Abstract
Occlusion therapy throughout early childhood is believed to be efficacious in treating deprivation amblyopia but has not been rigorously assessed in clinical trials. Further, tools to assess adherence to such therapy over an extended period of time are lacking. Using data from the Infant Aphakia Treatment Study, a randomized clinical trial of treatment for unilateral congenital cataract, we examined the use of quarterly 48-h recall interviews and annual 7-day prospective diaries to assess reported hours of patching in 114 children throughout the first 5 years of life. Consistency of data reported was assessed using correlation coefficients and intraclass correlation coefficients. Both interview and diary data showed excellent consistency with Cronbach’s Alpha’s ranging from 0.69 to 0.88 for hours of patching and 0.60 to 0.73 for hours of sleep. However, caregivers reported somewhat more adherence in prospective diaries than retrospective interviews. Completion rates, on the other hand, were substantially higher for telephone interviews than prospective diaries. For example, four years after surgery response rates to telephone interviews exceeded 75% versus completion rates of only 54% for diaries. In situations where occlusion dose monitors cannot be used for assessing adherence to occlusion therapy, such as in infants or over an extended period of time, quantitative assessments of occlusion therapy can be obtained by parental report, either as a series of prospective diaries or a series of recall interviews.
Keywords: Congenital cataract, Occlusion therapy, Adherence, Diary, Recall interview
Abbreviations: IOL, intraocular lens; CL, contact lens; IATS, Infant Aphakia Treatment Study; D, diopters; ODM, occlusion dose monitors
1. Introduction
Deprivation amblyopia, such as that caused by unilateral infantile cataract, requires long-term occlusion of the unaffected eye [1], [2]. Although occlusion is a standard part of treatment, its efficacy has not been rigorously assessed [3]. Further, because occlusion is often difficult for caregivers to administer [4], [5], there is a high-level of non-concordance between prescribed and achieved occlusion [6], [7], [8], [9], [10], [11]. Thus, the ability to assess adherence to occlusion therapy is important for clinicians caring for, as well as researchers.
Standard methods for assessing adherence to occlusion are not available. The assessment of adherence has utilized qualitative parental questionnaires [4], [6], [7], [10] parental report combined with attendance at clinic visits [12], diaries and recall interviews [8], [13], and occlusion dose monitors (ODMs) [14], [15], [16], [17], [18]. ODMs have the potential advantage of providing quantitative data [14], [15], [16], [18], [19] and have been successfully used over short periods of time (e.g., <6 months) in older children. However, these methods are often supplemented with patching diaries because of concerns about battery life and/or other technical problems [20]. Further, ODM use in infants may be unacceptable to caregivers, and may not be tolerated extended periods. ODMs also are less accurate in higher ambient temperatures and less reliable when the patch is worn under spectacles [21]. Finally, ODMs are not yet commercially available in the United States. Patching diaries can produce quantitative estimates of occlusion that correlate well with estimates obtained using ODMs [15], [16], but have been criticized for overestimating adherence [22] and for being unreliable [15], [23].
Thus, there is a need to develop and assess methods to assess adherence to prescribed patching over extended periods, starting in infancy. The Infant Aphakia Treatment Study (IATS) presented this opportunity in a well characterized sample of children. Our goals were to determine whether caregivers could reliably report adherence to prescribed patching in retrospective telephone interviews and/or prospective diaries, and the relative costs and benefits of assessing adherence using these two methods.
2. Methods
2.1. Subjects and methods
The overall design of the IATS and results of the visual acuity assessment at 4.5 years of age have previously been published [24], [25], [26]. Briefly, the IATS was a randomized controlled trial comparing two treatments for unilateral congenital cataract in infants undergoing cataract extraction between 28 days and 7 months of age: contact lens (CL) correction of aphakia versus primary intra-ocular lens (IOL) implantation with spectacle correction of residual refractive error if needed. The study was approved by the institutional review boards of all participating institutions and was in accordance with the tenets of the Declaration of Helsinki.
2.2. Prescribed patching and visual correction
Patching was prescribed for all patients until their fifth birthday. Starting the second week after cataract surgery, caregivers were instructed to have the child wear an adhesive occlusive patch over the fellow eye 1 h daily per month of age until the child was eight months old. Thereafter, caregivers were told to patch their child 50% of waking hours. Patches were provided to patients at no cost to minimize financial barriers to patching. Deviations from prescribed patching protocols, both over- and under-patching, were not considered to be protocol violations.
Refractive correction was prescribed for all children 100% of waking hours. Within a week after cataract surgery, patients randomized to the CL group were fitted with a silicone (Silsoft; Bausch & Lomb, Rochester, New York) or a rigid gas permeable contact lens with a 2.0-D overcorrection to provide a near-point correction. Parents were provided with a spare contact lens. Both daily wear and extended wear protocols were acceptable, given the preferences of the treating physicians. At 2 years of age, the eye was corrected to emmetropia using a CL, and spectacles were prescribed with a D segment bifocal lens with an add of +3.0 D for near focus.
