SUMMARY
BACKGROUND
Delayed diagnosis of tuberculosis (TB) increases mortality.
OBJECTIVE
To evaluate whether stool culture improves the diagnosis of TB in people living with the human immunodeficiency virus (PLHIV).
DESIGN
We analysed cross-sectional data of TB diagnosis in PLHIV in Cambodia, Thailand and Viet Nam. Logistic regression was used to assess the association between positive stool culture and TB, and to calculate the incremental yield of stool culture.
RESULTS
A total of 1693 PLHIV were enrolled with a stool culture result. Of 228 PLHIV with culture-confirmed TB from any site, 101 (44%) had a positive stool culture; of these, 91 (90%) had pulmonary TB (PTB). After adjusting for confounding factors, a positive stool culture was associated with smear-negative (odds ratio [OR] 26, 95% confidence interval [CI] 12–58), moderately smear-positive (OR 60, 95%CI 23–159) and highly smear-positive (OR 179, 95%CI 59–546) PTB compared with no PTB. No statistically significant association existed with extrapulmonary TB compared with no extrapulmonary TB (OR 2, 95%CI 1–5). The incremental yield of one stool culture above two sputum cultures (5%, 95%CI 3–8) was comparable to an additional sputum culture (7%, 95%CI 4–11).
CONCLUSION
Nearly half of the PLHIV with TB had a positive stool culture that was strongly associated with PTB. Stool cultures may be used to diagnose TB in PLHIV.
Keywords: Mycobacterium tuberculosis, HIV, diagnostic tests, routine, bacteriological techniques, SouthEast Asia
TUBERCULOSIS (TB) disproportionately affects persons living with the human immunodeficiency virus (PLHIV).1 Once infected with TB, PLHIV are more likely to progress to TB disease2 and to die from TB compared to persons without HIV.3,4 In 2011, of the 1.4 million TB deaths worldwide, 31% occurred in PLHIV.5 Early diagnosis of TB may reduce TB mortality. With advanced immunosuppression, PLHIV are more likely to have smear-negative and extrapulmonary TB disease,2 making it increasingly difficult to diagnose TB by sputum microscopy. PLHIV with TB may also lack respiratory symptoms and abnormalities on chest radiography (CXR).2
Stool cultures might offer an alternative method for TB diagnosis when sputum is difficult to obtain from PLHIV. Tuberculous bacteria are believed to be present in stool when bacteria are transported from the lungs to the oropharynx, are swallowed and then transit through the gastrointestinal tract.6 Mycobacterium tuberculosis is also found in the stool of patients with intestinal TB7 or in PLHIV with diarrhoea or enteritis.8–10 The examination of stool specimens may therefore facilitate TB diagnosis in PLHIV who are unable to produce sputum or who have TB in difficult-to-access extrapulmonary sites.
In a large study of TB diagnoses in PLHIV in SouthEast Asia, all enrolled patients had stool specimens collected and examined for TB along with sputum specimens and specimens from other non-pulmonary sites.11 Because of the potential utility of stool culture for diagnosing TB in PLHIV, we analysed data from this study to answer two questions: 1) Is a stool culture growing M. tuberculosis in PLHIV associated with a high bacillary burden of pulmonary TB (PTB), with gastrointestinal symptoms (and thus intestinal TB), with disseminated disease (positive culture from one or more extrapulmonary site) or more than one of these? 2) Does the examination of stool specimens increase TB detection in PLHIV? Answers to these questions would determine whether, and in which situations, stool specimens might be useful for diagnosing TB in PLHIV.
METHODS
Participants
We examined the microbiological results of PLHIV recruited to participate in a larger study evaluating combinations of symptoms, signs and diagnostic tests for confirming or ruling out TB.11 Eligible participants presenting to out-patient clinics in Cambodia, Thailand and Viet Nam were enrolled from 2006 to 2008. After standardised history taking and physical examination, each participant submitted three sputum specimens and one specimen each of blood, urine and stool for acid-fast bacilli (AFB) smear microscopy and culture. Lymph node aspirates were submitted for AFB smear microscopy and culture if an enlarged lymph node was present on physical examination. All participants with an uncontaminated stool culture result were included in the analysis.
