Abstract
Objective
To determine the association between cardiorespiratory fitness and sedentary behavior, independent of exercise activity.
Patients and Methods
We included 2,223 participants (ages 12-49 years, 47% female) without known heart disease who had both cardiovascular fitness testing and at least one day of accelerometer data from NHANES 2003-2004. From accelerometer data, we quantified bouts of exercise as mean minutes per day for each participant. Sedentary time was defined as <100 counts per minute in mean minutes per day. Cardiorespiratory fitness was derived from a sub-maximal exercise treadmill test. Multivariable-adjusted linear regression analyses were performed with fitness as the dependent variable. Models were stratified by gender, adjusted for age, BMI, wear time and included sedentary and exercise time.
Results
An additional hour of daily exercise activity time was associated with a 0.88 (0.37 to 1.39, P<.001) MET higher fitness for men and a 1.37 (0.43 to 2.31, P=.004) MET higher fitness for women. An additional hour of sedentary time was associated with a -0.12 (-0.02 to -0.22, P=.03) and a -0.24 (-0.10 to -0.38, P<.001) MET difference in fitness for men and women, respectively.
Conculsion
After adjustment for exercise activity, sedentary behavior appears to have an inverse association with fitness. These findings suggest that the risk related to sedentary behavior might be mediated, in part, through lower fitness levels.
Introduction
Cardiorespiratory fitness (CRF) is known to be one of the strongest predictors of cardiovascular health and longevity.1 Determinants of fitness are both non-modifiable (age, gender, genetics) and modifiable (body mass index and physical activity).2 Numerous prospective cohort studies have solidified the relationship between physical activity, CRF and reduced risk of cardiovascular disease, coronary heart disease and all-cause mortality.3 Therefore, current guidelines recommend at least 150 minutes per week of moderate-intensity physical activity or 75 minutes per week of vigorous-intensity aerobic physical activity, performed in bouts lasting at least 8-10 minutes.4 Despite the well-established benefits of exercise and release of these guidelines in 2008, the majority of adults do not meet these physical activity recommendations.5
Recent epidemiologic evidence suggests that the long-term health consequences related to a lack of moderate-to-vigorous physical activity (too little exercise) are distinct from those of habitual sedentary behavior3, 6-10 (sitting too much). However, less is known about the role of sedentary behavior in this context. Sedentary behavior is defined as behaviors that involve low levels of energy expenditure7 (1.0- 1.5 METs, including sitting, watching TV, reading and driving). In addition to the risks associated with low physical activity, the burden of sedentary behavior appears to be a separate risk factor that is independent of physical activity levels, with multiple observational studies showing increased risk for total all-cause and cardiovascular mortality for those individuals with increased sedentary time.8-10 For example, in a recent report from the Women's Health Initiative, women who reported more than 10 hours of sitting per day had an 18% increased risk of cardiovascular disease compared to women who sat less than 5 hours per day, regardless of physical activity levels.10 These data suggest that sedentary behavior is more than merely the lack of purposeful exercise.
The mechanism through which sedentary behavior may contribute to increased risk remains uncertain. Sedentary behavior has been associated with obesity, the metabolic syndrome, reduced lipoprotein lipase levels, insulin resistance and microvascular dysfunction.8, 11-17 However, to our knowledge, the association between sedentary behavior and CRF has not been studied. Because of the prognostic importance of CRF on health and mortality,1, 3 this knowledge could provide insight into the mechanisms through which sedentary behavior influences cardiovascular disease risk. Additionally, this would have potential implications for novel strategies designed to increase CRF. Therefore, we sought to characterize the associations between sedentary behavior and CRF using data from National Health and Nutrition Examination Survey (NHANES) 2003-2004.
Methods
Cohort description
NHANES is an ongoing series of surveys that have been conducted by the National Center for Health Statistics since the early 1960s to assess the health and nutritional status of the US civilian, non-institutionalized population. Fifteen geographic locations are selected annually and sampled to represent the general population with a complex, multi-stage probability design. The three main components of the study include an interview in the participants' home, a medical exam completed at a mobile examination center, and several medical and laboratory tests. The interview includes demographic, socioeconomic, dietary, and health-related questions. Co-morbidities are assessed by self-report. NHANES 2003-2004 included a CRF test component for participants aged 12-49 years. All participants were also eligible for physical activity monitoring using an accelerometer device. The National Center for Health Statistics Ethics Review Board approved the protocols, and informed consent was obtained from all subjects.