For infants randomized to the IOL group, spectacles were prescribed by the 1-month postoperative visit if any of the following conditions existed: hyperopia of more than 1.0 D, myopia of more than 3.0 D, or astigmatism of more than 1.5 D. In children younger than 2 years, the aim was to correct the refractive error to 2.0 D of myopia, whereas in children 2 years or older, the aim was emmetropia at distance with a near correction of +3.0 D. The phakic eye for both groups was corrected with spectacles if 1 of the following conditions existed: hyperopia > 5.0 D, myopia > 5.0 D, astigmatism > 1.5 D, or refractive esotropia. The aim was to correct the refractive error to the range of 0 to +3.0 D in the phakic eye. When required, spectacles were to be worn 100% of waking hours.
2.3. Assessment of adherence
Adherence to prescribed patching and refractive correction, and hours of sleep were reported by caregivers using two different methodologies: a retrospective telephone interview every 3 months and an annual prospective diary. The telephone interview and diary collected similar information. In the diary parents reported sleep patterns, patch use, contact lens wear and spectacle wear over a seven day prospective period (see appendix). Diaries were completed two months after surgery and at 13, 25, 37 and 49 months of age. The diary was mailed to the caregiver, who returned the diary to the DCC following completion. A staff member called the caregiver after mailing the diary to ensure that the diary had been received and to remind the caregiver to begin documenting use of the patch.
The telephone interviews were completed quarterly, starting 3 months after surgery and continuing until the child was five years of age, and used a semi-structured interview to elicit the same information as reported on the patching diary for the previous 48-h. The timing of the interview was determined using an algorithm that distributed the preferred day of the call evenly throughout the week since patching has been reported to differ on weekdays and weekend days [18]. Caregivers were not informed in advance about the specific day or times of the interview. The interviews were conducted by one of three trained interviewers (one English-speaking, one Spanish-speaking, and one Portuguese-speaking) in the caregiver’s primary language so that the caregiver was interviewed by the same person on each occasion. The vast majority of interviews (>95%) were performed by the English-speaking interviewer. The interviewers were located at the Data Coordinating Center to minimize the possibility that the respondent would exaggerate their adherence or that the interviewer’s interpretation of the information would be biased by knowledge of the child’s visual acuity or treatment.
Measures of adherence were derived from caregivers’ reports regarding the times that patch was placed and removed. Similar data were available regarding CL wear, spectacle wear and sleep. We used this information to calculate the number of hours per day that the patch was worn, and the number of hours that the child slept each day. For diaries, this represented an average over the 7 days covered by the diary; for interviews, this represented an average over 48 h. For purposes of the current analysis, we did not assess adherence to contact lens and spectacle wear because of the wide variety of prescribed regimens associated with refractive correction.
2.4. Analytic methods
Analyses were conducted using SPSS 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp) and SAS 9.2 (SAS Institute Inc. Cary, NC) statistical packages. Intraclass correlation coefficients were used to assess the consistency of reports of daily hours of patching and sleep over time. Pearson’s correlation coefficients were used to compare daily hours of patching and sleep reported on each diary to the same information reported on the closest 48-h interview. Similarly, we estimated the mean difference in number of hours of patching reported on diaries to the same information reported on the closest 48-h interview to determine whether information reported on diaries differed systematically from information reported in the interviews.
3. Results
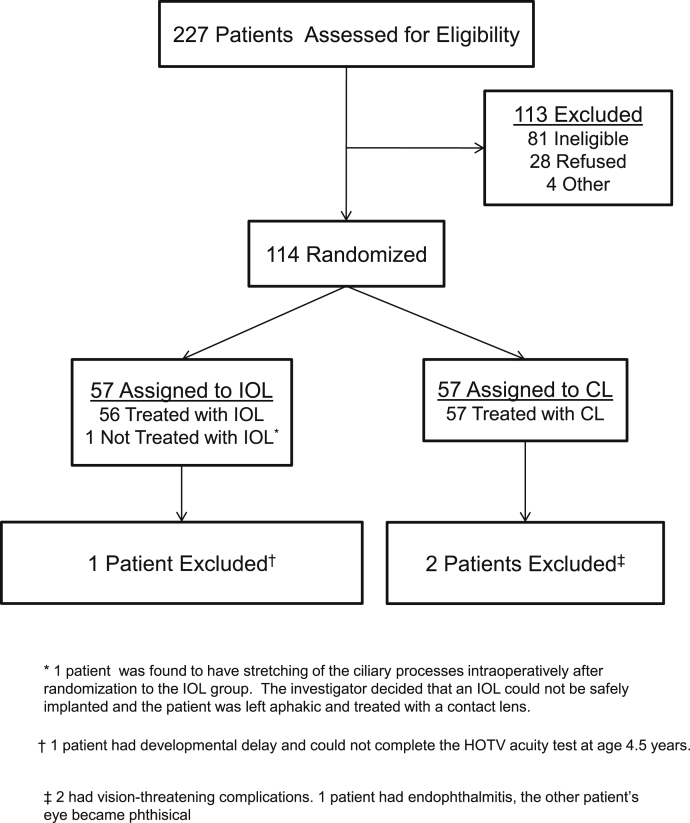
114 infants participated in IATS: 57 were randomized to receive an IOL at the time of cataract extraction and 57 were randomized to remain aphakic (Fig. 1). Surgery was performed on all infants between 28 days and 7 months of life. Additional details regarding the population are provided in other publications [25], [26]. The current analyses exclude two children for whom patching of the fellow eye was discontinued because of adverse events resulting in loss of visual potential in the treated eye (n = 2) and a third child with Stickler’s Syndrome who had better vision in the treated eye than in the fellow eye.
