Abstract
In this article, we present the case of a 57-year-old man with cervical and mediastinal tumor mass, normal blood count as well as virusological status. Cervical tumor tissue biopsy revealed cells positive for CD34, CD13, LCA, CD33, and CD163 but negative for T-cell and B-cell markers, NK-cell markers, plasmacytic markers and anaplastic large cell lymphoma markers. These features were consistent with myeloid sarcoma of the neck with involvement of the mediastinum. We discussed differential diagnosis and therapy of isolated myeloid sarcoma and suggest that clinical presentation, cell morphology, complete immunophenotype, and specific genotypic lesions in some cases, must be evaluated.
Keywords: Isolated myeloid sarcoma, Neck, Chest, Immunohistochemistry
1 Introduction
Myeloid sarcoma (MS) is a tumor mass consisting of myeloid blasts with or without maturation occurring at an anatomical site other than bone marrow [1]. In the literature, MS is also known as granulocytic/monoblastic sarcoma, extramedullary myeloid tumor, or chloroma, and may occur de novo, precide or coincide with acute myeloid leukemia (AML), represent blastic transformation of myeloprolipherative neoplasm or myelodisplastic syndrome or can be the initial manifestation of relapse in patients with previously diagnosed AML [1,2,3]. The term myeloid sarcoma was accepted by the World Health Organization (WHO) in 2002, but the disease was first recognized in the 19th century by Burns [4] and was later described by King and Rappaport [5,6].
Involvement of various tissues, including the skin, periosteum, intestine, lymph nodes, reproductive system, central nervous system, heart, lungs and oral cavity, has been previously reported [7–12]. Diagnosis of MS can be confirmed with cytochemical and immunophenotypic analyses that allow the distinction of MS from lymphomas, small cells tumors and blastic plasmacytoid dendritic cell neoplasm [1,13,14]. In this study, we present the case of a 57-year-old man diagnosed with isolated myeloid sarcoma of the neck and chest and discuss the factors involved in a timely differential diagnosis bearing in mind that previous studies have reported that in the absence of hematological disorders, 46–75% of patients are initially diagnosed with a condition other than MS, most commonly non-Hodgkin’s lymphoma (NHL) [15,16].
2 Case Report
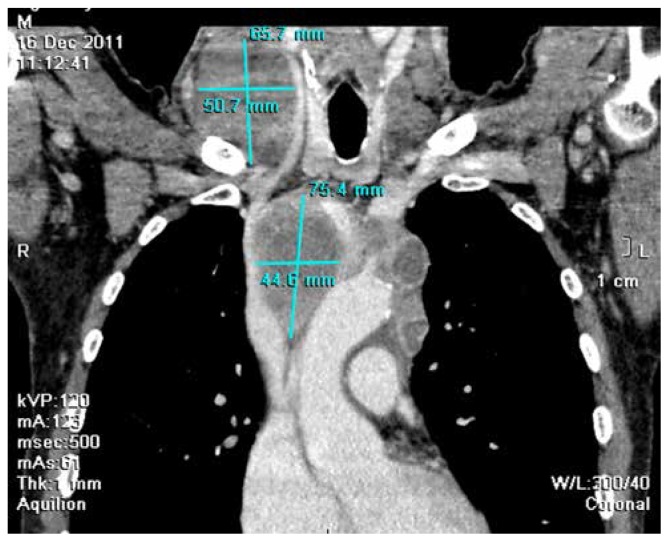
A 57-year-old man with past medical history significant for cerebrovascular stroke presented with intermittent chest pain and discomfort, which had persisted for 3 months. Physical examination revealed right supraclavicular tumor mass (7 × 5 cm), with no additional significant findings. The blood cell count revealed a hemoglobin level of 121 g/L; white blood cell count, 5.6/L (74% neutrophils, 2% eosinophils, 3% basophils, 15% lymphocytes, and 6% monocytes); and platelet count, 264 × 109/L. The complete biochemical panel was normal, except for an elevated LDH level (680 U/L). Chest radiography showed a widened mediastinum. Neck and thorax computed tomography (CT) revealed a tumor mass of the right supraclavicular region as well as right anterior mediastinum that was connected in the level of superior thoracic aperture; the mass surrounded the great vessels, with compression of the structures (Figure 1). Findings of abdominal ultrasonography and CT were normal.