Microbiology
All specimens were sent to the reference laboratory in each country for concentrated Ziehl-Neelsen AFB smear microscopy and mycobacterial culture. Stool specimens were prepared for culture by 1) emulsifying 1 g of stool with sterile glass beads in 10 ml sterile water, 2) filtration through sterile gauze, and 3) decontamination. All other specimens were decontaminated and processed according to procedures previously described.12 Specimens with 4–99 AFB per 100 examined microscope fields were graded as 1+ smear-positive (moderately smear-positive), while specimens with >100 bacilli/100 examined microscope fields were graded as 2+ or more smear-positive (highly smear-positive). Specimens with 1–3 AFB/100 examined fields were defined as smear-negative based on the known low correlation with positive culture.13,14
Cultures for and the identification of M. tuberculosis were performed on solid Löwenstein-Jensen (LJ) medium or in liquid culture using BACTEC™ MGIT™ 960 or BACTEC 9050/9120 (BD, Sparks, MD, USA).12 Any specimens with concurrent AFB and contamination were subcultured to isolate the AFB. Any specimens with contamination and no AFB were discarded. In Thailand and Viet Nam, where both solid and liquid culture were available, each processed specimen was divided into three; the first two aliquots were cultured on solid medium and the third aliquot was cultured on liquid medium. In Cambodia, where only solid culture was available, each processed specimen was divided into two aliquots for LJ culture.
Definitions
PTB was defined as a participant with at least one positive sputum culture for M. tuberculosis. Extra-pulmonary TB (EPTB) was defined as a participant who had at least one positive M. tuberculosis culture from a non-stool extra-pulmonary source (blood, lymph node aspirate or urine). Any participant with stool culture growing M. tuberculosis was defined as having a positive stool culture. We defined a subgroup of PLHIV for whom all submitted processed sputum specimens were of saliva-like consistency or totalled a volume of <3 ml, as persons with low-quality, low-volume sputum (i.e., persons who were unable to produce adequate sputum).
Statistical analysis
Statistical differences between groups were measured using Pearson’s χ2 and Wilcoxon’s rank-sum tests. We used exact logistic regression (with Firth’s Penalized Maximum Likelihood Estimate)15 to measure univariate associations of each sign, symptom and form of TB (PTB or EPTB) with the outcome of a positive stool culture, as small cell counts precluded the use of traditional logistic regression.
Characteristics that had an association with a positive stool culture at a P value of <0.10, possible confounding characteristics or a priori evidence suggesting epidemiologic relevance, were used to construct a multivariable logistic regression model. We designed the model to simultaneously measure the association of PTB and EPTB with the outcome of positive stool culture. Variables confounding the relationship between PTB and a positive stool culture, variables that were statistically significant or variables that were important for epidemiological relevance, were included in the logistic regression model. The relationship between each continuous variable and the outcome of a positive stool culture was explored by quantising the continuous variable and maintaining its original format before selecting the final format for the continuous variable in the logistic regression model. P < 0.05 was considered statistically significant. Incremental yield (IY) for stool culture was defined as the number or percentage of participants with positive stool cultures who were not positive by the baseline diagnostic method (e.g., sputum culture) divided by the total number of participants determined to have TB.
Ethics
Ethical approval was obtained from the human subjects committee of the US Centers for Disease Control and Prevention and collaborating institutions in each country. Informed consent was obtained from all participants for participation in the parent study.
RESULTS
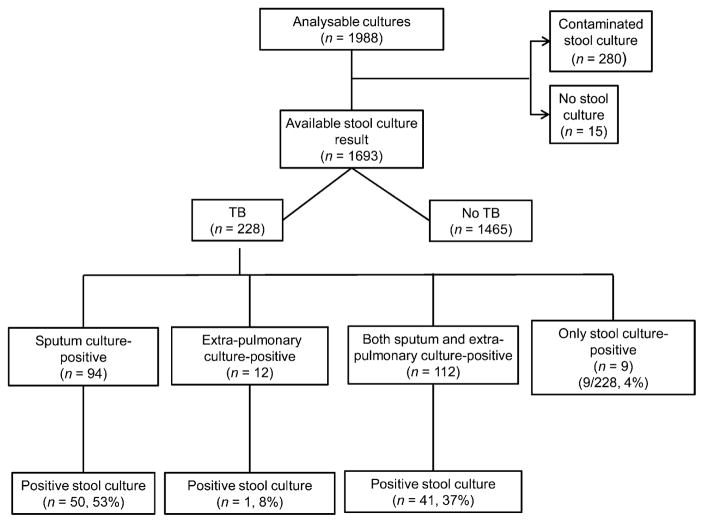
Of the 1988 eligible participants with at least one evaluable pulmonary or extra-pulmonary culture (Figure 1), 1693 (85%) had a positive or negative stool culture result. The other 295 participants either did not submit a stool specimen for culture (n = 15) or the submitted specimens were contaminated (n = 280). The proportion of participants with TB did not differ between participants with evaluable and non-evaluable stool cultures. Among the 1693 participants with available stool culture results, 228 (13%) had culture-confirmed TB of any site and 94 (41% of 228) had PTB without evidence of EPTB. Of the 228 participants with TB, 101 (44%) had a positive stool culture.