Fig. 1.
Consort diagram for the Infant Aphakia Treatment Study.
3.1. Completion of interviews and diaries
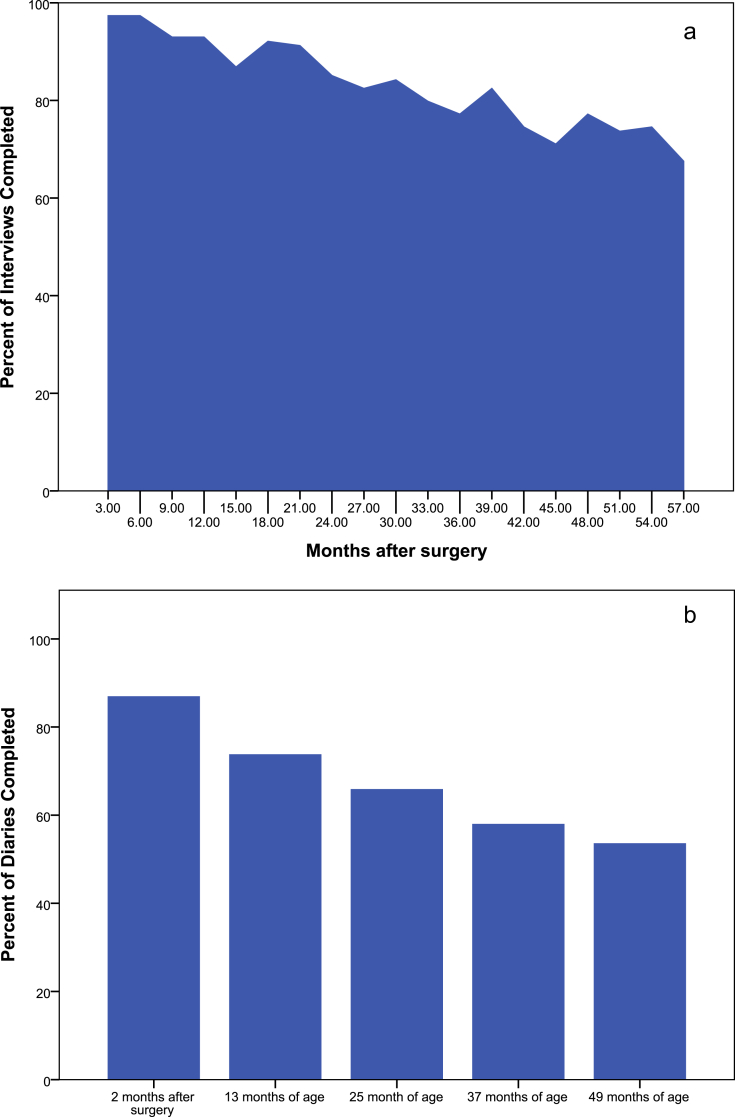
Fig. 2 shows completion rates for the 48-h interviews by time since surgery (panel a) and the diaries by age (panel b). On average, completion rates were higher for the 48-h recall interviews than for the prospective diaries. For example, 87% of caregivers completed the first diary two months after surgery; one month later the completion rate for the 48-h interview was ten percent higher (97%). The completion rates of both interviews and diaries declined over time. Even so, nearly five years after surgery, the interviews were completed by nearly three-quarters of caregivers (73%) while only about half (54%) of the caregivers returned the diary when the children were four years of age. Forty-three (38%) of the caregivers completed all five diaries and an additional 24 (21%) completed four of five diaries. Eight (7.0%) caregivers did not complete any of the diaries. On the other hand, all caregivers completed at least four of the 48-h recall interviews and nearly two-thirds (n = 71, 62.2%) completed 17 or more interviews. All participants had adherence data available for the first year following surgery with three-quarters (n = 89, 78.1%) completing all five possible assessments in this first year. Although there was some tendency for caregivers who did not provide adherence data at subsequent time points to report less adherence with occlusion than those who did provide data, these differences were neither consistent nor did they approach statistical significance (data not shown).
Fig. 2.
Completion Rates for Interviews (panel a) and Diaries (panel b) by Time since Surgery.
Substantial effort was required to collect these data. Half of all interviews were completed within two phone calls, regardless of the time since surgery. However, numerous attempts were required to successfully collect data on some participants. For example, at nearly all time points, more than 10 contact attempts were required in order to collect data on one or more participants.