From NHANES participants in 2003-2004, 4,902 individuals aged 12-49 years were examined at the mobile examination center. Among them, 1,439 participants met pre-specified exclusion criteria for fitness testing because of one or more of the following reasons: physical limitations that would prevent them from using the treadmill (n=328); history of cardiovascular disease or active conditions or symptoms (n=336); asthma or other lung and breathing conditions or symptoms (n=291); pregnancy > 12 weeks (n=203); use of β blockers, anti-arrhythmics, calcium channel blockers, nitrates or digitalis (n=97); refused fitness testing (n=67); or other reasons (n=117). Among individuals who were eligible to participate in the fitness test, 415 did not have their fitness level estimated. For 387 of these, the test was terminated prematurely due to pre-defined early stopping criteria (symptoms and/or safety concerns). There was missing data to estimate maximal oxygen consumption (VO2 max) in 12 participants. In 16 additional participants, technical problems or technician errors were cause for inability to estimate fitness. After excluding an additional 825 participants lacking one valid day of physical activity monitoring (a valid day is defined as ≥10 hours of accelerometer wear time), we were left with 2,223 participants with both CRF testing and sufficient accelerometer data.
Accelerometry
Participants were asked to wear a single axis ActiGraph model 7164 accelerometer (ActiGraph, LLC, Pensacola, FL) on their right hip during all waking hours for seven consecutive days (except when exposed to water - bathing, showering, swimming, etc.). Details of the accelerometer protocol are available.18 The data collected by the physical activity monitor reflects the intensity of activity as counts in a set period of time (1-minute intervals) and were analyzed using SAS syntax provided by the National Cancer Institute.19 Wear time was determined by subtracting non-wear time from 24 hours. Non-wear time was defined by an interval of at least 60 consecutive minutes of zero activity counts, with allowance of up to 2 minutes of counts between 0 and 100. Intensity-threshold criteria for adults have been previously established: 2,020 counts for moderate intensity (equivalent to 3 METs) and 5,999 counts for vigorous intensity (equivalent to 6 METs). For youth ages 12-17, different activity count thresholds are used to adjust for the higher resting energy expenditure of this age group.20-21 Bouts of exercise were defined as at least 8 of 10 minutes above these count thresholds and quantified as the mean minutes of activity bouts per day for each participant. Sedentary time was defined as < 100 counts per minute of wear time in mean minutes per day22. Sedentary time was quantified in three different ways: 1) averge daily sedentary time (hours per day); 2) proportion of total wear time that was sedentary; and 3) average count intensity during sedentary time, an indicator of stillness during sedentary time.
Cardiorespiratory fitness testing
CRF was our outcome variable, assessed by a sub-maximal treadmill exercise test. Participants were assigned to 1 of 8 treadmill test protocols on the basis of their expected VO2 max, which was predicted from gender, age, body mass index, and self-reported level of physical activity by using the formula developed by Jackson, et al.23-24 Each protocol included a 2-minute warm-up, two 3-minute exercise stages, and a 2-minute cool-down period. The goal of each protocol was to elicit a heart rate that was approximately 75% of the age-predicted maximum (220 - age) by the end of the second exercise stage.23
The heart rate was monitored continuously via 4 electrodes connected to the trunk and abdomen of the participant and was recorded at the end of warm-up, each exercise stage, and each minute of recovery. Blood pressure was measured at the end of each stage by an STBP-780 automated sphygmomanometer (Colin Medical Instruments Corporation, San Antonio, Texas). VO2 max (mL/kg/minute) was estimated by extrapolation to an expected age-specific maximal heart rate by using measured heart rate responses to the two 3-minute exercise stages. This assumes that the relation between heart rate and oxygen consumption is linear during treadmill exercise.23
Fitness was categorized into three levels. A low level of CRF is defined as an estimated VO2 max at or below the 20th percentile of the Aerobics Center Longitudinal Study data for the same gender and age; moderate fitness is defined as a value between the 20th and 59th percentile, and high fitness is defined as at or above the 60th percentile. For adolescents 12-19 years, standards are based on criteria from the FITNESSGRAM program.23
Other measures
The physical activity questionnaire section in NHANES includes questions related to daily activities, leisure time activities, and sedentary activities at home. In particular, participants were asked to qualitatively categorize their average daily activity into one of four discreet groups: 1) sits during the day and does not walk very much, 2) stands or walks frequently during the day, but does not have to carry or lift things often, 3) lifts lights loads or has to climb hills or stairs often, and 4) does heavy work or carries heavy loads. This question was used to compare participants' self-reported physical activity profiles with accelerometer-derived sedentary and exercise time.