Figure 1.
Flowchart of participant inclusion.
We compared the 101 PLHIV with stool culture growing M. tuberculosis with 127 PLHIV with negative stool cultures and another positive culture (Table 1). Those with positive stool cultures were slightly younger, more likely to have a concurrent positive extra-pulmonary culture and more likely to have a higher sputum bacillary concentration than persons with negative stool cultures, although they were not more likely to have PTB. PLHIV with positive stool culture had a lower median CD4 count than those with TB in sites other than stool (75 vs. 139 cells/μl). Neither respiratory nor gastrointestinal symptomatology alone was associated with positive stool cultures. The nine participants with positive stool cultures and no additional positive cultures were less likely to have an abnormal CXR (22% vs. 68%, P = 0.009) compared to persons with TB in sites other than stool.
Table 1.
Demographic and clinical variables associated with positive stool culture compared to other TB in sites other than stool*
| Variable | Positive stool culture (n = 101) n (%) |
TB in sites other than stool (n = 127) n (%) |
P value† |
|---|---|---|---|
| Age, years, median [IQR] | 29 [26–37] | 32 [28–40] | 0.04‡ |
| Male sex | 73 (72) | 78 (61) | 0.08 |
| Country of evaluation | |||
| Cambodia | 40 (40) | 58 (46) | |
| Thailand | 10 (10) | 22 (17) | |
| Viet Nam | 51 (51) | 47 (37) | 0.08 |
| Persons hospitalised | 9 (9) | 5 (4) | 0.12 |
| Symptoms | |||
| Cough | 74 (73) | 94 (74) | 0.90 |
| Fever | 74 (73) | 87 (69) | 0.43 |
| Diarrhoea | 32 (32) | 44 (35) | 0.64 |
| Nausea or vomiting | 32 (32) | 50 (39) | 0.23 |
| Abdominal pain | 44 (43) | 53 (42) | 0.78 |
| Night sweats | 42 (41) | 60 (47) | 0.39 |
| Difficulty breathing | 61 (60) | 61 (48) | 0.06 |
| Chest pain | 49 (49) | 64 (50) | 0.78 |
| Weight loss | 79 (78) | 86 (68) | 0.08 |
| Fatigue | 78 (77) | 94 (74) | 0.58 |
| Chills | 48 (47) | 63 (50) | 0.75 |
| BMI, kg/m2, median [IQR] | 18 [17–20] | 19 [17–21] | 0.05 |
| Enlarged lymph nodes | 33 (33) (n =100) | 35 (28) | 0.37 |
| Performance status: Karnofsky score ≥90§ | 49 (49) | 76 (60) | 0.09 |
| Chest radiograph with any abnormality | 71 (72) (n = 99) | 73 (61) (n = 120) | 0.09 |
| Cavity | 13 (13) | 11 (9) | 0.30 |
| Pulmonary TB | 91 (90) | 115 (91) | 0.91 |
| Sputum bacillary burden | (n = 91) | (n = 115) | |
| Minimal (smear-negative) | 31 (34) | 81 (70) | |
| Moderate (1+) | 30 (33) | 23 (20) | |
| Most (2–3+) | 30 (33) | 11 (10) | <0.001‡ |
| Extra-pulmonary TB | 42 (42) | 33 (27) (n = 122) | 0.02‡ |
| CD4 count, cells/μl, median [IQR] | 75 [20–213] | 139 [47–281] | 0.004‡ |
If n is not specified for the row, column totals apply.
