Abstract
Substantial evidence suggests a facilitatory influence of cannabinoid CB1 receptors in the modulation of ethanol consumption by rodents. Studies performed in rats selectively bred for high alcohol preference point to an involvement of CB1 receptors in the nucleus accumbens (NAC), ventral tegmental area (VTA) and medial prefrontal cortex (mPFC) in the modulation ethanol self-administration. However, the neural mechanisms through which CB1 receptors regulate ethanol intake in out-bred Wistar rats have not been investigated. The present study evaluated alterations in ethanol self-administration induced by localized infusions of the CB1 receptor antagonist SR141716A (0, 1 and 3 μg/side) into the NAC, anterior and posterior VTA and mPFC. Separate groups of Wistar rats were trained to operantly respond for an oral ethanol solution and prepared with bilateral injection cannulae aimed at each brain region. Results revealed significant decreases in ethanol intake following intra-NAC SR141716A administration, consistent with our prior observation of ethanol-induced increases extracellular 2-arachidonoyl glycerol (2-AG) in this brain region. We also observed a significant dose-dependent reduction in ethanol intake following SR141716A administration into the posterior, but not anterior VTA, consistent with evidence of a specific involvement of the posterior VTA in the regulation of ethanol intake. Ethanol consumption was unaltered following intra-mPFC SR141716A administration and ethanol self-administration did not induce robust changes in anandamide or 2-AG levels in mPFC microdialysates. These findings implicate an involvement of CB1 receptors in the NAC and posterior VTA, but not anterior VTA and mPFC in the regulation of ethanol self-administration behavior by outbred Wistar rats.
INTRODUCTION
The behavioral and reinforcing effects of ethanol (EtOH) are mediated through multiple neurotransmitter systems including glutamate, GABA, monoamines and a variety of neuroactive peptides [1–9]. Increasing evidence also implicates a role for the endogenous cannabinoid (EC) system in the physiologic and behavioral effects produced by EtOH. Anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) are ECs synthesized from postsynaptic membranes following neuronal depolarization. In general ECs act as retrograde messengers exerting essential modulatory influence on presynaptic neurotransmitter release via activation of cannabinoid receptors (i.e. CB1 and CB2). Termination of EC signaling is accomplished through a carrier-mediated uptake mechanism and subsequent hydrolysis by enzymes such as fatty acid amidehydrolase (FAAH) or monoacylglycerol lipase (MAGL) [10–12].
Voluntary EtOH self-administration is accompanied by increases in interstitial 2-AG levels in the nucleus accumbens (NAC) [13] suggesting that ECs might influence EtOH consumption via cannabinoid receptor activation. A wide variety of evidence supports this hypothesis. For example, EtOH consumption is reduced in CB1 knockout mice [14–15] and the CB1 receptor antagonist SR141716A (Rimonabant) reduces EtOH intake by alcohol-preferring C57BL/6 mice, Sardinian alcohol-preferring (sP) rats, Long-Evans rats, and both non-dependent and EtOH-dependent Wistar rats [16–21]. In addition, compared with alcohol avoiding DBA/2 mice alcohol-accepting C57BL/6 mice are characterized by significantly higher CB1 agonist-stimulated [35S] GTPγS binding [22] and altered expression of FAAH [23–24, 25]. Collectively, these observations provide strong evidence that EC signaling exerts a modulatory influence on EtOH preference and consumption.
Although EC signaling has been implicated in the modulation of EtOH intake, little is known about the neural substrates and mechanisms through which this process occurs. Recently, it has been shown that blockade of NAC CB1 receptors reduces EtOH intake by Wistar and alcohol-preferring (AA) rats [13, 26]. CB1 inhibition prevents EtOH-induced increases in dopamine (DA) cell firing in NAC and ventral tegmental area (VTA) [27–28] and EtOH-induced increases in NAC DA are absent in CB1 KO mice [29]. Furthermore, it has been shown that EtOH self-administration by AA rats is reduced following intra-VTA CB1 antagonist administration [28]. Collectively these findings suggest that the motivational effects of EtOH are modulated in part through a CB1 receptor-mediated influence on the activity of the mesolimbic DA system. In addition, EtOH self-administration by AA rats is reduced by administration of a CB1 receptor antagonist into the medial prefrontal cortex (mPFC) [30]. CB1 receptors also participate in an EtOH-induced inhibition of BLA projection neurons [31], which may contribute to the EtOH-induced decrease in the excitability of NAC GABAergic neurons [28].