Figure 1.
Neck and thorax computed tomography
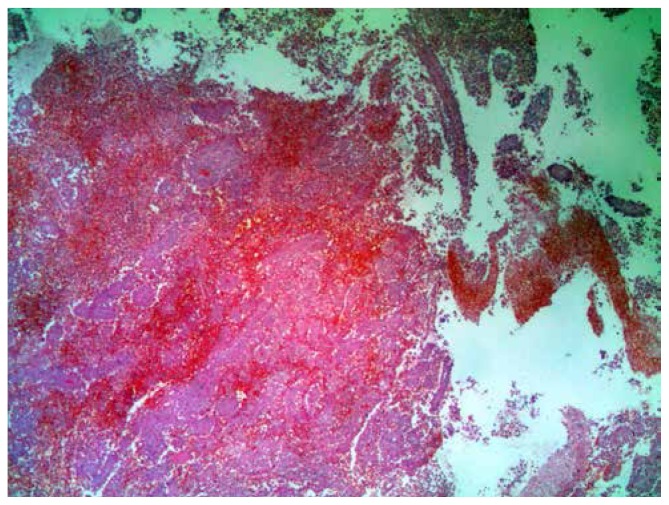
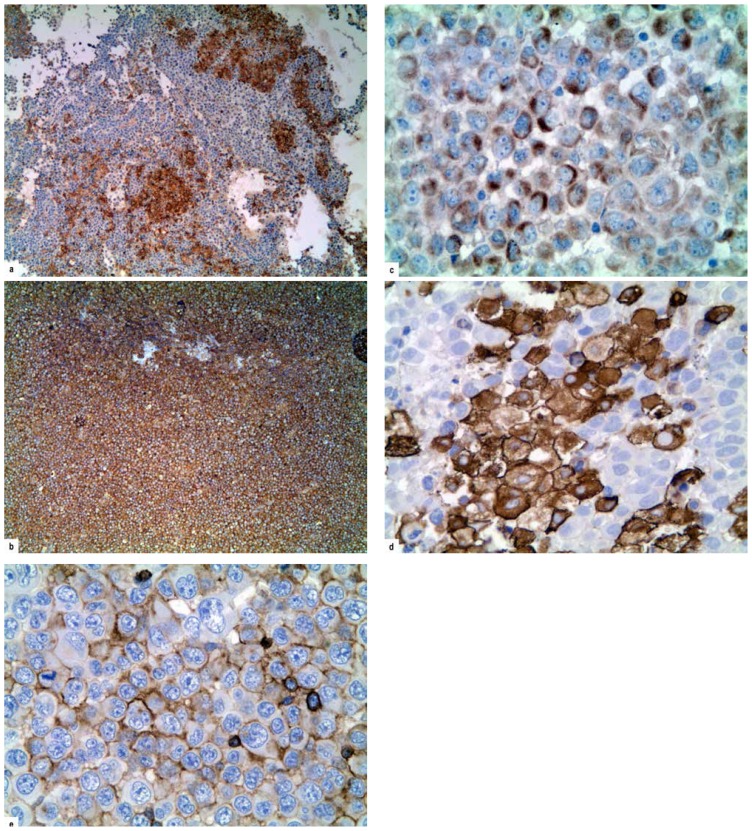
Supraclavicular tumor mass biopsy revealed monomorphic large-sized discohesive tumor cells with moderate eosinophilic cytoplasm, oval cleaved nuclei containing prominent nucleoli, and moderate mitotic activity. Tumor tissue also showed large necrotic fields and surrounded rare residual small lymphocytes, histiocytes and plasma cells (Figure 2). Immunohistochemistry analysis confirmed that tumor cells were positive for CD33, CD34, CD13, CD163 and LCA (Figure 3a–e) but negative for MPO, T-cell and B-cell markers (CD20 and CD79a), NK-cell markers (CD56), plasmacytic markers (CD138), and anaplastic large cell lymphoma markers (CD30 and ALK1). Findings of bone marrow biopsy and aspirate were normal, as were those of flow cytometric analysis. Cytogenetic examination of the bone marrow cells revealed a normal karyotype.
