/j.tcm.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 195.Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339:143–51. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.He W, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:21110–5. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Daskalopoulos EP, Hermans KC, Janssen BJ, Matthijs Blankesteijn W. Targeting the Wnt/frizzled signaling pathway after myocardial infarction: a new tool in the therapeutic toolbox? Trends Cardiovasc Med. 2013;23:121–7. doi: 10.1016/j.tcm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 198.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jaquet K, et al. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism? Stem Cells Dev. 2005;14:299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- 200.Chen L, Qin F, Ge M, Shu Q, Xu J. Application of adipose-derived stem cells in heart disease. J Cardiovasc Transl Res. 2014;7:651–63. doi: 10.1007/s12265-014-9585-1. [DOI] [PubMed] [Google Scholar]
- 201.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–99. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 202.Qian L, Srivastava D. Direct cardiac reprogramming: from developmental biology to cardiac regeneration. Circ Res. 2013;113:915–21. doi: 10.1161/CIRCRESAHA.112.300625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ye L, Zimmermann WH, Garry DJ, Zhang J. Patching the heart: cardiac repair from within and outside. Circ Res. 2013;113:922–32. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 205.Bonauer A, et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science. 2009 doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 206.Gurman P, et al. Recombinant tissue plasminogen activators (rtPA): a review. Clin Pharmacol Ther. 2015;97:274–85. doi: 10.1002/cpt.33. [DOI] [PubMed] [Google Scholar]
- 207.Shuros AC, et al. Ventricular preexcitation modulates strain and attenuates cardiac remodeling in a swine model of myocardial infarction. Circulation. 2007;116:1162–9. doi: 10.1161/CIRCULATIONAHA.107.696294. [DOI] [PubMed] [Google Scholar]
- 208.Bugyei-Twum A, et al. High glucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol. 2014;13:89. doi: 10.1186/1475-2840-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159:1061–9. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 210.Tank J, et al. Single-target RNA interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J Mol Cell Cardiol. 2014;66:141–56. doi: 10.1016/j.yjmcc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 211.Szabo Z, et al. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–40. doi: 10.1161/HYPERTENSIONAHA.114.03279. [DOI] [PubMed] [Google Scholar]
- 212.Mirkovic S, et al. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. Br J Pharmacol. 2002;135:961–8. doi: 10.1038/sj.bjp.0704539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kagitani S, et al. Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens. 2004;22:1007–15. doi: 10.1097/00004872-200405000-00024. [DOI] [PubMed] [Google Scholar]
- 214.Koitabashi N, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–12. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim J, et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol. 2006;48:1378–84. doi: 10.1016/j.jacc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 216.Bujak M, et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rodriguez-Pascual F, Busnadiego O, Gonzalez-Santamaria J. The profibrotic role of endothelin-1: is the door still open for the treatment of fibrotic diseases? Life Sci. 2014;118:156–64. doi: 10.1016/j.lfs.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 218.Luo K, et al. The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev. 1999;13:2196–206. doi: 10.1101/gad.13.17.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cunnington RH, et al. Antifibrotic properties of c-Ski and its regulation of cardiac myofibroblast phenotype and contractility. Am J Physiol Cell Physiol. 2011;300:C176–86. doi: 10.1152/ajpcell.00050.2010. [DOI] [PubMed] [Google Scholar]
- 220.Espira L, et al. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol. 2009;47:188–95. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 221.Balasubramanian S, et al. beta3 integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLoS One. 2012;7:e45076. doi: 10.1371/journal.pone.0045076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shivshankar P, et al. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J Mol Cell Cardiol. 2014;76:84–93. doi: 10.1016/j.yjmcc.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yi SL, Liu XJ, Zhong JQ, Zhang Y. Role of caveolin-1 in atrial fibrillation as an anti-fibrotic signaling molecule in human atrial fibroblasts. PLoS One. 2014;9:e85144. doi: 10.1371/journal.pone.0085144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cunnington RH, Nazari M, Dixon IM. c-Ski, Smurf2, and Arkadia as regulators of TGF-beta signaling: new targets for managing myofibroblast function and cardiac fibrosis. Can J Physiol Pharmacol. 2009;87:764–72. doi: 10.1139/Y09-076. [DOI] [PubMed] [Google Scholar]
- 225.Czubryt MP. Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair. 2012;5:19. doi: 10.1186/1755-1536-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tourkina E, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 227.Varr BC, Maurer MS. Emerging role of serelaxin in the therapeutic armamentarium for heart failure. Curr Atheroscler Rep. 2014;16:447. doi: 10.1007/s11883-014-0447-8. [DOI] [PubMed] [Google Scholar]
- 228.Neverova N, Teerlink JR. Serelaxin : a potential new drug for the treatment of acute heart failure. Expert Opin Investig Drugs. 2014;23:1017–26. doi: 10.1517/13543784.2014.924504. [DOI] [PubMed] [Google Scholar]
- 229.Squecco R, et al. Inhibitory effects of relaxin on cardiac fibroblast-to-myofibroblast transition: an electrophysiological study. Exp Physiol. 2015;100:652–66. doi: 10.1113/EP085178. [DOI] [PubMed] [Google Scholar]
- 230.Sassoli C, et al. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PLoS One. 2013;8:e63896. doi: 10.1371/journal.pone.0063896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–32. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2012;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cardin S, et al. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5:1027–35. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 234.Patrick DM, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–6. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.US National Library of Medicine. ATHENA clinical trial. ClinicalTrials.gov. 2016 http://www.clinicaltrials.gov/NCT02136862.