3.2. Agreement between patching reports
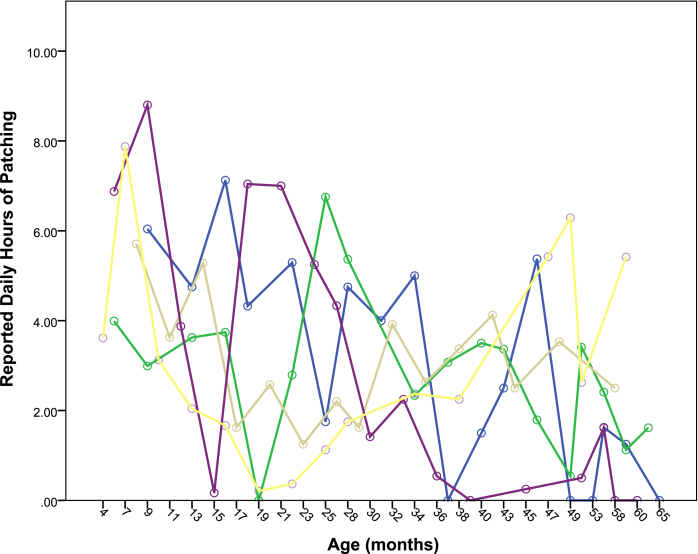
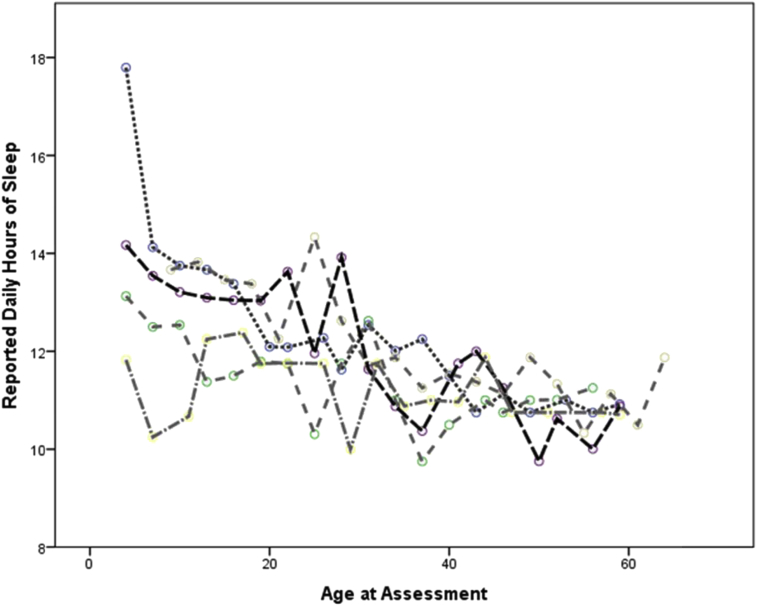
There was substantial inter- and intra-subject variation in the amount of patching reported. For example, in the first twelve months after surgery, the range of average hours of patching per day ranged from 0.37 to 7.63 and the interquartile range of reported adherence ranged from just under 3 h per day to nearly 5 ½ hours. The intra-subject variation in reported hours of patching per day is demonstrated in Fig. 3 which shows the reported hours of patching by age for the five participants who completed at least sixteen recall interviews and for whom the overall average hours patched per day was between 3.8 and 4.2 h, which was approximately the median amount of patching reported. Such variation was not present for reported hours of sleeping per day (see appendix).
Fig. 3.
Reported daily hours of patching by age at assessment for 5 selected participants with reported patching that averaged between 3.8 and 4.2 h per day.
Even considering the high degree of intra-subject variation in patching, data on adherence to patching were highly correlated with the same data reported three months later and were more strongly associated than were reports of hours of sleep per night (correlation coefficients ranging from 0.59 to 0.83 for occlusion and from 0.13 to 0.60 for hours of sleep). Further, the data reported on interviews and data reported on diaries were highly consistent. For example, the Cronbach’s Alpha for the four interviews conducted within the first twelve months after surgery is 0.69 for reported hours of patching per day and 0.67 for reported hours of sleep each night (Table 1). Additionally, daily hours of patching reported on each diary was strongly correlated with data reported by the caregivers on an interview conducted within 3 months (Table 2). However, in general, caregivers reported more patching on diaries than on interviews, even when these assessments were reported around the same age, and the 95% confidence interval for this difference excluded the null at 13 and 25 months of age.
Table 1.
Consistency of reported average waking hours patched per day by method of data collection.
| N | Waking hours patched per day |
||
|---|---|---|---|
| Median (IQR) | Cronbach’s alpha (95% CI) | ||
| Interviews conducted in 1st year post surgerya | 93 | 4.09 (2.80,5.44) | 0.69 (0.58,0.8) |
| Interviews conducted in 2nd year post surgeryb | 86 | 3.41 (1.43,5.00) | 0.85 (0.79,0.90) |
| Interviews conducted in 3rd year post surgeryc | 71 | 3.51 (1.96,5.46) | 0.88 (0.82,0.92) |
| Interviews conducted in 4th year post surgeryd | 62 | 3.41 (1.73,5.52) | 0.87 (0.82,0.92) |
| All Diariese | 43 | 3.79 (1.73,5.00) | 0.86 (0.78,0.92) |
Interviews conducted 3, 6, 9 and 12 months after surgery.
Interviews conducted 15, 18, 21 and 21 months after surgery.
Interviews conducted 24, 27, 30, and 33 months after surgery.
Interviews 36, 39, 42 and 45 months after surgery.
Diaries 2 months after surgery and at 14, 26, 38 and 50 months of age.
Table 2.