Pearson’s χ2 or Wilcoxon rank sum.
P < 0.05.
Karnofsky score measures the extent of impairment (performance status) secondary to illness.
TB = tuberculosis; IQR = interquartile range; BMI = body mass index.
When adjusted for age, sex, country of evaluation, body mass index, abnormal CXR and CD4 cell count, there was a graded relationship between PTB with increasing bacillary concentration and stool culture positivity (Table 2). The adjusted odds ratio (OR) with 95% confidence interval (CI) for positive stool culture was 26 (95%CI 12–58) for smear-negative PTB, 60 (95%CI 23–159) for 4–99 AFB per 100 fields examined, and 179 (95%CI 59–546) for >99 AFB/100 fields when compared to the referent group of no PTB.
Table 2.
Factors associated with positive stool culture
| Factor | n | Stool culture positivity OR (95%CI) |
Stool culture positivity aOR* (95%CI) |
P values for aOR |
|---|---|---|---|---|
| Pulmonary TB | 1693 | |||
| No pulmonary TB | 1487 | Reference | Reference | |
| Smear-negative TB | 112 | 56.1 (25.7–132.8) | 26.3 (11.9–57.9) | <0.001† |
| Smear-positive: 1+‡ | 53 | 188.0 (78.9–486.0) | 60.1 (22.8–158.7) | <0.001† |
| Smear-positive: ≥2+§ | 41 | 386.9 (145.9–>1000.0) | 178.7 (58.5–545.6) | <0.001† |
| Extra-pulmonary TB | 1672 | 32.9 (18.9–57.9) | 2.1 (1.0–4.6) | 0.052 |
| Age, year | 1693 | 1.0 (1.0–1.0) | 1.0 (0.9–1.0) | 0.24 |
| Sex | 1693 | |||
| Female | 804 | Reference | Reference | |
| Male | 889 | 2.5 (1.6–4.0) | 1.2 (0.7–2.3) | 0.53 |
| Study site | 1693 | |||
| Cambodia | 720 | Reference | Reference | |
| Thailand | 618 | 0.3 (0.1–0.6) | 0.9 (0.4–2.3) | 0.88 |
| Viet Nam | 355 | 2.8 (1.8–4.5) | 3.9 (1.8–8.2) | <0.001† |
| BMI, kg/m2, median, [IQR] | 1692 | 0.8 [0.7–0.8] | 0.9 [0.8–1.0] | 0.04† |
| CXR with any abnormality | 1651 | 11.7 (7.3–19.2) | 1.7 (0.9–3.4) | 0.12 |
| CD4 count, cells/μl | 1693 | |||
| ≥500 | 274 | Reference | Reference | |
| 200–<500 | 733 | 1.6 (0.6–5.4) | 1.1 (0.3–3.5) | 0.91 |
| 100–<200 | 238 | 4.1 (1.4–14.5) | 1.5 (0.4–5.2) | 0.54 |
| <100 | 448 | 8.0 (3.2–25.8) | 1.6 (0.5–5.0) | 0.44 |
Adjusted for sex, age, study site, BMI, abnormal CXR and CD4 count.
P < 0.05.
1+ = smear-positive, 4–99 AFB/100 fields.
≥2+ = smear-positive, >100 AFB/100 fields.
OR = odds ratio; CI = confidence interval; aOR = adjusted OR; TB = tuberculosis; BMI = body mass index; IQR = interquartile range; CXR = chest X-ray; AFB = acid-fast bacilli.
