Abstract
Aims
Low midlife fitness is associated with higher risk for heart failure (HF). However, it is unclear to what extent this HF risk is modifiable and mediated by the burden of cardiac and non-cardiac co-morbidities. We studied the effect of cardiac and non-cardiac co-morbidities on the association of midlife fitness and fitness change with HF risk.
Methods & Results
Linking individual subject data from the Cooper Center Longitudinal Study (CCLS) with Medicare claims files, we studied 19,485 subjects (21.2% women) who survived to receive Medicare coverage from 1999 to 2009. Fitness estimated by Balke treadmill time at mean age of 49 years was analyzed as a continuous variable (in metabolic equivalents [METs]) and according to age- and sex-specific quintiles. Associations of midlife fitness and fitness change with HF hospitalization after age 65 were assessed by applying a proportional hazards recurrent events model to the failure time data with each co-morbidity entered as time-dependent covariates. After 127,110 person-years of Medicare follow up, we observed 1,038 HF hospitalizations. Higher midlife fitness was associated with a lower risk for HF hospitalization [HR 0.82 (0.76–0.87) per MET] after adjustment for traditional risk factors. This remained unchanged after further adjustment for the burden of Medicare-identified cardiac and non-cardiac co-morbidities [HR 0.83 (0.78–0.89)]. Each 1 MET improvement in midlife fitness was associated with a 17% lower risk for HF hospitalization in later life [HR 0.83 (0.74–0.93) per MET].
Conclusions
Midlife fitness is an independent and modifiable risk factor for HF hospitalization at a later age.
Keywords: Heart failure, Cardiorespiratory Fitness, Fitness change
INTRODUCTION
Heart failure (HF) represents an increasingly important health problem due to the aging of the population and improved survival after acute coronary events. (1) Therefore, a broader understanding of HF prevention is needed. Recently, several observational studies have reported that both physical activity and cardiorespiratory fitness in healthy adults are inversely associated with HF risk. (2–6) The mechanisms through which exercise might lower HF risk in healthy adults have not been established, and it remains uncertain to what extent there may be a direct effect of exercise on HF risk, independent of established HF risk factors. Although most prior studies adjust for the effects of current risk factors, few studies allow for the adjustment of subsequent, downstream risk factors acquired after physical activity/fitness measurement. It is also unknown whether the risks related to physical inactivity are modifiable through exercise training, as there are no adequately powered studies designed to test this question in healthy adults.
In order to better characterize the effects of exercise on HF risk, we linked the Cooper Center Longitudinal Study (CCLS) with individual claims data from the Center for Medicare and Medicaid Statistics. We sought to compare the association between both baseline fitness levels in middle age, and changes in fitness levels, with subsequent HF risk. We also sought to account for the influence of antecedent cardiovascular (e.g. acute myocardial infarction, hypertension) and non-cardiovascular (e.g. diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease) co-morbidities on these associations. We hypothesized that both baseline and fitness change values would be associated with a lower HF risk independent of the interval development of established risk factors for HF.
METHODS
Subject Population
Among 73,439 participants in the CCLS who underwent a complete clinical examination at the Cooper Clinic in Dallas, TX, between 1970 and 2009, 24,872 were eligible to receive Medicare coverage between 1999 and 2009 as described previously.(7) (8) After excluding 3,885 participants lacking Part A and B Medicare and HMO coverage, 819 individuals whose CCLS examination occurred after enrollment into Medicare Fee-for-Service, 55 participants with early Medicare benefits (< age 65 due to Medicare coverage for disability, end-stage renal disease, etc.), and 628 participants with a self-reported history of myocardial infarction or stroke at study entry, 19,485 CCLS participants remained in the final study sample for the present analysis. A subgroup of 8,683 participants underwent a second fitness examination a mean of 7.7 years after the initial examination and was included in the analyses of fitness changes. Participants were followed from the date of initiating Medicare coverage or 1999 (if already receiving Medicare coverage prior to 1999) until death or end of follow-up on December 31, 2009. No individual was excluded based on his or her performance on the exercise treadmill portion of the examination.