Correlationa between adherence reported on the 7-day prospective diary and adherence reported on the 48-h telephone recall interview closest in time to the diary.
| N | Days between diary and interviewb mean + SD (range) | Correlationc | Reported waking hours patched |
Mean difference (95% CI) | ||
|---|---|---|---|---|---|---|
| 48-h interview | 7-Day diary | |||||
| 2 months after surgeryd | 98 | −19.07 ± 13.03 (−64,33) | 0.384 | 4.27 ± 2.03 | 3.91 ± 1.65 | 0.36 (−0.05,0.77) |
| 13 months of age | 84 | −1.00 ± 30.41 (−50,54) | 0.765 | 3.68 ± 2.36 | 4.22 ± 1.87 | −0.54 (−0.88,−0.21) |
| 25 months of age | 73 | −8.30 ± 22.42 (−57,46) | 0.752 | 3.64 ± 2.66 | 4.15 ± 2.32 | −0.59 (−0.93,−0.09) |
| 37 months of age | 56 | −1.84 ± 25.34 (−45,53) | 0.848 | 4.10 ± 2.98 | 3.89 ± 2.35 | 0.21 (−0.22,0.63) |
| 49 months of age | 55 | −3.96 ± 27.30 (−57,58) | 0.647 | 3.22 ± 2.79 | 3.51 ± 2.49 | −0.29 (−0.91,0.32) |
All correlations were statistically significant, p < 0.001.
Diary - Interview.
Pearson’s Correlation Coefficient between data reported on the 48-Hour Telephone Interview and data reported on the 7-Day Diary.
Note: According to the patching protocol, patching in the 7-day diary conducted 2 months after surgery should be, on average, 1 h less than patching at the 1st interview which was conducted 3 months after surgery.
4. Discussion
The IATS experience provides information on assessing adherence to occlusion and visual correction in very young children in a clinical trial of treatment for unilateral congenital cataracts. Our findings suggest that caregivers can report information that can be used to distinguish families who are able to adhere to prescribed post-surgical treatment from those who struggle with patching. Such data are of importance in both research and clinical practice. In particular, a recent Cochrane Review recently recommended clinical trials be conducted to evaluate the efficacy of occlusion therapy for children with stimulus deprivation amblyopia, such as caused by unilateral cataract [3]. The methods we investigate here would allow for a quantitative estimation of adherence to occlusion therapy in such a trial. However, our data suggest that collecting these data requires a significant investment of resources in order to provide a relatively complete picture of adherence in young children.
These findings confirm reports that there is a high degree of intra- and inter-individual variability in reported adherence to patching [5], which is likely to reflect true variability in adherence day to day, as well as over more extended time periods such as the five year follow-up reported here. Further, our data highlight the potential differences between the dose rate, defined as the average number of hours of patching per day, and the accumulated dose of occlusion, defined as the total amount of patching experienced by a child [15]. Thus, for conditions, such as unilateral cataract, where occlusion therapy is prescribed for months, or even years, collecting these data over time provides a fuller picture of accumulated dose of patching.
Frequent contact with families demonstrated to caregivers that the study had a sincere interest in their child and allowed strong relationships between caregivers and study staff to develop, which may be an additional benefit of collecting these data on a regular basis in clinical trials. Specifically, in the IATS study, follow-up of participants was 100% for the assessment of visual acuity at twelve months of age [27] and 99% at age 4.5 years [26]. We ascribe at least some of the successful follow-up of these participants to the interpersonal relationships that families developed with the staff who completed the 48-h interviews every 3 months.
These data demonstrate that caregivers can continue to successfully patch their young children over an extended period of time, even if during specific periods, they achieve only minimal patching. As evidenced in Fig. 2, a number of caregivers reported very little patching at some points, but reported being able to successfully occlude their child on subsequent assessments. The message that suboptimal patching at one point in time does not mean that the child cannot achieve an adequate accumulated dose of patching is an important message for caregivers, particularly during the second and third years of life when young children may express more resistance to patching [7], [28].
A number of investigators have suggested that adherence to patching is more accurately estimated using Occlusion Dose Monitors (ODM) than using parental report. They further suggest that parental reports overestimate adherence to occlusion, are not free from bias and require observation of entire treatment period [15]. It is possible that the amount of patching that we report here is overestimated. However, previous reports have shown good relationships between occlusion measured via parental diary and ODM. Further, in the current study, caregivers reported an average of three to 4 h of occlusion per day, a level which is similar to the average adherence to occlusion reported in a number of studies which used ODMs to assess adherence [16]. However, the amount of patching that we report is somewhat higher than that provided by other reports [29], [30]. This may reflect the support, such as newsletters and free patches that were provided to caregivers as part of IATS.
Our methods can be used in situations in which ODMs might not be appropriate or acceptable. In our own study, for example, ODMs were not deemed acceptable for use with infants and other young children because of parental and IRB concerns about risks associated with the devices. Additionally, we monitored adherence over a nearly five year period. It may be difficult to use ODMs over this extended time. Finally, using parental reports, we were able to successfully monitor adherence on both occlusion and use of contact lenses and spectacles. As currently configured, ODMs are only able to monitor adherence to the use of eye patches for occlusion. Methods to separately monitor adherence to spectacle use and occlusion are under development [21].