Figure 2.
The H/E staining of the tumor tissue (x25).
Figure 3.
Tumor cells were positive for a) CD33 (x50), b) CD34 (x50), c) CD13 (x200), d) CD163 (x200), e) LCA (x200).
These features were consistent with those of MS of the neck, with probable involvement of the mediastinum. The patient received standard AML induction chemotherapy (cytarabine 100 mg/m2 for 7 days and daunorubicin 50 mg/m2 for 3 days). On day 8, the patient experienced aplasia, which was complicated by a prolonged fever despite administration of intensive antibacterial and anti-fungal therapy. The patient remained febrile without a confirmed infection, and passed away 14 days after beginning therapy. During that period there was not chest X-ray confirmation of mediastinal lymphadenopathy regression.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent
Informed consent has been obtained from all individuals included in this study.
3 Discussion
There is little in the literature about isolated myeloid sarcoma presenting with tumor mass of neck and chest. In contrast to our case, few reports describe patients misdiagnosed as malignant lymphoma while two patients with a lymph node lesion exhibiting extensive lymph node infarction were diagnosed after AML development [17–19].
Therefore, diagnosis of MS poses a major challenge, because the expression of multiple markers must be analyzed to confirm the myeloid origin of neoplastic cells. Small round cell tumors, undifferentiated carcinoma or melanoma, lymphoproliferative disease, extramedullary localizations of chronic myeloproliferative diseases, and some nonmalignant lesions can be considered in the differential diagnosis of non infectious lymphadenopathy in adult patients. To distinguish between these diagnoses and myeloid sarcoma, clinical presentation, including tumor location, cell morphology, complete immunophenotype, and specific genotypic lesions in some cases, must be evaluated [1–3,13,14].
In our patient localization of tumor mass initially pinpointed to suspect lymphoprolipherative disease. However, differential diagnosis between MS and lymphoproliferative diseases can be complicated [1,13,14] especially in poorly differentiated MS in which the morphology may resemble large-cell non-Hodgkin lymphoma when the cells are MPO-negative and weakly stained. Based on this evidence, we believe that detailed immunohistochemistry is essential for accurate diagnosis of MS. Several studies have reported that the immunophenotypic profile of MS includes MPO, CD68, CD43, and lysozyme; however, none of these markers are MS specific. To establish a definitive diagnosis, we recommend that the minimum immunohistochemical marker panel should include the previously mentioned markers, in addition to CD33, CD34, and CD117. The coexpression of some markers, as well as CD4, CD7 or CD56, causes difficulties in interpretation because they are positive in a minority of cases and may lead to erroneous diagnosis of T/NK cell lymphoma or blastic plasmacytoid dendritic cell neoplasm (BPDCN). A diagnosis of undifferentiated carcinoma or melanoma can be eliminated using the same approach. Extramedullary localizations of chronic myeloproliferative diseases without blastic crises occur mainly in the lymph nodes and are characterized by a lack of CD34-positive cells showing blast morphology. Pseudotumors created by erythromyeloid metaplasia of adipose tissue in the mediastinal and retroperitoneal areas can be easily recognized because of an absence of blasts, CD 34-positive cells, and maturation block [1,2,3,13,14]. Furthermore, because cytogenetic abnormalities are detected in approximately 55% of MS cases, the identification of an AML-associated abnormality (i.e., trisomy 8, t[8;21][q22;q22] or inv[16][p13.1q22]/t[16;16][p13.1;q22]) may be useful in obtaining an accurate diagnosis [3].