The reinforcing properties of EtOH are mediated in part through its actions in the mesocorticolimbic system, which includes dopaminergic projections from VTA to NAC and PFC [32–35]. CB1 receptors are expressed with relatively high density in each of these regions [36–38]. Recent evidence points to an influence of CB1 receptors in the mPFC and VTA of rats selectively bred for high alcohol preference and intake [30, 26] though it is unknown whether a similar influence is present in non-selected outbred rats. Thus, we investigated the effect of microinfusions of the CB1 receptor antagonist SR141716A (Rimonabant) into the mPFC, NAC, and anterior and posterior VTA on voluntary EtOH self-administration by non-selected Wistar rats. We found that EtOH self-administration is significantly reduced following localized SR141716A infusions in the NAC and posterior VTA, but not following CB1 antagonist infusions in the anterior VTA or mPFC. In addition we found that EtOH self-administration does not alter levels of either AEA or 2-AG in microdialysates collected from the mPFC. Collectively these findings point to a regionally selective influence of brain CB1 receptors in the modulation of EtOH self-administration behavior.
MATERIALS AND METHODOLOGY
Subjects
Male Wistar rats (225–250 g; Charles River Laboratories, Wilmington, MA) were housed in groups of 2–3 in a temperature-controlled vivarium (22°C) with a 12 h light/dark cycle (lights off at 10 A.M.) and given ad libitum access to food and water. The group sizes for the drug infusion tests were as follows: intra-mPFC (n=8), intra-NAC (n=12), intra-VTA (anterior) (n=7), intra-VTA (posterior) (n=8). For the microdialysis test, the group size was 8. The studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the National Institutes of Health.
Drugs and Reagents
EtOH (10% w/v) was prepared from 95% Ethyl alcohol and water. Saccharin (Sigma, St. Louis, MO) was dissolved in water and used for the sweet-fading procedure employed to establish operant EtOH self-administration [39]. SR141716 was generously provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Washington, D.C.) and was dissolved in a vehicle of EtOH:emulphor:saline (1:1:18). AEA, 2-AG, 1(3)-arachidonoylglycerol (1-AG), and (S)-(+)-arachidonyl-2′-hydroxy-1′-propylamide (S-2 methanandamide) were used as chromatographic standards (Cayman Chemical, Ann Arbor, MI). All other reagents were of the highest grade from Sigma-Aldrich (St. Louis, MO).
EtOH Self-Administration
Operant EtOH self-administration was established under a fixed-ratio 1 (FR-1) timeout 20 s (TO-20 s; during which lever pressing behavior was recorded but not reinforced) schedule of reinforcement using a modified sweet solution fading procedure [39]. Animals lever-pressed to obtain 0.1 ml aliquots of liquid delivered into a sipper cup for oral consumption during 30-min sessions. The final EtOH reinforcer concentration was 10% (w/v).
Self-administration training and testing were performed in standard operant chambers housed in sound-attenuated and ventilated cubicles (Coulbourn Instruments, Allentown, PA) described in detail in [40]. Briefly, training sessions were conducted 5 days a week during the dark phase of the light cycle. Animals were considered to have stable baseline self-administration behavior when the total number of reinforcers earned per session stabilized to within ± 10% of the mean for 3 consecutive days. Post-session blood alcohol levels were determined during the final week of self-administration training. Blood samples were collected 60 minutes after termination of the self-administration sessions by clipping the tail approximately 5 mm from the tip. Approximately 100 μl of blood was collected into a microtube containing anticoagulant (4 μl heparin; 1000 USP units/ml) and were subsequently centrifuged at 2000 g for 10 min. Serum was extracted and assayed for EtOH content using the Alcohol Oxidase (AOD) method (Analox Instrument LTD, Lunenburg, MA, USA).
Surgery
Intracerebral Infusion Cannulas
Once animals acquired a stable operant self-administration behavior, they were anesthetized with isoflurane (1.5–2%) and implanted with bilateral microinfusion guide cannulas (22 gauge, 12 mm length, stainless steel) that terminated 2 mm above either the mPFC (from bregma: AP +2.7 mm, ML ± 0.6 mm; and DV −3.6 mm from dura) or the NAC (from bregma: AP +1.7 mm, ML ± 0.9 mm; and DV −5.4 mm from dura). Cannulas targeting either the anterior or the posterior VTA terminated 2 mm above their ventral surface as follows: anterior VTA (from bregma: AP −4.8 mm, ML ± 2.4 mm; and DV −6.5 mm from skull at a 10° angle to the vertical), posterior VTA (from bregma: AP −5.6 mm, ML ± 2.4 mm; and DV −6.5 mm from skull at a 10° angle to the vertical) [41]. Animals were allowed to recover from surgery for a minimum of 5 days before further experimentation. Post-surgical self-administration training sessions continued until stable self-administration behavior was achieved prior to the start of treatment drug testing.