CCLS Clinical Examination
Details of the clinical examination and the study cohort have been published previously. (9,10) Participants underwent a comprehensive clinical examination, which included a self-reported personal and family history, standardized medical examination by a physician, fasting blood levels of total cholesterol, triglycerides, and glucose, as well as a maximal treadmill exercise test. Body mass index was calculated from measured height and weight. Fitness was measured in the CCLS by a maximal treadmill exercise test using a Balke protocol as described previously. (9–11) In this protocol, treadmill speed is set initially at 88 m/min. In the first minute, the grade is set at 0% followed by 2% in the second minute and an increase of 1% for every minute thereafter. After 25 minutes, the grade remains unchanged but the speed is increased 5.4 m/min for each additional minute until the test is terminated. Participants were encouraged not to hold onto the railing and were given encouragement to exert maximal effort. The test was terminated by volitional exhaustion reported by the participant or by the physician for medical reasons. The test time using this protocol has been shown to correlate highly with directly measured maximal oxygen uptake (r = 0.92). (12,13)
In accordance with standard approaches to the analysis of fitness data (9,10), treadmill times were compared with age- and sex-specific normative data on treadmill performance within the CCLS, allowing each individual’s treadmill time to be classified into an age- and sex-specific quintile of fitness. These quintiles of fitness measures were then combined into three, mutually exclusive fitness groupings: “low fitness”: quintile 1; “moderate fitness”: quintiles 2–3; “high fitness”: quintiles 4–5. Also, using well-characterized regression equations, treadmill times from the Balke protocol allow for estimation of fitness level in metabolic equivalents (METS). (7,12)
The measurement of other baseline variables in the CCLS has been well described. (9,10,14) Body mass index was calculated from measured height and weight. Seated resting blood pressure was obtained with a mercury sphygmomanometer. Fasting venous blood was assayed for serum cholesterol and glucose using standardized, automated techniques.
Medicare claims data
Medicare inpatient claims data were obtained from the Center for Medicare and Medicaid Services (CMS) for surviving participants who were 65 years of age or older and who were thus eligible for Medicare benefits during the period from 1999 — the first year CMS data are currently available for public use — through 2009. CMS data contain 100% of claims paid by Medicare for covered health care services. A beneficiary may be tracked over time to elicit a history of all the utilization of health care services. Inpatient hospitalization files from CMS provide all individual records for each medical service billed to Medicare, the date of service, primary diagnosis and up to eight secondary diagnoses (ICD-9 codes), and procedure codes (ICD-9 procedure codes).
In accordance with standard approaches, HF hospitalization was defined as a primary diagnosis of HF as indicated by ICD-9 codes 428, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, and 404.93 (15). Co-morbidities such as acute myocardial infarction (AMI), hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obstructive sleep apnea (OSA) and obesity were also determined using the data from CMS. As described previously, AMI hospitalization was defined by ICD-9 codes 410.0 to 410.9 as either a primary or secondary diagnosis (15–17) from the hospitalization files (i.e. MedPAR). The CMS Chronic Condition Warehouse (CCW) was used to identify the presence of DM, CKD and COPD during the Medicare follow up period (7). The presence of hypertension was identified in study participants when ICD-9 codes 401.xx was listed as an outpatient claim on two occasions at least thirty days apart within two years or when listed as a single inpatient claim.(18–20) OSA was identified when participants had at least one ICD-9 code from the following set: 786.03, 786.04, 780.51, 780.53, 780.57, and 327.2 listed in the Medicare claims files. (21,22) Obesity was identified when participants had at least one claim using ICD codes 278.xx listed in the Medicare claims files. (23,24)
Statistical analysis
Since patients enter the Medicare claims data at various ages and for varying durations, the data is subject to censoring on the right and the left, with the possibility of multiple events per patient. Therefore, we estimated the intensity of recurrent events using the conditional model of Prentice, Williams, and Peterson. (25) This model stratifies the at-risk population by the number of HF hospitalizations. At the time a participant develops a HF hospitalization, they advance to the next at-risk stratum. Multiple HF hospitalizations within a 30-day time interval for a subject were counted as a single event. The presence of co-morbidity was defined when a condition, based on its respective aforementioned definitions, was noted prior to or within 365 days after a study participant’s first HF hospitalization. Associations between midlife cardiorespiratory fitness and HF hospitalization after age 65 were assessed by applying a proportional hazards recurrent events model to the failure time data with MI, DM, hypertension, COPD, CKD, OSA and obesity entered as time-dependent covariates. We used attained age as the time scale, and entered mid-life fitness as a continuous covariate. Models were also adjusted for age at baseline examination, gender, smoking, total cholesterol, baseline systolic blood pressure, and baseline body mass index.
The association between midlife fitness and HF hospitalization was characterized according to the aforementioned mutually exclusive fitness groupings in three separate models. Model 1 was adjusted for fitness and baseline characteristics (age, sex, BMI, cholesterol, baseline Diabetes status, smoking and systolic blood pressure). Model 2 included all variables listed in model 1 and all co-morbidities (DM, hypertension, acute MI, COPD, CKD, Obesity and OSA) observed during Medicare follow up. A variation of model 2 included baseline risk factors as well as the overall burden of co-morbidities (i.e. 1, 2, 3, or 4+). Finally, change in fitness was defined based on the transition of subjects from one fitness group to another (i.e. low fitness to not low fitness) and continuously as the change in METs between the two examinations. The association between the continuous change in fitness (i.e. per MET) and HF hospitalization was assessed by applying proportional hazards recurrent events model to the failure time data and adjusted for age, traditional risk factors, baseline fitness, as well as interval development of comorbidities. All fitness change models were additionally adjusted for the time between each fitness examination.