We suggest some caution in interpreting our findings and in applying them in other contexts. We were able to collect adherence data from all caregivers in the first year after surgery, 111 caregivers in the second year, 95 caregivers in the third year, 94 caregivers in the fourth year and 91 caregivers in the fifth year of follow up. However, for some specific time periods, the number of respondents is relatively small. This is particularly true for the diary at around four years of age, which was completed by only about half of participants. Additionally, only 43 (38%) caregivers completed all five diaries. If higher levels of adherence are reported by caregivers who returned all the diaries, our estimates of the number of hours of patching reported by caregivers could be overestimated. We have little evidence to suggest that this occurred, however, as the reported number of hours of patching on each diary was not significantly associated with the likelihood of responding to the all diaries (See Supplemental Table 2).
We also suggest caution in applying our estimates of the amount of patching achieved by caregivers to other contexts. We expect that adherence to both patching and visual correction obtained in IATS may be higher than is usual because the IATS provided glasses, contact lenses and patches to families. Thus, there may have been fewer financial barriers to adhering to prescribed post-surgical treatment than in other situations. Also the quarterly interviews and clinic visits may have provided additional motivation and support to families to continue occlusion therapy.
We further note that the caregivers were aware that these data were collected independently of the clinical staff that was monitoring their children’s visual acuity and ocular health. It is thus possible that the data from caregivers of IATS participants were less subject to social desirability bias than that obtained in other contexts. Further, these data are unlikely to be comparable to qualitative data assessed by clinicians in an office context where both social desirability and the results of visual acuity assessments might impact the assessment.
In summary, we believe that our findings show that adherence to patching and visual occlusion can be successfully reported by caregivers of infants and young children over an extended period of time. We further conclude that, although either prospective diaries or retrospective telephone interviews may be used to gather quantitative estimates of patching, obtaining data via quarterly telephone interview may be preferred given the higher completion rates obtained. Additionally, the frequent telephone contact may have an added benefit of enhancing retention in clinical research. Thus, it is possible that if we had performed additional telephone reminders the completion rates for the diaries might have improved. However, our findings suggest that collecting these data requires a substantial commitment of resources on the part of the investigators.
Funding/support
Supported through a cooperative agreements from the National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY006360 and Research to Prevent Blindness, Inc, New York, New York.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.conctc.2016.05.009.
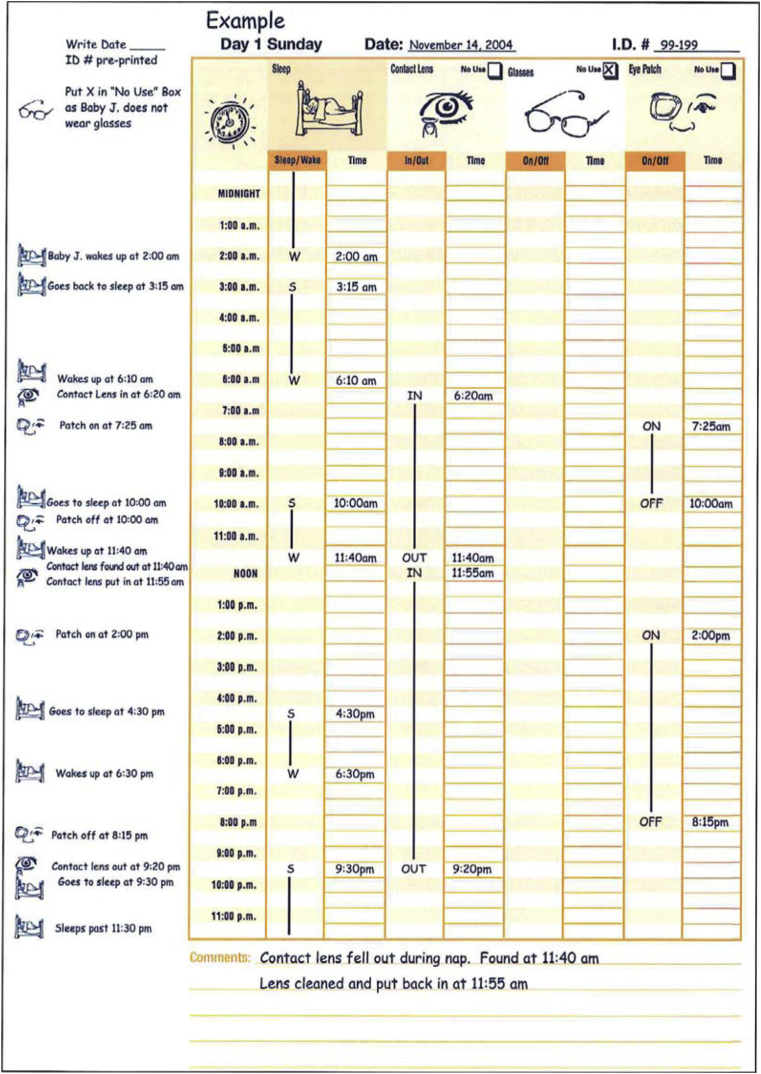
Appendix 1. Example of data collection for diaries and interviews
Appendix 2. Reported daily hours of sleep by age at assessment for 5 selected participants with reported patching that averaged between 3.8 and 4.2 h per day
Appendix 3. The Infant Aphakia Treatment Study Group
Administrative Units
Clinical Coordinating Center (Emory University): Scott R. Lambert, MD (Study Chair); Lindreth DuBois, MEd, MMSc (National Coordinator).