Intracerebral Microdialysis Cannulas
After operant self-administration training, animals were anesthetized (isoflurane 1.5–2%) and implanted with a microdialysis guide cannula (21 gauge, Plastics One inc, Roanoke, VA) that terminated 3 mm above the ventral surface of the mPFC (from bregma: AP +2.7, ML ± 0.6, DV −2.8 from dura) [41]. A minimum of 5 days postoperative recovery was allowed prior to continued self-administration sessions to re-establish stable self-administration behavior.
Intracerebral Microinfusions
Animals were habituated to the microinfusion procedure by a singular insertion of the microinjectors into the cannulae 30-min before an ethanol self-administration session. This procedure also produced the initial tissue damage from injector insertion. In subsequent tests intra-mPFC, −NAC, −VTA (anterior), and −VTA (posterior) infusions of either vehicle or SR141716 (1, 3 μg/side) were performed via bilateral 33 gauge microinjectors that extended 2 mm beyond the tip of the guide cannulae. Infusions were delivered in 0.5 μl volumes over a period of 30 sec, followed by an additional 1 min to allow drug diffusion before injector removal. Stylets were replaced in the guide cannulas, and 30 min later animals were allowed access to EtOH self-administration. Drug infusion tests were performed at weekly intervals (self-administration training continued 5d/week between each infusion test) and each animal was tested with each dose condition. The dose order presentation was randomized between animals following a Latin square design and each animal received a total of three infusions.
In Vivo Microdialysis
Animals trained to self-administer EtOH were habituated to the head tether required for microdialysis by attaching the head lead to the guide cannula prior to each daily self-administration session. This habituation minimizes disruption of the self-administration behavior during the microdialysis test. The evening before the experiment, animals were anesthetized (isoflurane 1.5–2%), and a microdialysis probe (3 mm polyethyl sulfone membrane, 15 kDa MW cutoff; SciPro Inc.) was inserted and secured to the previously implanted guide cannula. The probes were perfused with artificial CSF (aCSF) composed of the following: 145 mM NaCL, 2.8 mM KCL, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.4 mM D-Glucose, 0.25 mM ascorbic acid at a rate of 0.2 μl/min. The next morning, aCSF containing 30% (w/v) hydroxypropyl-β-cyclodextrin (HP-β-CD) was perfused at a rate of 0.6 μl/min. EC sampling efficiency is substantially increased by inclusion of HP-β-CD in the perfusate solution [13, 42, 43]. Following a 90 min period for equilibration dialysate samples were collected at 10 min intervals over a 60 min baseline period, during the subsequent 30 min operant EtOH self-administration session, and for 90 min post-session.
Liquid Chromatography Coupled with Mass Spectrometry (LC-MS)
Five μl microdialysate aliquots were spiked with 5 μl of 50 nM of S-2 methanandamide as an internal standard. The samples were subsequently loaded onto a precolumn (1.0 × 10 mm, Haisil HL C18 5μm; Higgins Analytical Inc., Mountain View, CA, USA) using a refrigerated autoinjector and 10% MeOH (v/v) mobile phase delivered at 47 μl/min. Following a 4 min wash period, mobile phase flow through the precolumn was reversed via a switching valve and the retained substances were delivered to a 0.5 × 150 mm microbore analytical column (Haisil HL C18 3μm; Higgins Analytical Inc., Mountain View, CA) and separated using an isocratic mobile phase consisting of 76% MeOH (v/v) delivered at 9 μl/min. The analytical column eluent was delivered via a nanoelectrospray interface into the mass spectrometer (1100MSD) (Agilent Technologies, Santa Clara, CA, USA) that was run in positive Selected Ion Monitoring mode to maximize sensitivity. The following mass/charge (m/z) ratios were used: AEA, 370.3 (M+1Na); 2-AG and 1-AG, 401.3 (M+1Na); S-2 methanandamide, 384.3 (M+1Na). External calibration curves were constructed from a minimum of three standard concentrations (each run in duplicate) and new calibrations were generated daily. Under these conditions the limits of quantitation were approximately 0.1 nM for each analyte.
Histology
Placement of infusion injectors and microdialysis probes were determined by comparing 45 μm brain slices with the atlas of Paxinos and Watson [41].
Statistical Analyses
The effects of SR141716 administration into the mPFC, NAC, VTA (anterior) and VTA (posterior) on EtOH self-administration were evaluated using a within-subjects design with repeated measures analysis of variance (ANOVA). AEA and 2-AG dialysate concentrations were transformed to percentages of average baseline dialysate concentration for evaluation of changes in dialysate EC content during and following EtOH self-administration by ANOVA with repeated measures over time. Evaluations of altered dialysate EC levels during and after EtOH self-administration were also performed by comparing averaged data from 30-min intervals for comparison with pre-self-administration baseline.