All statistical analyses were performed using SAS for Windows (release 9.2; SAS Institute, Inc., Cary, NC). This work was supported by the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center and grant 13GRNT14560079 from the American Heart Association to PI (JB). The authors are solely responsible for the study design, analyses, preparation of the manuscript and its final contents.
RESULTS
Baseline characteristics for the study population demonstrate a low burden of traditional risk factors at the time of study enrollment, with a lower burden of traditional risk factors across higher levels of fitness (Table 1).
Table 1.
Baseline characteristics according to category of baseline fitness from data acquired at the baseline clinical examination.
| Cohort Characteristics | Low Fitness (n=3,400) |
Moderate Fitness (n=7,978) |
High Fitness (n=8,107) |
|---|---|---|---|
| Age at Baseline exam, years | 46.4 (8.6) | 48.9 (8.8) | 51.1(8.5) |
| Females (%) | 16.6 | 19.3 | 24.8 |
| Age at Medicare Entry, years | 67.8 (4.8) | 67.9 (4.9) | 67.6 (4.7) |
| Systolic BP, mm Hg | 123.6 (14.9) | 121.5 (14.5) | 120.9 (14.8) |
| Total cholesterol, mg/dL | 220.6 (41.1) | 216.0 (39.4) | 209.6 (37.5) |
| BMI, kg/m2 | 28.0 (4.8) | 26.1 (3.5) | 24.4 (2.9) |
| Current Smokers (%) | 28.5 | 17.4 | 7.9 |
| Baseline Diabetes (%) | 5.0 | 2.8 | 1.6 |
| METS | 8.0 (1.4) | 9.9 (1.5) | 12.2 (2.2) |
Data presented as mean (SD) or % except as noted
METS: metabolic equivalents; BMI
After 12,7110 person years of Medicare follow up, among 19,485 participants we observed 1,038 HF hospitalizations as well as a large number of co-morbidities, including DM (N= 3,838), hypertension (N= 11,591), acute MI hospitalization (N= 666), CKD (N= 2,666), COPD (N= 2,369), OSA (N= 2,448), and Obesity (N= 1,648).
Lower fitness measured in middle-age was associated with a higher burden of HF and other co-morbidities after age > 65 years. For example, compared to low midlife fitness (quintile 1), high fitness (quintiles 4–5) was associated with a lower burden of HF hospitalization (5.8% vs. 1.7%), acute MI hospitalization (4.9% vs. 2.5%), and DM (34.3% vs. 11.9%) (Table 2).
Table 2.
Prevalence of heart failure and other co-morbidities after age >65 as observed on Medicare follow up stratified by mid-life fitness quintiles.
| Co-morbidity* | Low Fitness (n=3,400) |
Moderate Fitness (n=7,978) |
High Fitness (n=8,107) |
|---|---|---|---|
| DM (%) | 34.3 | 21.4 | 11.9 |
| HTN (%) | 69.6 | 62.7 | 52.0 |
| COPD (%) | 20.6 | 12.3 | 7.8 |
| CKD (%) | 21.3 | 14.1 | 10.1 |
| Obesity (%) | 16.1 | 9.5 | 4.3 |
| OSA (%) | 17.0 | 14.1 | 9.2 |
| Acute MI hospitalization (%) | 4.9 | 3.7 | 2.5 |
| HF hospitalization (%) | 5.8 | 3.3 | 1.7 |
Each co-morbidity has been defined based on medicare claims data as follows:
Acute MI hospitalization was defined by ICD-9 codes 410.0 to 410.9 listed as a diagnosis from the hospitalization files (i.e MedPAR).
DM, CKD and COPD were identified using the CMS Chronic Conditions Warehouse.
Hypertension was identified using ICD-9 codes 401.xx listed as an outpatient claim on two occasions within two years or when listed as a single inpatient claim.
OSA was identified using at least one ICD-9 code from the following set: 786.03, 786.04, 780.51, 780.53, 780.57, and 327.2 listed in the Medicare claims files
Obesity was identified using ICD-9 codes 278.xx listed in the Medicare claims files.