- 236.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–42. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 237.Boon RA, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–10. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 238.Bang C, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–41. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 240.Chatelier A, et al. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol. 2012;590:4307–19. doi: 10.1113/jphysiol.2012.233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nakajima H, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–9. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 242.Verheule S, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wright M, Narayan SM. Ablation of atrial fibrillation. Trends Cardiovasc Med. 2015;25:409–419. doi: 10.1016/j.tcm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Moore JP. Arrhythmia management for the adult patient with congenital heart disease: an update and analytical review. Minerva Pediatr. 2014;66:415–39. [PubMed] [Google Scholar]
- 245.Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The Living Scar – Cardiac Fibroblasts and the Injured Heart. Trends Mol Med. 2016;22:99–114. doi: 10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rouillard AD, Holmes JW. Coupled agent-based and finite-element models for predicting scar structure following myocardial infarction. Prog Biophys Mol Biol. 2014;115:235–43. doi: 10.1016/j.pbiomolbio.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 247.Koomalsingh KJ, et al. Optimized local infarct restraint improves left ventricular function and limits remodeling. Ann Thorac Surg. 2013;95:155–62. doi: 10.1016/j.athoracsur.2012.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fomovsky GM, Clark SA, Parker KM, Ailawadi G, Holmes JW. Anisotropic reinforcement of acute anteroapical infarcts improves pump function. Circ Heart Fail. 2012;5:515–22. doi: 10.1161/CIRCHEARTFAILURE.111.965731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 250.Ifkovits JL, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction: a 5-year update. J Am Coll Cardiol. 2011;58:2615–29. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 252.Voorhees AP, et al. Building a better infarct: Modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol. 2015;85:229–39. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Purcell BP, et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater. 2014;13:653–61. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Eckhouse SR, et al. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med. 2014;6:223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chung ES, et al. Effect of peri-infarct pacing early after myocardial infarction: results of the prevention of myocardial enlargement and dilatation post myocardial infarction study. Circ Heart Fail. 2010;3:650–8. doi: 10.1161/CIRCHEARTFAILURE.110.945881. [DOI] [PubMed] [Google Scholar]
- 256.Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. Anat Rec. 2001;264:1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
- 257.Shimazaki M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moldovan NI, et al. Reoxygenation-derived toxic reactive oxygen/nitrogen species modulate the contribution of bone marrow progenitor cells to remodeling after myocardial infarction. J Am Heart Assoc. 2014;3:e000471. doi: 10.1161/JAHA.113.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Norris RA, Gourdie RG, O’Quinn MP, RR M. Periostin Inhibitory Compositions for Myocardial Regeneration, Methods of Delivery, and Methods of Using Same. US20100291188 A1. US Patent Office Application. 2008
- 260.Bramwell B. A Case of Heart-Block, with Fibrous Degeneration and Partial Obliteration of the Bundle of His. Br Med J. 1909;1:995–6. doi: 10.1136/bmj.1.2521.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kohl P. Structural and functional recoupling of atrial and ventricular myocardium: new conduits for electrical flow. J Am Coll Cardiol. 2014;64:2586–8. doi: 10.1016/j.jacc.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 262.Lefroy DC, et al. Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: surface electrocardiographic features and estimated prevalence. Am J Cardiol. 1998;82:444–50. doi: 10.1016/s0002-9149(98)00359-2. [DOI] [PubMed] [Google Scholar]