RESULTS
On average animals in this study received 37.0 ± 11.5 operant training sessions with a 10% EtOH reinforcer prior to the establishment of stable operant behavior. During the final stages of self-administration training the average EtOH intake during a single self-administration session was 0.38 ± 0.04 g/kg resulting in post-session blood alcohol concentrations of 29.9 ± 4.8 mg/dl (determined from intermittent testing during the self-administration training period).
Intra-NAC SR141716 infusion dose-dependently decreases EtOH self-administration
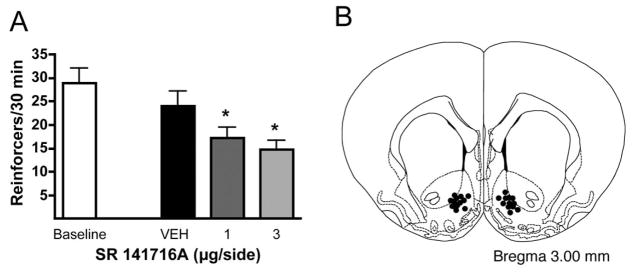
Rats displayed reliable and stable lever pressing for 10% (w/v) EtOH with an average of 29.0 ± 3.1 EtOH rewards earned per 30 min baseline session. Intra-NAC infusion of vehicle led to a slight non-significant reduction in self-administration behavior (F1,11 = 4.771, n.s.). In contrast, intra-NAC SR141716 administration dose-dependently decreased EtOH self-administration relative to the vehicle condition (F2,20 = 8.587, p < 0.01) (Fig. 1a) with significant reductions observed following 1 and 3 μg/side infusions. Fig. (1b) shows the placements of each NAC microinfusion.
Fig. 1.
Effect of intra-NAC microinfusion of the CB1 receptor antagonist SR141716A on EtOH self-administration by Wistar rats. Bilateral SR141716A (1 and 3 μg/side) infusions into the NAC significantly reduced EtOH self-administration as compared with EtOH intake following vehicle infusion (panel A; n = 12). Data are presented as the mean of reinforcers ± SEM. Asterisks denote significant differences, *p < 0.05. Placements of the microinjector tips in the NAC are depicted in panel B (coronal brain slice 3.00 mm anterior to Bregma [39]).
EtOH self-administration is decreased by SR141716A infusions into the posterior, but not anterior VTA
The average number of EtOH rewards earned per session during baseline self-administration training was 33.1 ± 7 and 29.4 ± 3.1 for animals with injection cannulae aimed at the anterior or posterior VTA, respectively. There was no significant alteration in EtOH intake following vehicle infusion into either anterior (F1,6 = 1.216, n.s.) or posterior (F1,7 = 0.379, n.s.) VTA. Moreover, there was no significant effect of any of the tested SR141716A doses when infused into the anterior VTA (F2,12 = 0.688, n.s.) (Fig. 2c). In contrast, a dose-dependent reduction in EtOH self-administration was observed following SR141716A infusion into the posterior VTA (F2,14 = 6.641, p < 0.01) with a significant reduction observed following administration of the 3 μg/side dose (Fig. 2a). Placements for posterior and anterior VTA microinfusions are represented in Fig. (2b, d), respectively.
Fig. 2.
Effect of intra-VTA microinfusion of the CB1 receptor antagonist SR141716A on EtOH self-administration by Wistar rats. Infusions of SR141716A (1 & 3 μg/side) into the posterior-VTA dose dependently reduced EtOH self-administration (Panel A; n = 8) while infusion of these same antagonist doses into the anterior VTA did not alter EtOH intake (Panel C; n = 7). Data are presented as the mean of reinforcers ± SEM. Asterisks denote significant differences, *p < 0.05. Localizations of the drug injector tips into the posterior (−5.6 to −5.8 mm from Bregma) and anterior (−4.8 to 5.2 mm from Bregma) VTA are shown in Panels B and D, respectively [39].
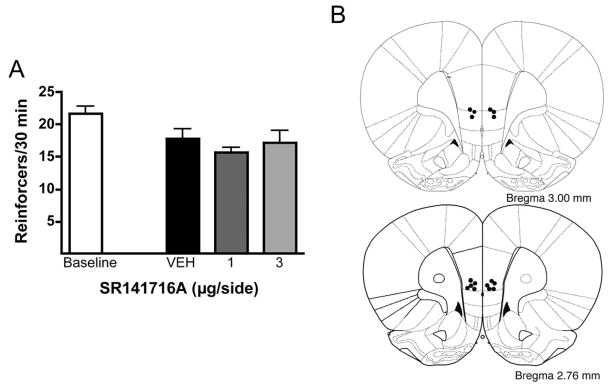
EtOH self-administration is unaltered by intra-mPFC infusion of SR141716A
During baseline training the average number of EtOH rewards earned per session by this group of animals was 21.7 ± 1.1 and this level of intake was slightly but significantly reduced following intra-mPFC vehicle infusion (F1,7 = 5.648, p < 0.05). However, relative to the vehicle condition EtOH self-administration was unaltered by intra-mPFC SR141716A infusion (F2,14 = 0.725, n.s.) (Fig. 3a). Fig. (3b) shows the placements of each mPFC microinfusion.