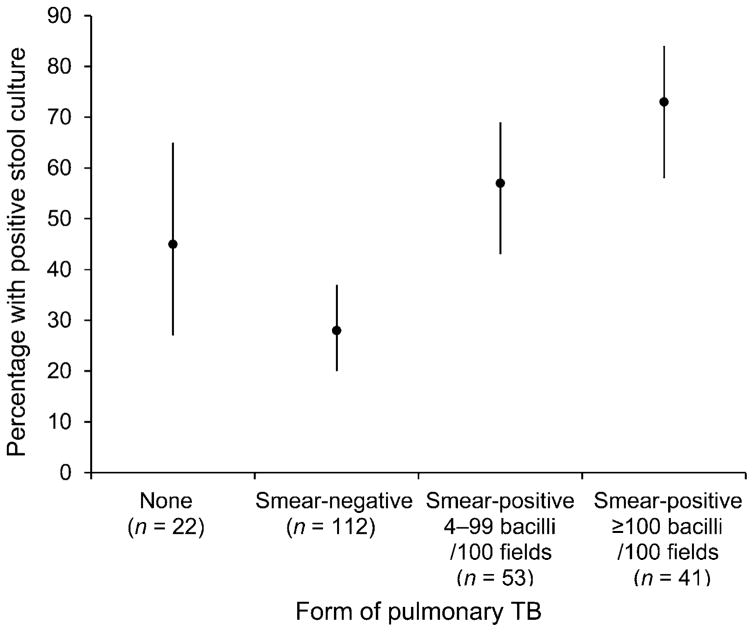
The sensitivity of stool culture for PTB was 44% (91/206, or 37% if contaminated stool cultures were included in the denominator). As the sputum bacillary concentration increased, the likelihood of stool culture positivity also increased (Figure 2, Cochran-Armitage test for trend P < 0.001). Although only 24 (24%) participants with a positive stool culture had positive stool smear microscopy, we found that as the bacillary burden of stool specimens increased, the bacillary burden of sputum cultures increased (trend P < 0.001). The specificity of stool smear microscopy as compared to stool culture was 99% (1581/1589).
Figure 2.
Relationship between sputum bacillary concentration and positive stool culture in persons with pulmonary TB. Cochrane-Armitage test for trend P < 0.001. TB = tuberculosis.
For the entire group of 228 participants with TB, one stool smear had an IY of 3% (95%CI 1–6) over two sputum smears, similar to the IY of a third sputum smear (3%, 95%CI 1–6). One stool culture had an IY of 5% (95%CI 3–8) over two sputum cultures, while an additional sputum culture had an IY of 7% (95%CI 4–11; Table 3). For persons who produced low quality or inadequate sputum, the IY of a stool smear or culture over two sputum smears or cultures was respectively 2% (95%CI 1–7) and 7% (95%CI 3–13). A third sputum smear or culture in this population had an IY of respectively 0% (95%CI 0–3) and 3% (95%CI 1–8). None of the differences measured between the IY of testing a stool specimen vs. an additional sputum specimen were statistically significant.
Table 3.
Incremental yield of stool specimen as compared to an additional pulmonary specimen
| Incremental yield n (%) |
95%CI | |
|---|---|---|
| Patients with tuberculosis (n = 228) | ||
| 1 sputum smear (yield = 66) | ||
| 1 stool smear | 8 (4) | 2–7 |
| 1 additional sputum smear | 18 (8) | 5–12 |
| 2 sputum smears (yield = 84) | ||
| 1 stool smear | 6 (3) | 1–6 |
| 1 additional sputum smear | 6 (3) | 1–6 |
| 1 sputum culture (yield = 162) | ||
| 1 stool culture | 23 (10) | 7–15 |
| 1 additional sputum culture | 29 (13) | 9–18 |
| 2 sputum cultures (yield = 191) | ||
| 1 stool culture | 11 (5) | 3–8 |
| 1 additional sputum culture | 15 (7) | 4–11 |
| Tuberculosis patients with low quality or low volume sputum* (n = 106) | ||
| 1 sputum smear (yield = 30) | ||
| 1 stool smear | 4 (4) | 2–9 |
| 1 additional sputum smear | 11 (10) | 6–18 |
| 2 sputum smears (yield = 41) | ||
| 1 stool smear | 2 (2) | 0.5–6.6 |
| 1 additional sputum smear | 0 | 0–4 |
| 1 sputum culture (yield = 71) | ||
| 1 stool culture | 11 (10) | 6–18 |
| 1 additional sputum culture | 14 (13) | 8–21 |
| 2 sputum cultures (yield = 85) | ||
| 1 stool culture | 7 (7) | 3–13 |
| 1 additional sputum culture | 3 (3) | 1–8 |
Volume <3 ml and saliva-like appearance.
CI = confidence interval.