Current smoking status was assessed by self-report. For adolescents 12-19 years old, current smoking was defined as an affirmative response to the question: have you used tobacco or nicotine in the last 5 days?
Statistical analysis
Baseline characteristics and accelerometer-derived variables were compared across strata of fitness levels separately for men and women, using the Jonckheere-Terpstra test for trend.25 Multivariable-adjusted linear regression analyses were performed with CRF as the dependent variable, measured both as a continuous variable (VO2 max) and as an ordinal variable (low, moderate, and high fitness). All models were stratified by gender and adjusted for age, BMI, and mean wear time. Exposure variables included accelerometer-derived sedentary time as well as moderate and vigorous exercise time. Accelerometer-derived sedentary time was quantified in three different ways (average daily sedentary time, percent sedentary time, and average sedentary count intensity). Self-reported activity profiles were compared across tertiles of accelerometer-derived sedentary time to examine the validity of accelerometer-derived measures of sedentary behavior.
Results
The mean age of the study population was 22.4 ± 10.3 years with 47% female. Baseline characteristics of men and women, stratified by fitness level, are shown in Table 1. The duration of accelerometer wear-time did not differ across fitness groups for all participants. Women were more sedentary than men with 7.0 ± 2.1 hours/day compared to 6.6 ± 2.4 hours/day, P<.001. Women also had less average total daily moderate and vigorous activity time when compared to men (0.13 ± 0.22 hours/day verses 0.28 ± 0.37 hours/day respectively, P<.001).
Table 1. Baseline Characteristics of Men and Women Stratified by Fitness Level.
| MEN (n=1170) | ||||
|---|---|---|---|---|
| Low Fitness (n=336) |
Intermediate Fitness (n=494) |
High Fitness (n=340) |
P trend | |
| Age (years) | 19.6 (±8.6) | 22.0 (±10.1) | 26.4 (±10.8) | <.001 |
| BMI (kg/m2) | 26.8 (±6.5) | 24.0 (±5.1) | 24.9 (±5.1) | <.001 |
| SBP (mm Hg) | 115.5 (±10.6) | 113.3 (±11.2) | 115.3 (±10.7) | .58 |
| DBP (mm Hg) | 63.3 (±13.6) | 62.5 (±13.8) | 65.3 (±13.1) | .07 |
| Total cholesterol (mg/dL) | 173.5 (±40.1) | 171.9 (±39.6) | 178.0 (±40.2) | .15 |
| HDL cholesterol (mg/dL) | 48.0 (±11.6) | 50.7 (±12.8) | 52.2 (±13.9) | <.001 |
| Fasting glucose (mg/dL) | 96.1 (±27.1) | 94.3 (±18.7) | 92.6 (±10.5) | 0.16 |
| Current smoking (n)* | 62 (19.2) | 104 (22.2) | 94 (28.1) | .006 |
| Wear time (hours/day) | 14.1 (±2.1) | 14.2 (±2.0) | 14.3 (±1.9) | .06 |
| Wear time (days) | 4.6 (±1.9) | 4.8 (±1.9) | 4.9 (±1.9) | .11 |
| Sedentary time (hours/day) | 7.0 (±2.3) | 6.5 (±2.4) | 6.4 (±2.3) | <.001 |
| Moderate & Vigorous Activity time (hours/day) | 0.20 (±0.31) | 0.31 (±0.41) | 0.33 (±0.37) | <.001 |
| Estimated VO2 max (mL/kg/min) | 35.7 (±3.7) | 43.8 (±4.6) | 53.5 (±10.8) | <.001 |
| WOMEN (n=1053) | ||||
|---|---|---|---|---|
| Low Fitness (n=364) |
Intermediate Fitness (n=372) |
High Fitness (n=371) |
P trend | |
| Age (years) | 19.4 (±8.3) | 21.5 (±9.8) | 26.9 (±11.4) | <.001 |