Fig. 3.
Effect of intra-mPFC microinfusion of the CB1 receptor antagonist SR141716A on EtOH self-administration by Wistar rats. Neither 1 nor 3 μg/side infusion of SR141716A into the mPFC altered EtOH self-administration (Panel A; n = 8). Data are presented as the mean of reinforcers ± SEM. Panel B depicts coronal slices (3.00 and 2.76 mm anterior to bregma, [39]) showing the location of each microinjector tip within the mPFC.
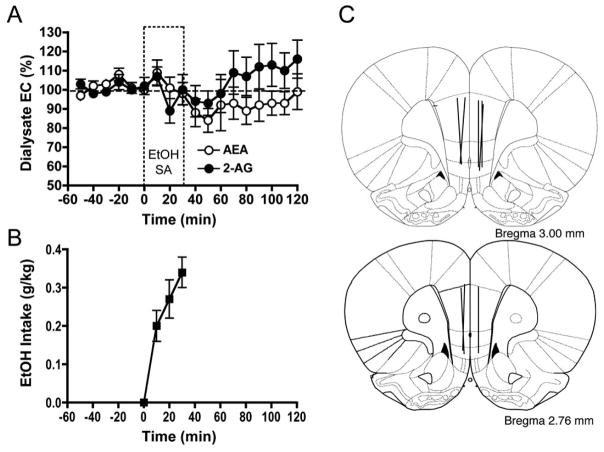
EtOH self-administration induces modest alterations in microdialysate EC levels collected from the mPFC
Baseline AEA levels in mPFC dialysates were 1.42 ± 0.08 nM and 2-AG levels in the same samples were 8.53 ± 1.01 nM. During the 30-minute period of self-administration access the animals consumed 0.34 ± 0.04 g/kg, with the greatest intake occurring in the first 10 minutes of the session (Fig. 4b). There were no significant differences from baseline in the average AEA level determined during the self-administration period (t = 0.960, n.s.), or during the three 30-minute periods following drug access (t = 2.054; t = 1.595; t = 1.169, respectively). Similarly, there were no significant changes from baseline in the average 2-AG level determined during the self-administration period (t = 0.619), or during the three 30-minute periods following drug access (t = 1.093; t = −0.74; t = −1.245, respectively. Fig. (4c) depicts the placements of dialysis probes in the mPFC.
Fig. 4.
Effect of EtOH self-administration on EC levels in mPFC microdialysates. EtOH self-administration induced subtle, non-significant alterations in microdialysate EC levels within the mPFC (Panel A; n = 8). Although modest changes in dialysate AEA and 2-AG content were observed during the 90-minute post-session sampling period, there was no significant change in dialysate levels as compared with pre-EtOH baseline. Dialysate AEA and 2-AG levels are expressed as the percentage of change from baseline ± SEM. The cumulative EtOH intake (g/kg; mean ± SEM) during the self-administration session is shown in Panel B. Schematic representation of the active dialysis membrane placements within the mPFC is shown in Panel C (3.00 and 2.76 mm anterior to bregma, [39]).
DISCUSSION
The present data demonstrate that localized infusions of the CB1 receptor antagonist SR141716A into the NAC and posterior VTA but not mPFC or anterior VTA reduce EtOH self-administration in out-bred Wistar rats. These results are further supported by our observations showing that EtOH self-administration is accompanied by a substantial increase in interstitial 2-AG levels in the NAC [13] but only subtle alterations in EC levels in the mPFC. Previous work from our lab and others has shown that operant responding for sucrose, cocaine and water are not disrupted by intra-NAC or −VTA SR141716A infusions [26, 13, 44], suggesting that the present observations do not result from a non-specific disruption of operant behavior.