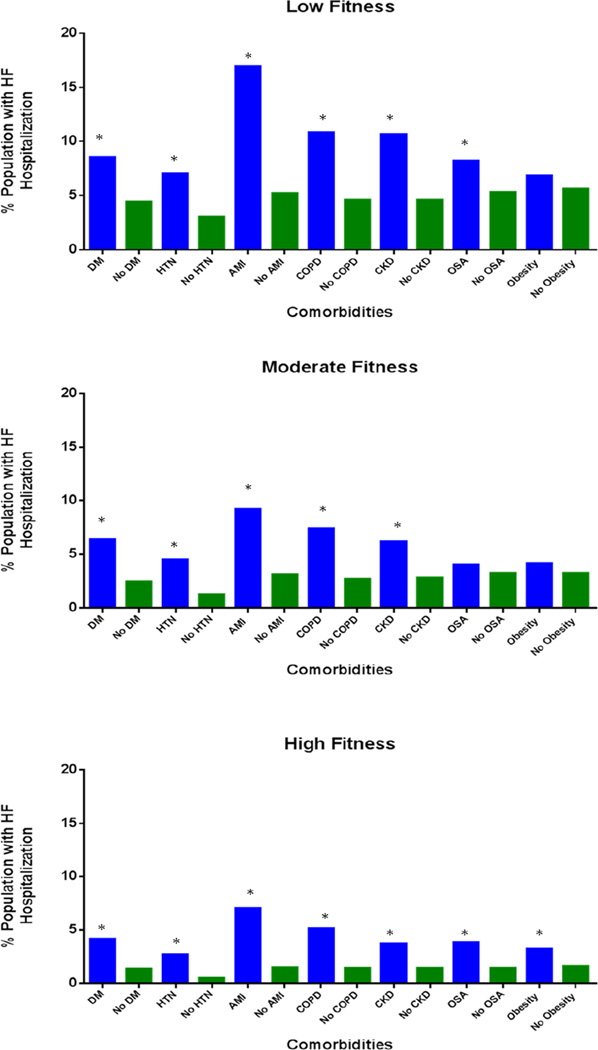
In analyses stratified by fitness levels and individual co-morbidities, both lower fitness levels measured at study entry and the presence of co-morbidities at age > 65 years were associated with a higher rate of HF hospitalization. This association was preserved across all fitness quintiles and across all co-morbidities. For example, among participants with low midlife fitness, the presence of an interval hospitalization for acute MI at age >65 was associated with a higher rate of HF hospitalization compared to participants with low midlife fitness but without an interval acute MI (16.9% vs. 5.2%). Similarly, among participants with high midlife fitness, we observed a similar pattern of results for those with and without an interval acute MI (HF hospitalization: 7.0% vs. 1.5%) (Figure 1).
Figure 1.
Prevalence of heart failure in participant groups with or without other co-morbidities as seen on Medicare follow up across mid-life fitness quintiles. * P-value < 0.05
After adjustment for traditional risk factors measured at the baseline examination, higher midlife fitness was associated with a lower risk for HF hospitalization [HR 0.82 (0.76–0.87) per MET; Table 3, model 1]. After adjustment for both traditional risk factors and interval development of co-morbidities, the association between fitness and HF hospitalization remained unchanged [HR 0.83 (0.78–0.89)]. In this fully adjusted model DM, acute MI, and COPD, but not HTN, CKD, OSA and obesity were independently associated with HF hospitalization (Table 3, model 2).
Table 3.
Multivariable adjusted hazard ratios for HF hospitalization.
| Model 1# | Model 2¥ | |||
|---|---|---|---|---|
| HR (95%CI) | Chi-Square | HR (95%CI) | Chi-square | |
| Fitness (per MET) | 0.82 (0.76–0.87)* | 37.9 | 0.83 (0.78–0.89) * | 29.80 |
| DM | - | - | 1.59 (1.26–1.96) * | 16.96 |
| HTN | - | - | 1.03 (0.77–1.37) | 0.04 |
| Acute MI | - | - | 1.64 (1.16–2.32) * | 7.85 |
| COPD | - | - | 1.37 (1.09–1.72) * | 7.07 |
| CKD | - | - | 1.14 (0.89–1.47) | 1.03 |
| Obesity | - | - | 0.82 (0.58–1.15) | 1.30 |
| OSA | - | - | 1.31 (0.97–1.75) | 3.00 |
Model 1: Adjusted for fitness and age, sex, BMI, cholesterol, baseline DM, smoking and systolic BP.
Model 3: Adjusted for variables in Model 1+ interval DM, HTN, acute MI, COPD, CKD, Obesity and OSA.
P value<0.05; Hazards are Yes vs. No for co-morbid conditions (DM, HTN, COPD, Acute MI, Obesity, OSA)
Each co-morbidity has been defined based on medicare claims data as follows:
Acute MI hospitalization was defined by ICD-9 codes 410.0 to 410.9 listed as a diagnosis from the hospitalization files (MedPAR).
DM, CKD and COPD were identified using the CMS Chronic Conditions Warehouse.
Hypertension was identified using ICD-9 codes 401.xx listed as an outpatient claim on two occasions within two years or when listed as a single inpatient claim.
OSA was identified using at least one ICD-9 code from the following set: 786.03, 786.04, 780.51, 780.53, 780.57, and 327.2 listed in the Medicare claims files.
Obesity was identified using ICD-9 codes 278.xx listed in the Medicare claims files.