| BMI (kg/m2) | 26.3 (±7.2) | 24.5 (±5.7) | 25.2 (±6.0) | .14 |
| SBP (mm Hg) | 108.3 (±10.6) | 107.6 (±10.6) | 108.9 (±11.6) | .84 |
| DBP (mm Hg) | 63.2 (±10.6) | 63.7 (±10.8) | 65.3 (±11.3) | .01 |
| Total cholesterol (mg/dL) | 170.7 (±34.4) | 173.5 (±36.0) | 178.9 (±36.0) | .002 |
| HDL cholesterol (mg/dL) | 56.8 (±13.9) | 58.0 (±13.9) | 58.0 (±15.1) | .20 |
| Current smoking (n)* | 41 (12.0) | 51 (14.4) | 72 (23.9) | <.001 |
| Fasting glucose (mg/dL) | 89.5 (±11.7) | 88.9 (±7.5) | 89.6 (±15.0) | .51 |
| Wear time (hours/day) | 13.9 (±1.8) | 14.0 (±1.8) | 13.9 (±1.7) | .86 |
| Wear time (days) | 4.8 (±1.9) | 4.5 (±2.0) | 4.9 (±1.9) | .48 |
| Sedentary time (hours/day) | 7.3 (±2.1) | 7.1 (±2.1) | 6.6 (±2.1) | <.001 |
| Moderate & Vigorous Activity time (hours/day) | 0.09 (±0.18) | 0.14 (±0.23) | 0.15 (±0.22) | <.001 |
| Estimated VO2 max (mL/kg/min) | 30.1 (±3.6) | 36.5 (±4.3) | 46.3 (±9.7) | <.001 |
Means ± standard deviations for continuous variables
Current smoking shown as n (percent of respondents); missing responses in 44 men and 55 women.
Low level of cardiovascular fitness is defined as an estimated VO2 max below the 20th percentile of the ACLS data for the same gender and age; moderate fitness is defined as a value between the 20th and 59th percentile, and high fitness is defined as at or above the 60th percentile. For adolescents 12-19 years, standards are based on criteria from the FITNESSGRAM program. BMI is body mass index. SBP and DBP are baseline, resting systolic and diastolic blood pressure, respectively. HDL is high-density lipoprotein. VO2 max is the maximal oxygen consumption, as estimated by the submaximal treadmill test.
Activity profiles defined by accelerometer varied according to measured fitness levels, with lower fitness levels associating with a higher burden of sedentary time and a lower amount of time spent in moderate/vigorous physical activity. For example, when compared to men with high fitness, men with low fitness spent approximately 36 more minutes per day in sedentary time (7.0 vs. 6.4 hours, P<.001 unadjusted) and 8 minutes less per day in exercise time (12.0 vs. 19.8 minutes, P<.001 unadjusted). Similar trends were observed in women.
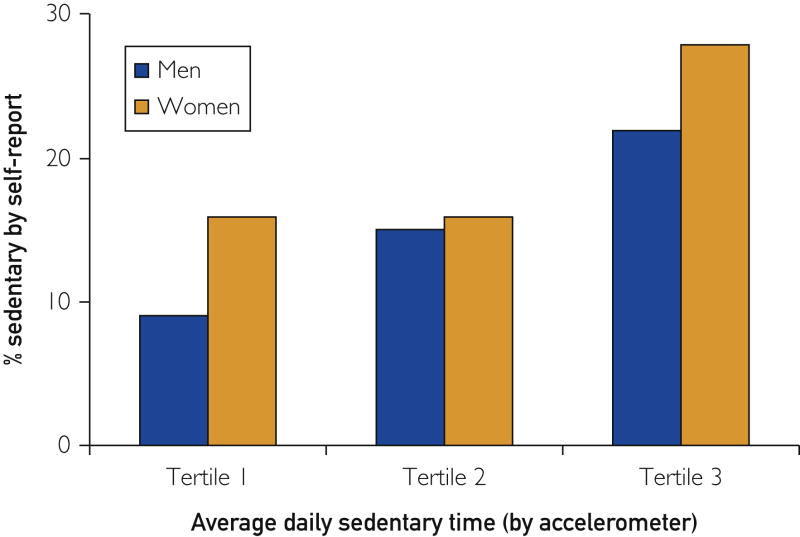
Participants were asked to best describe their usual daily activities, selecting from one of four qualitative descriptions (815 men and 714 women responded). The self-reported sedentary profile was compared to accelerometer-derived sedentary time tertiles. The percentage of participants with a self-reported sedentary lifestyle was associated with a higher amount of accelerometer-derived average daily sedentary time (P trend, P<.001 for men and P=.002 for women; see Figure 1). For example, 50% of respondents with the highest burden of accelerometer-derived sedentary time (tertile 3, greater than 7.5 hours/day) reported a sedentary lifestyle compared to 25% of respondents in the lowest burden of sedentary time (tertile 1, less than 6 hours/day). The accelerometer-derived assessments of sedentary time provide external validity for this self-report.