- 263.Hager A, et al. Congenital and surgically acquired Wolff-Parkinson-White syndrome in patients with tricuspid atresia. J Thorac Cardiovasc Surg. 2005;130:48–53. doi: 10.1016/j.jtcvs.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 264.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 265.Goldsmith EC, et al. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–94. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 266.Rother J, et al. Crosstalk of cardiomyocytes and fibroblasts in co-cultures. Open Biol. 2015;5 doi: 10.1098/rsob.150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kohl P, Camelliti P. Fibroblast-myocyte connections in the heart. Heart Rhythm. 2012;9:461–4. doi: 10.1016/j.hrthm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 268.He K, et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92:39–47. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 269.Veeraraghavan R, et al. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 2015 doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rhett JM, Veeraraghavan R, Poelzing S, Gourdie RG. The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc Med. 2013;23:222–8. doi: 10.1016/j.tcm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res. 2003;93:381–3. doi: 10.1161/01.RES.0000091364.90121.0C. [DOI] [PubMed] [Google Scholar]
- 272.Kohl P, Noble D. Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc Res. 1996;32:62–8. [PubMed] [Google Scholar]
- 273.MacCannell KA, et al. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–32. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–25. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 276.Thodeti CK, Paruchuri S, Meszaros JG. A TRP to cardiac fibroblast differentiation. Channels (Austin) 2013;7:211–4. doi: 10.4161/chan.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Benamer N, et al. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. Am J Physiol Heart Circ Physiol. 2013;304:H1231–9. doi: 10.1152/ajpheart.00878.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol. 2008;294:H2040–52. doi: 10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng. 2008;36:41–56. doi: 10.1007/s10439-007-9405-8. [DOI] [PubMed] [Google Scholar]
- 280.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys J. 2009;97:2179–90. doi: 10.1016/j.bpj.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Xie Y, et al. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: A computational study. Heart Rhythm. 2009;6:1641–9. doi: 10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rutherford SL, Trew ML, Sands GB, LeGrice IJ, Smaill BH. High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. Circ Res. 2012;111:301–11. doi: 10.1161/CIRCRESAHA.111.260943. [DOI] [PubMed] [Google Scholar]
- 283.Ashihara T, et al. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram-based catheter ablation. Circ Res. 2012;110:275–84. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gonzales MJ, Vincent KP, Rappel WJ, Narayan SM, McCulloch AD. Structural contributions to fibrillatory rotors in a patient-derived computational model of the atria. Europace. 2014;16(Suppl 4):iv3–iv10. doi: 10.1093/europace/euu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.McDowell KS, et al. Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PLoS One. 2015;10:e0117110. doi: 10.1371/journal.pone.0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2009;6:87–97. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Saba S, et al. Prevention of adverse electrical and mechanical remodeling with biventricular pacing in a rabbit model of myocardial infarction. Heart Rhythm. 2008;5:124–30. doi: 10.1016/j.hrthm.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mahoney V. New York University PhD Thesis. 2015. Connexin 43 mediates electrotonic conduction across non-myocyte lesions in the intact heart. [Google Scholar]
- 289.Quinn TA, et al. Cell-Specific Expression of Voltage-Sensitive Protein Confirms Cardiac Myocyte to Non-Myocyte Electrotonic Coupling in Healed Murine Infarct Border Tissue. Circulation. 2014;130:A11749. [Google Scholar]
- 290.Choi YH, et al. Cardiac conduction through engineered tissue. Am J Pathol. 2006;169:72–85. doi: 10.2353/ajpath.2006.051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 292.Yankelson L, et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–31. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 293.Anderson DM, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Smyth JW, Shaw RM. Autoregulation of Connexin43 Gap Junction Formation by Internally Translated Isoforms. Cell Reports. 2013;5:611–618. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ul-Hussain M, et al. Internal ribosomal entry site (IRES) activity generates endogenous carboxyl-terminal domains of Cx43 and is responsive to hypoxic conditions. J Biol Chem. 2014;289:20979–90. doi: 10.1074/jbc.M113.540187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lindsey ML, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–28. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 297.Soares AR, et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:13243. doi: 10.1038/srep13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lindsey ML, et al. Transformative Impact of Proteomics on Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000226. [DOI] [PubMed] [Google Scholar]