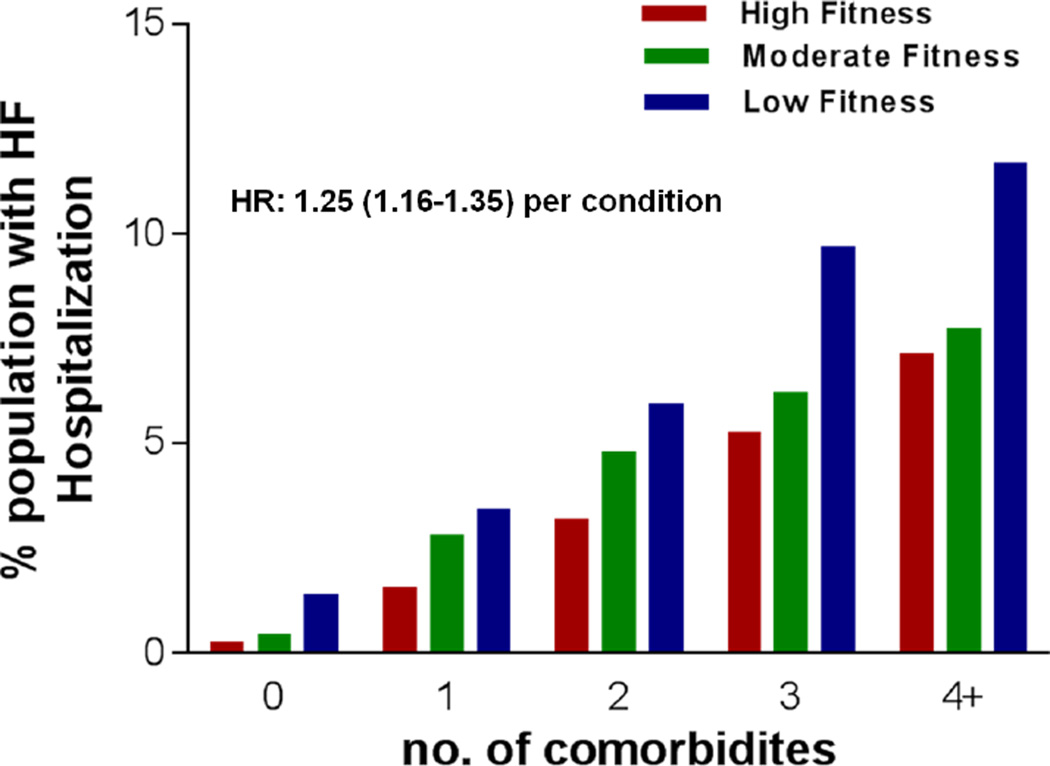
When the individual co-morbidities were added as an integer, we observed a higher risk for subsequent HF hospitalization among participants with 1, 2, 3, or 4 or more co-morbidities (Figure 2). This association was apparent across all fitness/co-morbidity subgroups, with the highest risk among participants with low baseline fitness and 4 or more co-morbidities in older age. Even after adjustment for all baseline risk factors and fitness, there was a 25% increase in risk for HF hospitalization for each, additional co-morbidity identified at age > 65 years (HR: 1.25; 95% CI: 1.16–1.35).
Figure 2.
Prevalence of heart failure among participant groups with increasing number of co-morbidities at age > 65 years. Their mid-life fitness levels have stratified the data for each subject group. Multivariable adjusted hazard ratio for HF hospitalization associated with total co-morbidity burden at age >65 years (defined as a continuous variable) is 1.25(1.16–1.35) per co-morbidity.
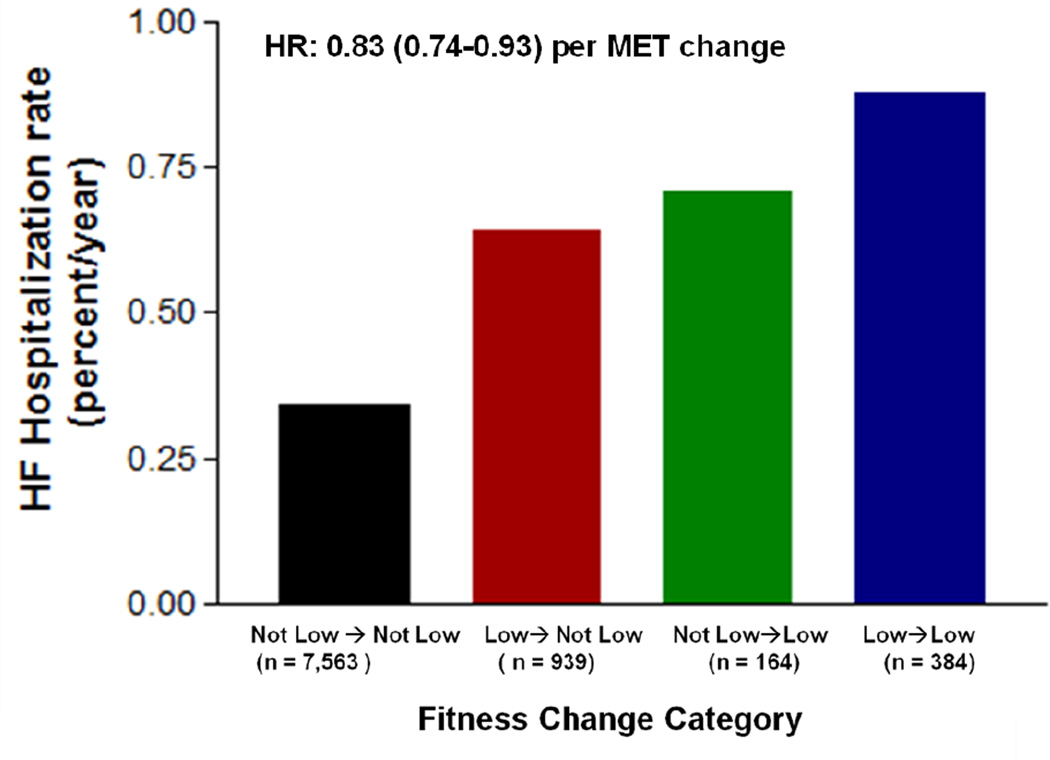
Finally, a subgroup of participants underwent a second fitness examination a mean of 7.7 years after the baseline examination, allowing an analysis of change in midlife fitness on HF risk in later life (Supplemental Table). Compared to individuals with persistently low fitness levels in middle age, individuals who increased their fitness levels from low fit (quintile 1) to not low fit (quintiles 2–5) had a lower rate of HF hospitalization (Figure 3). Patients with sustained higher fitness levels (quintiles 2–5) at follow-up had the lowest risk for HF hospitalization. A similar pattern of results was observed when change in fitness was examined as a continuous variable in the fully adjusted model, suggesting that the association between change in fitness and HF risk was consistent across the distribution of fitness change levels and was independent of the thresholds used to defined fitness quintiles [HR: 0.83 (95% CI: 0.74–0.93) per MET].
Figure 3.
Association between change in mid-life fitness and HF hospitalization rate at a later age. Fitness change categories are determined based on transition of subjects from one fitness group to other between their initial and follow up examinations: Not low Fit → Not low Fit: Q2–5→Q2–5; Low Fit → Not low Fit: Q1→Q2–Q5; Not low Fit → Low Fit: Q2–Q5→Q1; Low Fit →Low Fit: Q1→Q1. Multivariable adjusted hazard ratio for HF hospitalization associated with fitness change, defined continuously as difference in METS between the two examinations, is 0.83 (0.74–0.93) per MET.
DISCUSSION