Figure 1.
Percentage of Participants with a Self-Reported Sedentary Lifestyle Across Accelerometer-Derived Sedentary Time Tertiles. All participants were asked to best describe their usual daily activities, selecting from one of four descriptions: 1) sits during the day and does not walk very much, 2) stands or walks about a lot during the day, but does not have to carry or lift things often, 3) lifts lights loads or has to climb hills or stairs often, and 4) does heavy work or carries heavy loads. Shown here is the percentage of participant respondents (n=815 for men and n=714 for women) with a self-reported sedentary profile (i.e. profile 1 above) by sedentary time tertile. For men (in minutes): tertile 1 (26-327); tertile 2 (328-450); tertile 3 (451-1251). For women: tertile 1 (76-358); tertile 2 (359-477); tertile 3 (478-1000). The percentage of participants with a self-reported sedentary lifestyle correlates with accelerometer-derived average daily sedentary time across tertiles of sedentary time (P trend, P<.001 for men and P<.002 for women).
After multi-variable adjustment, each additional hour of combined moderate and vigorous average daily activity time was associated with a 0.88 (0.37 to 1.39, P<.001) MET higher fitness for men and a 1.37 (0.43 to 2.31, P=.004) MET higher fitness for women. Each additional hour of daily sedentary time was associated with a -0.12 (-0.02 to -0.22, P=.03) MET difference in fitness for men and a -0.24 (-0.10 to -0.38, P<.001) MET difference in fitness for women (Table 2).
Table 2. Multivariable-adjusted Linear Regression analyses in Men and Women.
| MEN (n=1170) | WOMEN (n=1053) | |||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Vigorous/moderate activity | 0.88 (0.37 to 1.39) | <.001 | 1.37 (0.43 to 2.31) | .004 |
| Sedentary activity | -0.12 (-0.02 to -0.22) | .03 | -0.24 (-0.10 to -0.38) | <.001 |
Fitness (in METs) is the dependent variable. β shown here as change in METs for each hour of activity (combined vigorous/moderate or sedentary) with confidence intervals. Models were stratified by gender and adjusted for age, BMI, mean wear time and included both sedentary and moderate-vigorous activity time.
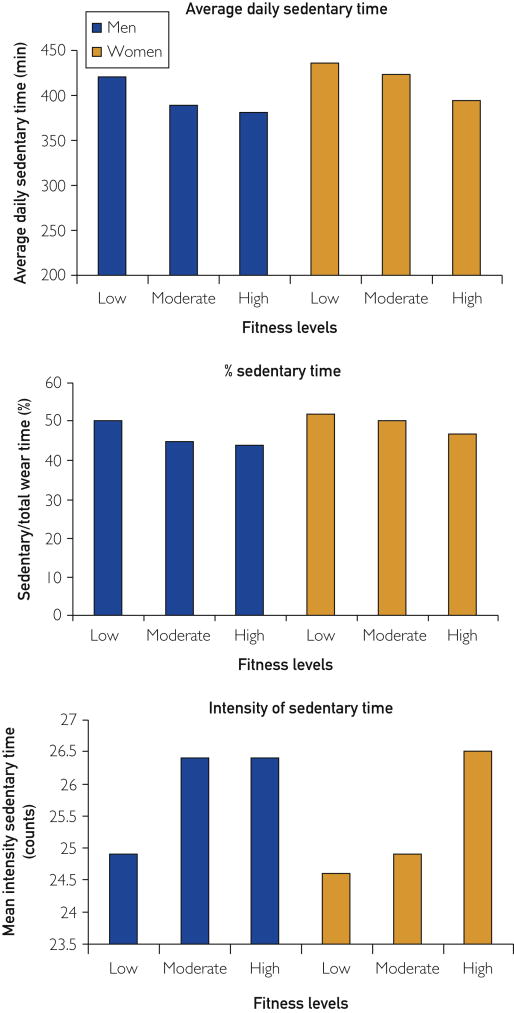
We observed a similar pattern of results whether sedentary time was quantified on an absolute scale (i.e. average daily sedentary time) (Figure 2 top, P<.001), a relative scale (i.e. percent sedentary time, figure 2 middle, P<.001), and as the average intensity of sedentary time, with lower intensities reflecting less movement during sedentary time (Figure 2 bottom, P<.001 in women, P=.01 in men).