The NAC is an important region for the mediation of the rewarding properties of EtOH [45–47] and evidence suggests that EtOH-induced increases in NAC dopamine signaling contributes to the rewarding properties of EtOH [48–51]. CB1 receptors are expressed in the NAC [36] where they are localized on afferent glutamatergic terminals [52–53]. Activation of CB1 receptors inhibits excitatory transmission to GABAergic medium spiny neurons in the NAC [54, 55], which exert an inhibitory control over dopaminergic neurons in the VTA. Thus, CB1 receptor activation would indirectly induce activity of mesolimbic dopamine neurons in the VTA, consistent with electrophysiological data demonstrating that EtOH-induced activation of VTA DA cells is modulated by NAC CB1 receptors [28]. Thus, it seems likely that EtOH-induced increases in NAC EC levels [13] would activate CB1 receptors providing an inhibitory influence on glutamatergic neurotransmission [52–53], decreasing GABA release in regions innervated by the NAC such as the VTA. The resulting disinhibition of VTA DA neurons may support EtOH reward by increasing DA release in the NAC. In the present study, EtOH self-administration was reduced by blockade of NAC CB1 receptors with SR141716A. Based on the evidence described above this may result from a restoration of inhibitory input to VTA DA cells which attenuates EtOH-induced increases in NAC DA release. This hypothesis is supported by electrophysiological data showing that SR141716A suppresses EtOH-induced activation of VTA dopamine neurons [28] and EtOH inhibition of artificially stimulated NAC GABAergic medium spiny neurons [28]. NAC CB1 receptors may also participate in EtOH-induced alterations in NAC output through DA-independent mechanisms as has been observed following opiate administration [40].
The VTA is an anatomically heterogenous structure in the rat and is comprised of five nuclei [56]. The VTA contains morphologically distinct dopaminergic neurons that display specialized axonal projections and topographical afferent and efferent projections including an organization of ascending projections from the VTA to the NAC [57–58]. These neuroanatomical distinctions suggest that the VTA is a functionally heterogenous structure and recent behavioral findings lend further support to this conclusion. For example, using intracranial drug self-administration Ikemoto et al. [59] demonstrated an anterior to posterior gradient in the self-administration of GABAA receptor antagonists into the VTA of Wistar rats, with drug infusions into the anterior but not posterior aspects of the VTA supporting operant behavior. In contrast, rats will self-infuse GABAA receptor agonists into the posterior, but not anterior VTA [60]. Similarly, both Wistar and alcohol-preferring rats will self-administer EtOH directly into the posterior but not the anterior VTA [61–64]. The reinforcing effects of EtOH in the posterior VTA are dependent on the activation of local DA neurons [63, 64] that may occur through direct effects of EtOH on DA cells and/or through the influence of serotonin receptors [64, 65]. Interestingly, EtOH-induced increases in VTA DA cell firing has been shown to be dependent on CB1 receptor function [28] and as such our present observations suggest that intra-VTA SR141716A administration reduces EtOH self-administration by preventing EtOH-induced activation of midbrain DA cells. The mechanism(s) underlying this process are not clearly understood. ECs in the VTA provide inhibitory influence at both GABAergic and glutamatergic terminals [66–68] and CB1-mediated reductions in GABA release from efferents to the VTA may result in disinhibition of VTA DA cells. It is also possible that CB1-mediated reductions in glutamate release diminish an excitatory influence on GABAergic interneurons, thereby also reducing inhibitory control over VTA DA cells. Thus, intra-VTA CB1 antagonist administration may reduce EtOH self-administration by preserving inhibitory control over DA cell firing, thereby reducing the excitatory effects of EtOH on the mesolimbic DA projection. Regardless of the mechanism, our present findings provide additional evidence of an important differential influence of the posterior versus anterior VTA in the regulation of EtOH consumption. Moreover, these observations demonstrate the feasibility of identifying regionally-restricted CB1 receptor influences using localized SR141716A infusions despite the lipophilic nature of this compound.
Previous work by Hansson and colleagues [30] has demonstrated that selectively bred alcohol-preferring AA rats are characterized by a regionally selective dysregulation of EC signaling. Specifically, they observed decreased expression and function of the hydrolytic enzymes FAAH and MAGL in the medial PFC of AA rats as compared with a similar rat line bred for lack of alcohol preference (ANA rats). FAAH and MAGL are the primary metabolic clearance routes for the ECs AEA and 2-AG, and thus decreased levels and function of these enzymes would be expected to result in increased EC levels. Consistently, AA rats were found to have significantly higher 2-AG levels in bulk PFC tissue as compared with PFC tissue from ANA rats, though there were no line differences in PFC AEA content. These observations led to the hypothesis that increased EC tone in the PFC confers enhanced EtOH preference and consumption, and this was supported by the observation that intra-PFC SR141716A administration dose-dependently reduced EtOH self-administration by AA rats. However, it is not known whether EC signaling in the PFC contributes to the motivational effects of EtOH in general, or whether this mechanism is specifically related to selectively bred lines of alcohol-preferring rats.