DISCUSSION
In this large study assessing the utility of stool culture for diagnosing TB, we found that TB could be identified using stool cultures in 44% of 228 PLHIV with TB diagnosed using conventional solid or liquid culture. The diagnosis of nine participants (4%) for whom stool culture was the only culture-positive specimen for TB indicates that, if available, stool cultures may increase the number of persons living with HIV diagnosed with TB. This value must be counterbalanced against the increased processing requirements and higher culture contamination rates associated with culturing stool. The graded, increasing association between sputum bacillary concentration and a positive stool culture, along with the lack of association between positive stool culture and EPTB or gastrointestinal symptoms suggest that swallowed pulmonary M. tuberculosis bacteria that survive transit through the gastrointestinal tract are largely responsible for positive stool cultures in PLHIV.
The sensitivity of 44% is too low to suggest that stool specimens should replace sputum specimens for TB diagnosis, and is lower than the sensitivity reported in other studies. In a series of 52 patients with TB who also had HIV, stool cultures grew M. tuberculosis in 52% of the 19 patients assessed, and AFB microscopy was positive in 42%,16 as compared to 44% stool culture positivity and 11% stool microscopy positivity in our study. Stool cultures and microscopy specimens were positive in 75% of persons in another series; however, the sensitivity may have been higher because most patients had advanced immune suppression indicated by acquired immune-deficiency syndrome or clinical stage C disease.17 Our IY analysis of all PLHIV with concurrent TB disease indicates that an additional stool specimen performed similarly to the corresponding additional sputum specimen; however, our analysis was not powered to assess this difference. We have not found any published studies assessing stool culture yield for PLHIV unable to expectorate sputum; this merits further evaluation, as this may be a population for whom stool culture may have utility. Stool specimens may also aid in the diagnosis of TB in children who cannot readily produce sputum; however, we were unable to assess this with our data as few children were enrolled.18–20
Our finding that the bacillary burden in stool correlates with the bacillary burden in sputum cultures suggests that bacteria are being swallowed, and that some proportion of those bacteria survive gastrointestinal transit and grow in culture. Stool polymerase chain reaction (PCR) may be a more appropriate diagnostic test for persons with low bacillary-burden PTB or smear-negative PTB, as non-viable bacteria might still be identified. Evaluation of stool PCR in PLHIV may be worthwhile to determine whether the IY is higher than what we found for culture. In settings where routine PCR is unfeasible, the Xpert® MTB/RIF assay may be performed with minimal infrastructure, has reduced running time to just under 2 h and is feasible for use in peripheral laboratories.21,22 Xpert MTB/RIF also has satisfactory performance on non-respiratory specimens23,24 and in PLHIV.22 With Xpert MTB/RIF demonstrating utility in children with pulmonary TB25 and stool PCR testing suggesting utility in children,20,26 it will be important to evaluate Xpert MTB/RIF on stool in children with and without HIV.
While our study found a significant relationship with PTB and stool cultures growing M. tuberculosis, we are limited in our ability to comment on the relationship between EPTB and positive stool cultures. Although each participant underwent extensive evaluations for EPTB (blood, urine, stool and lymph node aspiration, if lymphadenopathy was present), we may have missed cases of EPTB, as other sites were not cultured for TB. In our analysis, we did not include 295 PLHIV who did not have an evaluable stool culture. There was, however, no difference in the sputum bacillary concentration or prevalence of PTB and EPTB between participants with and those without evaluable stool cultures. We believe that the positive stool cultures in our study indicate bacilli of pulmonary origin; however, participants were not routinely evaluated for intestinal TB by recommended procedures such as endoscopy or abdominal imaging.27,28 Due to small numbers, we were limited in our ability to compare PLHIV with a positive stool culture with 1) participants with EPTB only, or 2) EPTB and PTB.
CONCLUSIONS
In this population of PLHIV, stool cultures demonstrated potential utility for the diagnosis of TB, although they did not perform better than additional sputum cultures, and the IY of diagnoses relative to the added burden on the laboratory was low. Sputum should remain the diagnostic specimen of choice for pulmonary TB; however, in persons who are unable to produce an adequate sputum specimen for analysis, stool culture may be a useful specimen for diagnosing TB in some settings. Prospective evaluations of stool testing, preferably with PCR-based techniques, should be undertaken in persons unable to produce adequate sputum, including PLHIV and children with and without HIV.