In the present study, we observed several important findings. First, in a cohort with low prevalence of traditional risk factors, both lower midlife fitness and increased co-morbidity burden after age > 65 were associated with a higher risk of HF hospitalization. Second, the inverse association between midlife fitness and HF hospitalization risk was independent of the presence of cardiac and non-cardiac co-morbidities after age > 65 years. Finally, an improvement in midlife fitness was associated with a lower risk of HF hospitalizations at a later age. Taken together, these findings suggest that the HF risks associated with low fitness are modifiable and may reflect the direct effects of exercise independent of antecedent HF risk factors.
Higher cardiorespiratory fitness and physical activity are associated with a lower risk for fatal cardiovascular mortality across life span. (9,26–29) Furthermore, higher levels of fitness have been shown to modify the relationship between body fatness and mortality. (30–33) However, the association between fitness and non-fatal cardiovascular events is not well understood, thereby limiting the understanding of the pathway through which fitness lowers cardiovascular mortality risk. Recently, we observed that the protective associations of higher fitness levels in middle-age were stronger for HF than for acute myocardial infarction (15), suggesting a unique role of HF risk in the pathway from low fitness to death. Similarly Khan et al (6) have reported a strong, dose dependent inverse association between cardiorespiratory fitness and HF risk. However, to our knowledge, the potential pathway through which higher fitness might lower HF risk has not been well established.
One potential mechanism for this observed association is through the indirect effects of lower fitness on both cardiovascular and non-cardiovascular risk factors whereby low fitness in middle-age predisposes to the accumulation of these established HF risk factors. In addition to the role of traditional HF risk factors (i.e. diabetes, hypertension, coronary disease), several recent studies have identified the influence of both antecedent cardiac and non-cardiac co-morbidities on incident HF in the community. (5,34–36) In the present study, we also observed similar association between the presence of cardiac and non-cardiac co-morbidities and risk for HF hospitalization, with marked differences in risk for HF among individuals with a higher burden of chronic disease prior to HF hospitalization. Even though low fitness in middle-age represents an important determinant of the burden of chronic disease in later life (7), we observed that lower mid-life fitness was associated with a higher risk for HF hospitalization independent of and across all levels of chronic disease burden. These findings suggest that the pathway through which higher fitness levels in middle-age confers a lower risk for HF hospitalization decades later in older age is at least in part independent of the development of future, established HF risk factors.