Contact Lens Committee: Buddy Russell, COMT; Michael Ward, MMSc.
Data and Safety Monitoring Committee: Robert Hardy, PHD (Chair); Eileen Birch, PhD; Ken Cheng, MD; Richard Hertle, MD; Craig Kollman, PhD; Marshalyn Yeargin-Allsopp, MD (resigned); Cyd McDowell; Donald F. Everett, MA (ex officio).
Data Coordinating Center (Emory University): Michael Lynn MS (Director), Betsy Bridgman, BS; Marianne Celano PhD; Julia Cleveland, MSPH; George Cotsonis, MS; Carey Drews-Botsch, PhD; Nana Freret, MSN; Lu Lu, MS; Seegar Swanson; Thandeka Tutu-Gxashe, MPH.
Eye Movement Reading Center (University of Alabama, Birmingham and Retina Foundation of the Southwest, Dallas, TX): Claudio Busettini, PhD, Samuel Hayley, Joost Felius, PhD.
Medical Safety Monitor: Allen Beck, MD.
Program Office (National Eye Institute): Donald F. Everett, MA.
Steering Committee: Scott R. Lambert, MD; Edward G. Buckley, MD; David A. Plager, MD; M. Edward Wilson, MD; Michael Lynn, MS; Lindreth DuBois, Med MMSc; Carolyn Drews-Botsch, PhD; E. Eugenie Hartmann, PhD; Donald F. Everett, MA.
Vision and Developmental Testing Center (University of Alabama, Birmingham): E. Eugenie Hartmann, PhD (Director); Anna K Carrigan, MPH; Clara Edwards;
Participating Clinical Centers (In order by the number of patients enrolled):
Medical University of South Carolina; Charleston, South Carolina (14): M. Edward Wilson, MD; Margaret Bozic, CCRC, COA.
Harvard University; Boston, Massachusetts (14): Deborah K. Vanderveen, MD; Theresa A. Mansfield, RN; Kathryn Bisceglia Miller, OD.
University of Minnesota; Minneapolis, Minnesota (13): Stephen P. Christiansen, MD; Erick D. Bothun, MD; Ann Holleschau, B.A.; Jason Jedlicka, OD; Patricia Winters, OD; Jacob Lang, O.D.
Cleveland Clinic; Cleveland, Ohio (10): Elias I. Traboulsi, MD; Susan Crowe, BS, COT; Heather Hasley Cimino, OD.
Baylor College of Medicine; Houston, Texas (10): Kimberly G. Yen, MD; Maria Castanes, MPH; Alma Sanchez, COA; Shirley York.
Emory University; Atlanta, Georgia (9): Scott R. Lambert, MD; Amy K. Hutchinson, MD; Lindreth Dubois, Med, MMSc; Rachel Robb, MMSc; Marla J. Shainberg, CO.
Oregon Health and Science University; Portland, Oregon (9): David T Wheeler, MD; Ann U. Stout, MD; Paula Rauch, OT, CRC; Kimberly Beaudet, CO, COMT; Pam Berg, CO, COMT.
Duke University; Durham, North Carolina (8): Edward G. Buckley, MD; Sharon F. Freedman, MD; Lois Duncan, BS; B.W. Phillips, FCLSA; John T. Petrowski, OD.
Vanderbilt University: Nashville, Tennessee (8): David Morrison, MD; Sandy Owings COA, CCRP; Ron Biernacki CO, COMT; Christine Franklin, COT.
Indiana University, Indianapolis, Indiana (7): David A. Plager, MD; Daniel E. Neely, MD; Michele Whitaker, COT; Donna Bates, COA; Dana Donaldson, OD.
Miami Children’s Hospital, Miami, Florida (6): Stacey Kruger, MD; Charlotte Tibi, CO; Susan Vega.
University of Texas Southwestern; Dallas, Texas (6): David R. Weakley, MD; David R. Stager Jr. M.D.; Joost Felius, PhD; Clare Dias, CO; Debra L. Sager; Todd Brantley, OD.