Figure 2.
Accelerometer-derived Sedentary Time Across Fitness Levels. Sedentary variables according to fitness level as determined by ACLS percentiles based on age and gender (low fitness is at or below the 20th percentile, moderate fitness is between the 20th and 59th percentile, and high fitness is at or above the 60th percentile). Average daily sedentary time is inversely proportional to cardiovascular fitness for men and women (P trend <.001). The proportion of total valid wear time that is sedentary is inversely proportional to cardiovascular fitness for men and women (P trend <.001). Average sedentary count intensity is directly proportional to cardiovascular fitness (P trend <.001 in women, P=.01 in men).
Discussion
In the present study, we observed consistent, inverse associations between sedentary behavior and fitness that were independent of exercise activity. Specifically, we observed that the negative impact of 6-7 hours of sitting on fitness was similar to the beneficial effect of 1 hour of moderate-intensity exercise. These findings suggest that the risks related to sedentary behavior may be mediated, in part, through lower fitness levels.
Clinical Implications
The associations between both physical inactivity and low CRF with cardiovascular mortality are well-established.1, 3, 26 In spite of this knowledge and policy initiatives designed to promote the value of physical activity, relatively little progress has been made in increasing physical activity in the general population. According to data from the BRFFS/CDC, the percentage of U.S. adults not meeting the physical activity guidelines (self-report) has remained at or around 50% for more than two decades.5 Accelerometer-derived physical activity levels from NHANES 2003-2004 concluded that as few as 5% of adults adhere to the recommended 30 min/day of sustained bouts of activity.20 Clearly, more effective strategies are needed to address the risks related to physical inactivity and low CRF.
The findings from the present study suggest that sedentary behavior represents an important determinant of CRF levels, independent of physical activity. Thus, strategies designed to decrease the burden of sedentary behavior may represent a novel companion strategy to address the risks related to low fitness. In a meta-analysis of thirty-three studies, each 1-MET increase in CRF was associated with a 13% and 15% reduction in all-cause mortality and cardiovascular events, respectively.27 Based on our results, a woman could not only increase her CRF 1 MET with 40 additional minutes of exercise, but could also do this by decreasing daily sedentary time by four hours. The 40 minutes of exercise does not have to be done in one session but rather can be broken into shorter periods of 10-minute bouts.4 Similarly, sedentary time can be broken up with low intensity standing and ambulating done intermittently throughout the day.28-32
Physicians should assess patients' physical activity and sedentary behavior profiles, even if only qualitatively, as the two are not mutually exclusive. In addition to increasing physical activity, making active efforts to reduce sedentary behaviors may be a more feasible goal as a companion strategy to improve fitness, particularly in those who do little or no exercise.
Current Study in Context
Prior studies suggest that sedentary behavior might be associated with cardiovascular disease through its effects on metabolic risk. In an animal model of sedentary behavior, just 4 hours of inactivity was associated with a rapid decrease in plasma HDL and a reduction in triglyceride uptake into muscle, coincident with a parallel reduction in lipoprotein lipase levels.8, 13 Although exercise was associated with some increase in lipoprotein lipase activity, the magnitude of the deleterious effects of sedentary behavior far exceeded that of the beneficial effects of exercise. Similar findings have been seen in human studies. Healthy athletes that underwent a 2-week detraining period (no exercise, with activities limited to only those of daily living) were found to have significant reductions in muscle lipoprotein lipase levels on biopsy. They also noted significant increases in fasting insulin levels with detraining.14,15
The present study extends this prior work, suggesting that low fitness levels may represent an additional mechanism through which sedentary behavior confers adverse health risk. Our findings are consistent with previous reports from the Dallas Bedrest Study, where 3 weeks of bedrest was associated with dramatic declines in CRF levels equivalent to 3 decades of aging in those same men.33 Lifestyle patterns characterized by a high amount of sedentary activity may represent a milder form of bedrest that translates into negative effects on CRF. Additional research is needed to characterize the underlying mechanisms through which sedentary behavior lowers CRF levels.