The present results indicate that EtOH self-administration behavior by outbred Wistar rats is not sensitive to CB1 receptor blockade in the mPFC. Doses of the CB1 receptor antagonist SR 141716A that significantly reduce EtOH self-administration when administered into the posterior VTA or NAC did not alter EtOH self-administration when administered into the mPFC. Although ineffective in Wistar rats, the highest dose tested in the present experiment (3 μg/side) was previously shown to significantly reduce EtOH self-administration when infused into the mPFC of AA rats [30]. It is possible that some procedural differences underlie the distinct results observed in the two lines of rats. For example different vehicles were employed for solubilization of SR141716A: the present study utilized an emulphor:EtOH:saline vehicle while the previous work employed 75% DMSO. It is unlikely, however, that the DMSO vehicle contributed substantially to the altered EtOH intake in the prior study as the behavioral changes were shown to be dose-responsive to SR141716A and regionally-selective effects were reported (e.g. there were no significant alterations in EtOH self-administration following SR141716A infusion into the dorsolateral striatum). It is also possible that slightly different placements of the drug infusion cannulae contribute to the differential findings between studies. In the previous study by Hansson and colleagues SR141716A infusions were delivered into the mid- to dorsal aspect of the prelimbic cortex [30] while in the present study the CB1 antagonist was infused more ventrally into the prelimbic/infralimbic transitional zone. It is presently unclear whether these somewhat different infusion placements contribute to the differential effects of intra-PFC SR141716A infusions on EtOH intake by alcohol-preferring vs outbred rats.
In the previous report from Hansson and colleagues [30], it was shown that intra-PFC SR141716A administration dose-dependently reduced EtOH self-administration by AA rats but did not significantly alter saccharin self-administration. This suggests that the behavioral effect of the antagonist was mediated through a blockade of EtOH-induced increases in EC formation in the PFC. We have previously observed that EtOH self-administration increases extracellular 2-AG levels in the NAC of Wistar rats and that EtOH self-administration behavior is reduced by intra-NAC SR141716A administration [13; present results], consistent with the hypothesis that EtOH-induced increases in NAC EC levels modulates self-administration behavior through a CB1 receptor mechanism. Accordingly, the lack of altered self-administration behavior following intra-PFC SR141716A administration suggests that EtOH intake does not alter EC formation in the mPFC of Wistar rats. Consistent with this hypothesis we find that EtOH self-administration induces only modest changes in mPFC microdialysate AEA and 2-AG levels even with levels of EtOH intake previously found to significantly increase extracellular 2-AG in the NAC [13]. Collectively, these data suggest that EtOH self-administration by Wistar rats does not significantly alter EC transmission in the mPFC. In contrast, EtOH self-administration by alcohol-preferring AA rats may result in increased PFC EC levels as a result of deficient EC clearance mechanisms specifically in the PFC of these rats [30]. Thus it is possible that increased EtOH-induced EC levels in the PFC characterizes an innate preference for EtOH and propensity toward increased EtOH consumption, an effect that is absent in non-selected Wistar rats and perhaps lines of rats bred for reduced EtOH preference (ANA). Consistent with this theory is the observation that intra-PFC administration of the FAAH inhibitor URB597 increases EtOH self-administration by non-selected Wistar rats [30].
CONCLUSION
Collectively our findings demonstrate that localized infusions of the CB1 receptor antagonist SR141716A into the NAC or posterior VTA reduces EtOH self-administration by non-selected Wistar rats. CB1 antagonist infusions into the anterior VTA did not alter EtOH intake and this observation provides further evidence of an important differentiation in the influence of the anterior vs posterior VTA in the regulation of EtOH consumption. In general these observations are consistent with recent evidence that CB1 receptors in the NAC and VTA modulate EtOH intake by selectively bred alcohol-preferring rats [26]. However, although previous results demonstrate that intra-PFC administration of SR141716A reduces EtOH intake by alcohol-preferring AA rats [30] we found no significant effect of this manipulation on EtOH intake by non-selected Wistar rats and found no robust alteration in PFC dialysate EC levels during or after EtOH self-administration. Because alcohol-preferring AA rats are characterized by reduced expression and activity of EC metabolic enzymes specifically in the mPFC [30] it is possible that EtOH-induced increases in EC formation are exaggerated in the mPFC of these animals.
Acknowledgments
This study was supported by grants RO1-AA014619 and P60-AA06420 from the National Institute on Alcohol Abuse and Alcoholism. This is publication 20166 from The Scripps Research Institute.
Footnotes
CONFLICT OF INTEREST
The authors listed on this manuscript do not have any potential conflicts of interest related to the subject of this report. Further, each of the authors on this manuscript is supported in full by NIH research grants and have not received, and do not anticipate receiving, any compensation for professional services from any source outside of the NIH.