Case Western Reserve, Cleveland, Ohio (1): Faruk Orge, M.D.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Beller R. Good visual function after neonatal surgery for congenital monocular cataracts. Am. J. Ophthalmol. 1981;91(5):559–565. doi: 10.1016/0002-9394(81)90053-2. [DOI] [PubMed] [Google Scholar]
- 2.Birch E.E., Stager D.R. Prevalence of good visual acuity following surgery for congenital unilateral cataract. Archi. Ophthalmol. 1988;106(1):40–43. doi: 10.1001/archopht.1988.01060130046025. [DOI] [PubMed] [Google Scholar]
- 3.Antonio-Santos A., Vedula S.S., Hatt S.R., Powell C. Occlusion for stimulus deprivation amblyopia. Cochrane Database Syst Rev. 2014;91(Issue 2) doi: 10.1002/14651858.CD005136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon-Woods M., Awan M., Gottlob I. Why is compliance with occlusion therapy for amblyopia so hard? A qualitative study. Arch. Dis. Child. 2006;91(6):491–494. doi: 10.1136/adc.2005.090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webber A.L. Effect of amblyopia on self-esteem in children. Optom. Vis. Sci. 2008;85(11):1074–1081. doi: 10.1097/OPX.0b013e31818b9911. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zuhaibi S. Compliance of amblyopic patients with occlusion therapy: a pilot study. Oman J. Ophthalmol. 2009;2(2):67. doi: 10.4103/0974-620X.53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua B.E.G., Johnson K. A retrospective review of the associations between amblyopia type, patient age, treatment compliance and referral patterns. Clin. Exp. Ophthalmol. 2004;32(2):175–179. doi: 10.1111/j.1442-9071.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- 8.Newsham D. Parental non-concordance with occlusion therapy. Br. J. Ophthalmol. 2000;84(9):957–962. doi: 10.1136/bjo.84.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Searle A. Psychosocial and clinical determinants of compliance with occlusion therapy for amblyopic children. Eye. 2002;16(2):150–155. doi: 10.1038/sj.eye.6700086. [DOI] [PubMed] [Google Scholar]
- 10.Searle A. Compliance with eye patching in children and its psychosocial effects: a qualitative application of protection motivation theory. Psychol. Health & Med. 2000;5(1):43–54. [Google Scholar]
- 11.Wallace M. Compliance with occlusion therapy for childhood amblyopia. Investig. Ophthalmol. Vis. Sci. 2013;54:6158–6166. doi: 10.1167/iovs.13-11861. [DOI] [PubMed] [Google Scholar]
- 12.Nucci P. Compliance in antiamblyopia occlusion therapy. Acta Ophthalmol. 1992;70(1):128–131. doi: 10.1111/j.1755-3768.1992.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd I. Modulation of amblyopia therapy following early surgery for unilateral congenital cataracts. Br. J. Ophthalmol. 1995;79(9):802–806. doi: 10.1136/bjo.79.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awan M., Proudlock F.A., Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Investig. Ophthalmol. Vis. Sci. 2005;46(4):1435–1439. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- 15.Fielder A. Compliance in amblyopia therapy: objective monitoring of occlusion. Br. J. Ophthalmol. 1995;79:585–589. doi: 10.1136/bjo.79.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fronius M. Occlusion treatment for amblyopia: assessing the performance of the electronic occlusion dose monitor. Strabismus. 2006;14(2):65–70. doi: 10.1080/09273970600700962. [DOI] [PubMed] [Google Scholar]
- 17.Loudon S. Psychological causes of non-compliance with electronically monitored occlusion therapy for amblyopia. Br. J. Ophthalmol. 2009;93(11):1499–1503. doi: 10.1136/bjo.2008.149815. [DOI] [PubMed] [Google Scholar]
- 18.Wallace M. Concordance with occlusion therapy for childhood amblyopia. Investig. Ophthalmol. Vis. Sci. 2013;54(15) doi: 10.1167/iovs.13-11861. 4979–4979. [DOI] [PubMed] [Google Scholar]
- 19.Fielder A. Compliance monitoring in amblyopia therapy. Lancet. 1994;343:547. doi: 10.1016/s0140-6736(94)91502-4. [DOI] [PubMed] [Google Scholar]
- 20.Loudon S.E., Polling J.-R., Simonsz H.J. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003;241(3):176–180. doi: 10.1007/s00417-002-0570-z. [DOI] [PubMed] [Google Scholar]
- 21.Januschowski K. Measuring wearing times of glasses and ocular patches using a thermosensor device from orthodontics. Acta Ophthalmol. 2013;91:e635–e640. doi: 10.1111/aos.12171. [DOI] [PubMed] [Google Scholar]
- 22.Simonsz H. Electronic monitoring of treatment compliance in patching for amblyopia. Strabismus. 1999;7(2):113–123. doi: 10.1076/stra.7.2.113.645. [DOI] [PubMed] [Google Scholar]
- 23.Moseley M. Personalized versus standardized dosing strategies for the treatment of childhood amblyopia: study protocol for a randomized controlled trial. Trials. 2015;16(1):189. doi: 10.1186/s13063-015-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert S.R. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132(6):676–682. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Infant Aphakia Treatment Study Group The Infant Aphakia Treatment Study: design and clinical measures at enrollment. Arch. Ophthalmol. 2010;128(1):21–27. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Infant Aphakia Treatment Study Group. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132(6):676–682. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Group, I.A.T.S A randomized clinical trial comparing contact lens to intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch. Ophthalmol. 2010;128(7):810. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen R., Speedwell L., Russell-Eggitt I. Long-term visual outcome after extraction of unilateral congenital cataracts. Eye. 2010;24(7):1263–1267. doi: 10.1038/eye.2009.295. [DOI] [PubMed] [Google Scholar]
- 29.Smith L. Factors affecting treatment compliance in amblyopia. J. Pediatr. Ophthalmol. Strabismus. 1994;32(2):98–101. doi: 10.3928/0191-3913-19950301-09. [DOI] [PubMed] [Google Scholar]
- 30.Hiscox F. Occlusion for amblyopia: a comprehensive survey of outcome. Eye. 1992;6(Pt 3):300–304. doi: 10.1038/eye.1992.59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.