For our primary analysis, we quantified physical activity and sedentary behavior using accelerometer data rather than self-report. Although both approaches represent valid measurement strategies, prior literature suggests that physical activity derived from questionnaires may be substantially overestimated.12, 20 Within our cohort, 18% of participants reported no regular exercise. However, based on accelerometer data, almost 40% did not log any moderate or vigorous-intensity exercise. Accelerometers provide objective measures about the frequency, intensity, and duration of activity patterns and have been shown to provide reliable measurements of both moderate and vigorous activity and sedentary behavior.34, 35
Limitations
Several limitations to our analyses should be noted. First, fitness testing in NHANES was restricted to a younger cohort (ages 12-49 years) without significant medical problems. This limits the generalizability of our findings to older adults who are at greatest risk for CVD. However, the risks related to sedentary behavior have been observed across multiple, diverse cohorts, suggesting that the risks related to sedentary behavior are consistent.9-11, 29 Thus, we would anticipate that the impact of sedentary behavior on fitness levels would also be present among older adults with a higher burden of chronic diseases. Second, this is a cross-sectional study, and therefore, it is possible that low fitness levels promote sedentary behavior directly. The Dallas Bedrest Study33 suggests, at a minimum, that extreme forms of sedentary behavior are causally related to declines in fitness. Nevertheless, additional prospective studies are needed. Third, submaximal treadmill testing was used to estimate maximal oxygen consumption and fitness. This test is inferior to symptom-limited maximum protocols because of its reliance on prediction formulas and the assumption of a linear heart rate response to exercise. However, previous studies have found moderate-to-high correlations between submaximal estimates of VO2 max and measured VO2 max in men and women (r = 0.76 to 0.92)36. A uniaxial accelerometer was used in this study. Although triaxial accelerometers were designed to capture more information from different types of activities (as they incorporate acceleration from three orthogonal directions), population studies in adolescents have concluded that these two types of accelerometers do not differ in their measurement of physical activity in population studies, and that either could be used.37 Finally, there is little consensus on activity count thresholds, with the most variability in the cut point definitions of time spent in moderate-vigorous exercise activity (rather than in sedentary activity). Our definitions are consistent with the majority of NHANES accelerometer publications (that use the NCI-supplied SAS syntax cut points) and are therefore, less likely to compromise the ability to make comparisons among studies.22
Conclusions
After adjustment for exercise activity, sedentary behavior appears to have an inverse association with fitness. These findings suggest that the risk related to sedentary behavior might be mediated, in part, through lower fitness levels. In addition to the benefits of regular exercise activity, avoiding sedentary behavior represents a potential strategy to improve health benefits independent of exercise activity. Additional research is needed to characterize the extent to which the detrimental effects of sedentary behavior can be reversed with alterations in sedentary lifestyle patterns. Efforts to reduce sedentary behavior are strongly needed, and future studies should also evaluate the efficacy of intervention strategies to achieve this goal.
Supplementary Material
Acknowledgments
Financial support: Dr. Berry receives funding from the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center; grant K23 HL092229 from the National Heart, Lung, and Blood Institute; and grant 13GRNT14560079 from the American Heart Association.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- CRF
cardiorespiratory fitness
- VO2 max
estimated maximal oxygen consumption
Footnotes
Disclosures: Dr. de Lemos has received honoraria from Astra Zeneca, consulting income from Janssen Pharmaceuticals, and serves on a Data Safety Monitoring Board for Novo Nordisk. Dr. Berry is a member of the Speaker's Bureau for Merck & Co. Dr. Blair serves on advisory boards for Technogym, Santech and the Clarity Project, receives book royalties from Human Kinetics and has unrestricted research grants from The Coca-Cola Company, Body Media, Technogym and the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry JD, Willis B, Barlow CE, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57(15):1604–10. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakoski SG, Barlow CE, Farrell SW, Berry JD, Morrow JR, Haskell WL. Impact of body mass index, physical activity, and other clinical factors on cardiorespiratory fitness (from the Cooper Center Longitudinal Study) Am J Cardiol. 2011;108(1):34–9. doi: 10.1016/j.amjcard.2011.02.338. [DOI] [PubMed] [Google Scholar]
- 3.Shiroma E, Lee I. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–52. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. [Accessed September 2013]; Health.gov website. http://www.health.gov/paguidelines/pdf/paguide.pdf.