Acknowledgments
This study was supported by a grant from the US Agency for International Development. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis control 2011. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.16. [Google Scholar]
- 2.Harries A, Maher D, Graham S. TB/HIV: a clinical manual. 2. Geneva, Switzerland: WHO; 2004. pp. 24–57. [Google Scholar]
- 3.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a West African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Cain KP, Kanara N, Laserson KF, et al. The epidemiology of HIV-associated tuberculosis in rural Cambodia. Int J Tuberc Lung Dis. 2007;11:1008–1013. [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis report 2012. Geneva, Switzerland: WHO; 2012. [Accessed May 2013]. WHO/HTM/TB/2012.6. http://www.who.int/tb/publications/global_report/en/index.html. [Google Scholar]
- 6.Cordova J, Shiloh R, Gilman RH, et al. Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol. 2010;48:1820–1826. doi: 10.1128/JCM.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin PY, Wang JY, Hsueh PR, et al. Lower gastrointestinal tract tuberculosis: an important but neglected disease. Int J Colorectal Dis. 2009;24:1175–1180. doi: 10.1007/s00384-009-0721-3. [DOI] [PubMed] [Google Scholar]
- 8.Conlon CP, Banda HM, Luo NP, et al. Faecal mycobacteria and their relationship to HIV-related enteritis in Lusaka, Zambia. AIDS. 1989;3:539–541. doi: 10.1097/00002030-198908000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Manatsathit S, Tansupasawasdikul S, Wanachiwanawin D, et al. Causes of chronic diarrhea in patients with AIDS in Thailand: a prospective clinical and microbiological study. J Gastroenterol. 1996;31:533–537. doi: 10.1007/BF02355053. [DOI] [PubMed] [Google Scholar]
- 10.Mwachari C, Batchelor BI, Paul J, Waiyaki PG, Gilks CF. Chronic diarrhoea among HIV-infected adult patients in Nairobi, Kenya. J Infect. 1998;37:48–53. doi: 10.1016/S0163-4453(98)90561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 12.Monkongdee P, McCarthy KD, Cain KP, et al. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am J Respir Crit Care Med. 2009;180:903–908. doi: 10.1164/rccm.200905-0692OC. [DOI] [PubMed] [Google Scholar]
- 13.de Kantor IN, Kim SJ, Frieden T, et al. Laboratory services in tuberculosis control. Part II: Microscopy. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 14.Rieder HL, Van Deun A, Kam KM, et al. Priorities for tuberculosis bacteriology services in low-income countries. 2. Paris, France: International Union Against Tuberculosis and Lung Disease; 2007. [Google Scholar]
- 15.Patetta M. Categorical data analysis using logistic regression: course notes. Cary, NC, USA: SAS Institute Inc; 2009. pp. 5.25–5.28. [Google Scholar]
- 16.Kramer F, Modilevsky T, Waliany AR, Leedom JM, Barnes PF. Delayed diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Am J Med. 1990;89:451–456. doi: 10.1016/0002-9343(90)90375-n. [DOI] [PubMed] [Google Scholar]
- 17.Murcia-Aranguren MI, Gomez-Marin JE, Alvarado FS, et al. Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogota, Colombia. BMC Infect Dis. 2001;1:21. doi: 10.1186/1471-2334-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberhelman RA, Soto-Castellares G, Caviedes L, et al. Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediatrics. 2006;118:e100–e106. doi: 10.1542/peds.2005-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donald PR, Schaaf HS, Gie RP, et al. Stool microscopy and culture to assist the diagnosis of pulmonary tuberculosis in childhood. J Trop Pediatr. 1996;42:311–312. doi: 10.1093/tropej/42.5.311. [DOI] [PubMed] [Google Scholar]
- 20.Oberhelman RA, Soto-Castellares G, Gilman RH, et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study. Lancet Infect Dis. 2010;10:612–620. doi: 10.1016/S1473-3099(10)70141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaitre N. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011;49:1772–1776. doi: 10.1128/JCM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extra-pulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49:1202–1205. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11:819–824. doi: 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf H, Mendez M, Gilman RH, et al. Diagnosis of pediatric pulmonary tuberculosis by stool PCR. Am J Trop Med Hyg. 2008;79:893–898. [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–999. [PubMed] [Google Scholar]
- 28.Gilinsky NH, Marks IN, Kottler RE, Price SK. Abdominal tuberculosis. A 10-year review S Afr Med J. 1983;64:849–857. [PubMed] [Google Scholar]