Another potential mechanism through which fitness in middle-age might lower HF risk in later life is through more direct effects of exercise on cardiovascular physiology. Physical activity and higher levels of fitness have been associated with favorable effects on cardiac structure and function. (37–39) Athletes and individuals with higher levels of physical activity have improved early diastolic filling (40–42). Studies have also observed that healthy but sedentary aging leads to prominent increase in left ventricular stiffness, a potential substrate for HF. (43) Furthermore, short term exercise training in previously sedentary subjects has been shown to improve cardiorespiratory fitness as well as physiological left ventricular remodeling. (44) Similarly, exercise training in high-risk individuals with concentric left ventricular hypertrophy and poorly controlled hypertension is associated with a reduction in left ventricular mass and wall thickness (45). Animal model studies have also observed a direct, favorable effect of exercise training on cardiac structure and function leading to a delayed onset of HF (46). In the present study, we extend these findings and show that improvement in fitness in middle-age was independently and inversely associated with HF at a later age. Taken together, these findings suggest a more direct, causal association between mid-life fitness and HF risk in later life.
Our study has several important clinical implications. HF is a significant public health burden with an estimated prevalence of 2.1% and an annual cost of $39.2 billion in 2010. (47,48) Risk of developing HF increases with advancing age and it is one of the most frequent causes of hospitalization in the elderly population. (49,50) Although there has been substantial advancement in the diagnosis and treatment of HF, not much progress has been made in the field of disease prevention. Current preventive strategies for HF are limited to modification of traditional risk factors such as hypertension, diabetes, and coronary artery disease. In this study, we have identified mid-life cardiorespiratory fitness as an independent and modifiable risk factor for HF, which could have implications for the development of future preventive therapies.
Improvement in physical activity levels as well as cardiorespiratory fitness has been associated with a lower risk of cardiovascular mortality.(52,53) Blair et al reported a 44% reduction in risk of mortality among CCLS subjects who had an improvement in their mid-life fitness levels. (10) Furthermore, recent studies have shown that improvement or maintenance in cardiorespiratory fitness is associated with lower risk for development of CVD risk factors as well as mortality independent of change in body mass index. (54,55) In the present study, we extend these findings and report a protective effect of improvement in mid-life fitness on HF risk at a later age. Implementation of more aggressive exercise training in middle-age individuals with low fitness could be an effective strategy to reduce the burden of HF in later life.
This study has several important limitations. First, our study population from the Cooper Center Longitudinal Study represents a unique cohort of predominantly white participants with a low risk burden and relatively high socioeconomic status compared to the general population. However, recent studies from our group has shown that the association between major risk factors and lifetime risk of CVD in the CCLS cohort is similar to that observed in general population. (27,56) These findings suggest that although the burden of risk factors in the CCLS may be lower than the general population, the effect of these risk factors on future cardiovascular disease is similar. The development of a national cardiorespiratory fitness registry with directly measured fitness levels during exercise testing in well established centers throughout United States would be extremely useful to better understand the role of fitness in modifying HF risk among participants with different ethnic and socioeconomic backgrounds. (51)
Second, we linked individual-level data with Medicare claims files to compare the association between fitness and HF hospitalization at age ≥ 65 years. We were not able to capture outcomes that occurred between study entry and the onset of Medicare eligibility. However, similar approach has been used previously to study the contribution of traditional risk factors on medicare outcomes. (57–59)
Finally, we used diagnoses from Medicare claims files rather than adjudicated clinical outcomes and therefore some events might have been missed or misclassified. However, measurement error tends to bias towards the null and encouragingly, the association between fitness and HF hospitalizations were present despite use of administrative data.
In summary, we observed that cardiorespiratory fitness in healthy, middle-aged adults is an independent and modifiable risk factor for development of HF at a later age. These findings highlight the importance of exercise training in middle-age individuals as an effective preventive strategy to reduce HF burden at a later age.
Acknowledgements
none
Funding Sources
This work was supported by the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center and grant 13GRNT14560079 from the American Heart Association to PI (JB). The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. All authors have read and agree to the manuscript as written.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All other authors report no conflicts of interest relevant to this manuscript.