References
- 1.Ferko AP. Interaction between L-glutamate and ethanol on the central depressant properties of ethanol in mice. Pharmacol Biochem Behav. 1994;47:351–54. doi: 10.1016/0091-3057(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 2.Boehm SL, II, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- 3.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–8. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 4.Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–59. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- 5.Colombo G, Serra S, Brunetti G, et al. The GABA(B) receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol. 2002;37:499–503. doi: 10.1093/alcalc/37.5.499. [DOI] [PubMed] [Google Scholar]
- 6.Fadda F, Garau B, Marchei F, Colombo G, Gessa GL. MDL72222, a selective 5-HT3 receptor antagonist, suppresses voluntary ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 1991;26:107–10. doi: 10.1093/oxfordjournals.alcalc.a045088. [DOI] [PubMed] [Google Scholar]
- 7.Hodge CW, Samson HH, Lewis RS, Erikson HL. Specific decreases in ethanol- but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205-930. Alcohol. 1993;10:191–6. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–71. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips TJ, Wenger CD, Dorow JD. Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 1997;21:691–702. [PubMed] [Google Scholar]
- 10.De Petrocellis L, Grazia Cascio M, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–74. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivates with cannabimetric properties: biochemistry and possible physiopathological relevance. Biochem Biophys Acta. 1998;1392:153–75. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 12.Freund TM, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 13.Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–4. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 15.Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–3. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Arnone M, Maruani J, Chaperon F, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 17.Economidou D, Mattioli L, Cifani C, et al. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 18.Colombo G, Agabio R, Fa M, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–30. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 19.Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- 20.Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on Ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–82. [PubMed] [Google Scholar]
- 21.Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR 141716A decreases operant ethanol self-administration in rats exposed to ethanol-vapor chambers. Acta Pharmacol Sin. 1999;20:1109–14. [PubMed] [Google Scholar]
- 22.Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate gammas binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429–36. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- 23.Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–44. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- 25.Vinod KY, Sangunio E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:143–233. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- 26.Malinen H, Hyytiä P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–83. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheer JF, Wassum KM, Sombers LA, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–95. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perra S, Giuliano P, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology. 2005;183:368–77. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- 29.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 30.Hansson AC, Bermúdez-Silva FJ, Malinen H, et al. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- 31.Perra S, Pillolla G, Luchicchi A, Pists M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res. 2008;32:443–49. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Roberts AJ, Schulteis G, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 33.Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res. 1998;22:176–82. [PubMed] [Google Scholar]
- 34.Pierce C, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas BF, Wei X, Martin BR. Characterization and autoradiographic localization of the cannabinoid binding site in the rat brain using [3H]11-OH-delta 9-THC-DMH. J Pharmacol Exp Ther. 1992;263:1383–90. [PubMed] [Google Scholar]
- 38.Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–68. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- 39.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 40.Caillé S, Parsons LH. Cannabinoid modulation of opioid reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–13. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Vol. 1998 San Diego: Academic Press; 1998. [Google Scholar]
- 42.Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrer B, Bermúdez-Silva FJ, Bilbao A, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caillé S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–49. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- 45.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 46.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–63. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 47.Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–96. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- 48.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 49.Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–6. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 51.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–58. [PubMed] [Google Scholar]
- 52.Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- 53.Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–16. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur J Neurosci. 2002;15:1795–802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 55.Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–48. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oades RA, Halliday GM. Ventral tegmental (A10) system: neurobiology. I. Anatomy and connectivity. Brain Res Rev. 1987;12:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 57.Kalen P, Skagerberg G, Lindvall O. Projections from the ventral tegmental area and mesencephalic raphé to the dorsal raphé nucleus in the rat. Exp Brain Res. 1988;73:69–77. doi: 10.1007/BF00279662. [DOI] [PubMed] [Google Scholar]
- 58.Tan Y, Brog JS, Williams ES, Zahm DS. Morphometric analysis of ventral mesencephalic neurons retrogradely labeled with Fluoro-Gold following injections in the shell, core and rostral pole of the rat nucleus accumbens. Brain Res. 1995;689:151–6. doi: 10.1016/0006-8993(95)00556-6. [DOI] [PubMed] [Google Scholar]
- 59.Ikemoto S, Kohl RR, McBride WJ. GABAA receptor blockade in the anterior ventral tegmental area increase extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69:137–43. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- 60.Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 61.Gatto GJ, McBride WJ, Murphy J, Lumeng L, Li T-K. Ethanol self-administration into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–64. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 62.Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–24. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- 63.Rodd ZA, Melendez RI, Bell RL, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–57. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodd ZA, Bell RL, Zhang Y, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2009;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- 65.Ding Z-M, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2009;204:381–90. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–78. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–61. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]