- 5.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 6.Haskell WL, Blair SN, Hill JO. Physical activity: health outcomes and importance for public health policy. Preventive Medicine. 2009;49(4):280–2. doi: 10.1016/j.ypmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Owen N, Sparling PH, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clinic Proc. 2010;85(12):1138–41. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7(6):e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomistek AK, Manson JE, Stefanick ML, et al. The relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women's Health Initiative. J Am Coll Cardiol. 2013;61(23):2346–54. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy GN, Matthews CE, Dunstan DW, Winkler E, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. European Heart Journal. 2011;32(5):590–7. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988-2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125(21):2595–602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity and low-intensity ambulatory activity. J Physiol. 2003;551(Pt 2):673–82. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagibori R, Kondo K, Suzuki Y, et al. Effect of 20 days' bed rest on the reverse cholesterol transport system in healthy young subjects. J Intern Med. 1998;243(4):307–12. doi: 10.1046/j.1365-2796.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 15.Simsolo RB, Ong JM, Kern PA. The regulation of adipose tissue and muscle lipoprotein lipase in runners by detraining. J Clin Invest. 1993;92(5):2124–30. doi: 10.1172/JCI116813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vascular Biol. 2007;27(12):2650–56. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey, laboratory procedures manual. [Accessed Sept. 2013]; Chapter 16. http://cdc.gov/nchs/data/nhanes/nhanes_03_04/lab_pm.pdf.
- 19.National Cancer Institute; SAS programs for analyzing NHANES 2003-2004 Accelerometer Data. website. http://appliedresearch.cancer.gov/tools/nhanes_pam/ Last updated 03 Sep 2013. [Google Scholar]
- 20.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 21.Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc. 2012;44(No.1S):S1–4. doi: 10.1249/MSS.0b013e3182399b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003-2006. Prev Chronic Dis. 2012;9:E113. doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. NHANES cardiovascular fitness procedures manual. [Accessed September 2013]; http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/current_nhanes_03_04.htm.
- 24.Jackson, Blair, Mahar, Wier, Ross, Stuteville Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc. 1990;22(6):863–70. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1956;41(1-2):133–45. [Google Scholar]
- 26.Blair SN, Kohl HW, III, Barlow CE, Paffenberger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 27.Kodama S, Aaito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 28.Franklin BA. Health implications of low cardiorespiratory fitness, too little exercise, and too much sitting time: changing paradigms and perceptions. American Journal of Health Promotion. 2011;25(4):exi–exv. doi: 10.4278/ajhp.25.4.exi. [DOI] [PubMed] [Google Scholar]
- 29.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 30.Van der Ploeg HP, Chey MT, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222,497 Australian adults. Arch Intern Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 31.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab. 2004;286:E675–85. doi: 10.1152/ajpendo.00562.2003. [DOI] [PubMed] [Google Scholar]
- 32.McCrady-Spitzer SK, Levine JA. Nonexercise activity thermogenesis: a way forward to treat the worldwide obesity epidemic. Surgery for Obesity and Related Diseases. 2012;8(5):501–6. doi: 10.1016/j.soard.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.McGuire DK, Levine BD, Williamson JW, et al. A 30-year follow-up of the Dallas bed rest and training study: II. Effect of age and cardiovascular adaptation to exercise training. Circulation. 2001;104(12):1358–66. [PubMed] [Google Scholar]
- 34.Healy GN, Bronwyn CK, Winkler EAH, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults' sedentary time in population-based studies. Am J Prev Med. 2011;41(2):216–27. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant S, Corbett K, Amjad A, Wilson J, Atichison T. A comparison of methods of predicting maximum oxygen uptake. Br J Sports Med. 1995;29(3):147–52. doi: 10.1136/bjsm.29.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanhelst J, Beghin L, Duhamel A, Bergman P, Sjostrom M, Gottrand F. Comparison of uniaxial and triaxial accelerometry in the assessment of physical activity among adolescents under free-living conditions: the HELENA study. BMC Med Res Methodol. 2012:12–26. doi: 10.1186/1471-2288-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.