REFRENCES
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. The New England journal of medicine. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. Journal of applied physiology. 2005;99:1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Tuomilehto J, Jousilahti P, et al. Occupational, commuting, and leisure-time physical activity in relation to heart failure among finnish men and women. Journal of the American College of Cardiology. 2010;56:1140–1148. doi: 10.1016/j.jacc.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 5.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of internal medicine. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 6.Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. European journal of heart failure. 2014;16:180–188. doi: 10.1111/ejhf.37. [DOI] [PubMed] [Google Scholar]
- 7.Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife Fitness and the Development of Chronic Conditions in Later Life. Archives of internal medicine. 2012;172:1–8. doi: 10.1001/archinternmed.2012.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defina LF, Willis BL, Radford NB, et al. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Annals of internal medicine. 2013;158:162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA : the journal of the American Medical Association. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 10.Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA : the journal of the American Medical Association. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 11.Willis BL, Morrow JR, Jr, Jackson AW, Defina LF, Cooper KH. Secular change in cardiorespiratory fitness of men: Cooper Center Longitudinal Study. Medicine and science in sports and exercise. 2011;43:2134–2139. doi: 10.1249/MSS.0b013e31821c00a7. [DOI] [PubMed] [Google Scholar]
- 12.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 13.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 14.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA : the journal of the American Medical Association. 1996;276:205–210. [PubMed] [Google Scholar]
- 15.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circulation Heart failure. 2013;6:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Normand SL, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: progress and continuing challenges. Circulation. 2010;121:1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 17.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open medicine : a peer-reviewed, independent, open-access journal. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 19.Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428. doi: 10.1161/HYPERTENSIONAHA.109.139279. [DOI] [PubMed] [Google Scholar]
- 20.Robitaille C, Dai S, Waters C, et al. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2012;184:E49–E56. doi: 10.1503/cmaj.101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou KT, Huang CC, Tsai DC, et al. Sleep apnea and risk of retinal vein occlusion: a nationwide population-based study of Taiwanese. American journal of ophthalmology. 2012;154:200–205. e1. doi: 10.1016/j.ajo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Javaheri S, Shukla R, Wexler L. Association of smoking, sleep apnea, and plasma alkalosis with nocturnal ventricular arrhythmias in men with systolic heart failure. Chest. 2012;141:1449–1456. doi: 10.1378/chest.11-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flum DR, Kwon S, MacLeod K, et al. The use safety and cost of bariatric surgery before and after Medicare's national coverage decision. Annals of surgery. 2011;254:860–865. doi: 10.1097/SLA.0b013e31822f2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Annals of surgery. 2008;247:21–27. doi: 10.1097/SLA.0b013e318142cb4b. [DOI] [PubMed] [Google Scholar]
- 25.Prentice R, Williams B, Peterson A. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–379. [Google Scholar]
- 26.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. The New England journal of medicine. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 27.Berry JD, Willis B, Gupta S, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. Journal of the American College of Cardiology. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA : the journal of the American Medical Association. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Progress in cardiovascular diseases. 2014;56:382–390. doi: 10.1016/j.pcad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Lavie CJ. Obesity and prognosis-just one of many cardiovascular paradoxes? Progress in cardiovascular diseases. 2014;56:367–368. doi: 10.1016/j.pcad.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Lavie CJ, Schutter AD, Archer E, McAuley PA, Blair SN. Obesity and prognosis in chronic diseases--impact of cardiorespiratory fitness in the obesity paradox. Current sports medicine reports. 2014;13:240–245. doi: 10.1249/JSR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 33.McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Progress in cardiovascular diseases. 2014;56:434–440. doi: 10.1016/j.pcad.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Lam CS, Lyass A, Kraigher-Krainer E, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. European journal of heart failure. 2012;14:414–422. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. Journal of the American Society of Nephrology : JASN. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 37.Prasad A, Popovic ZB, Arbab-Zadeh A, et al. The effects of aging and physical activity on Doppler measures of diastolic function. The American journal of cardiology. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study) The American journal of cardiology. 1990;65:371–376. doi: 10.1016/0002-9149(90)90304-j. [DOI] [PubMed] [Google Scholar]
- 39.Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart failure. 2014;2:238–246. doi: 10.1016/j.jchf.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 41.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 42.Turkbey EB, Jorgensen NW, Johnson WC, et al. Physical activity and physiological cardiac remodelling in a community setting: the Multi-Ethnic Study of Atherosclerosis (MESA) Heart. 2010;96:42–48. doi: 10.1136/hrt.2009.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 44.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kokkinos PF, Narayan P, Colleran JA, et al. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. The New England journal of medicine. 1995;333:1462–1467. doi: 10.1056/NEJM199511303332204. [DOI] [PubMed] [Google Scholar]
- 46.Miyachi M, Yazawa H, Furukawa M, et al. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension. 2009;53:701–707. doi: 10.1161/HYPERTENSIONAHA.108.127290. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 48.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 49.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 50.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 51.Kaminsky LA, Arena R, Beckie TM, et al. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 52.Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 53.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. The New England journal of medicine. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 54.Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–2490. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. Journal of the American College of Cardiology. 2012;59:665–672. doi: 10.1016/j.jacc.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. The New England journal of medicine. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daviglus ML, Liu K, Greenland P, et al. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. The New England journal of medicine. 1998;339:1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 58.Daviglus ML, Liu K, Pirzada A, et al. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Archives of internal medicine. 2005;165:1028–1034. doi: 10.1001/archinte.165.9.1028. [DOI] [PubMed] [Google Scholar]
- 59.Daviglus ML, Liu K, Yan LL, et al. Relation of body mass index in young adulthood and middle age to Medicare expenditures in older age. JAMA : the journal of the American Medical Association. 2004;292:2743–2749. doi: 10.1001/jama.292.22.2743. [DOI] [PubMed] [Google Scholar]