In approximately 90% of untreated MS patients who initially present without a hematological disorder, AML develops within 10.5 to 11 months. Currently, no data regarding to the optimal treatment are available from large-scale studies [7,9, 20–26]. Recently, Antic et al published the results of a meta-analysis that assessed the effects of systemic therapy, rather than local treatment (surgery or radiotherapy), in adults with isolated MS. The median overall survival (OS) for patients undergoing chemotherapy and hematopoietic stem cell transplantation (HSCT) was 28 months (95% CI, 7–48), whereas the median OS for patients receiving local treatment was 9 months (95% CI, 4–13). The difference in OS was not significant, but potentially represents an absolute benefit of 19 months (95% CI, 11–28). In patients aged <40 years, a difference in OS was observed between patients treated with HSCT (33 months) and those without HSCT (9 months; p = 0.031). There was no evidence of a difference in outcome related to age, sex, site, histology, extent of resection, tumor size, or exposure to radiotherapy [27].
Previous studies demonstrate the importance of employing a wide spectrum of antibodies including MPO, CD68, CD43, lysozyme, CD33, CD34, and CD117 during immunohistochemical work-up of tumor tissue in cases of suspected MS. Timely diagnosis has a major impact on treatment outcome (i.e., longer survival as a consequence of adequate therapy). Although additional data are required, chemotherapy with HSCT may be considered as an optimal therapy for patients with isolated MS. Surgery or radiotherapy could be considered for rapid symptomatic relief of lesion-associated pain as well as in older patients or patients with significant comorbidities.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Pileri SA, Orayi A, Falini B. Myeloid sarcoma. In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoetic and lymphoid tissues. Lyon: IARC; 2008. pp. 140–141. [Google Scholar]
- 2.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785–3793. doi: 10.1182/blood-2011-04-347229. [DOI] [PubMed] [Google Scholar]
- 3.Klco JM, Welch JS, Nguyen TT, Hurley MY, Kreisel FH, Hassan A, et al. State of the art in myeloid sarcoma. Int J Lab Hematol. 2011;33(6):555–565. doi: 10.1111/j.1751-553X.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 4.Burns A. Observation of surgical anatomy, head and neck. Edinburgh: Thomas Royce and co; 1811. pp. 364–366. [Google Scholar]
- 5.King A. A case of chloroma. Monthly J med. 1853;17:97. [Google Scholar]
- 6.Rappaport H. Tumors of the hematopoetic system. Armed Forces Inst Pathol. 1966:241–243. [Google Scholar]
- 7.Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, Perunicic M, et al. Is there a “gold” standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother. 2013;67(1):72–77. doi: 10.1016/j.biopha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson RE, Kurec AS, Davey FR. Granulocytic sarcoma. Clin Lab Med. 1990;10:889–901. [PubMed] [Google Scholar]
- 9.Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47(12):2527–2541. doi: 10.1080/10428190600967196. [DOI] [PubMed] [Google Scholar]
- 10.Antic D, Verstovsek S, Elezovic I, Grujicic D, Gotic M, Bila J, et al. Spinal epidural granulocytic sarcoma in non-leukemic patient. Int J Hematol. 2009;89(1):95–97. doi: 10.1007/s12185-008-0227-8. [DOI] [PubMed] [Google Scholar]
- 11.Antic D, Elezovic I, Bogdanovic A, Vukovic NS, Pavlovic A, Jovanovic MP, et al. Isolated myeloid sarcoma of the gastrointestinal tract. Intern Med. 2010;49(9):853–856. doi: 10.2169/internalmedicine.49.2874. [DOI] [PubMed] [Google Scholar]
- 12.Colović N, Jurišić V, Terzić T, Jevtovic D, Colović M. Alveolar granulocytic sarcoma of the mandible in a patient with HIV. Onkologie. 2011;34(1–2):55–8. doi: 10.1159/000317351. [DOI] [PubMed] [Google Scholar]
- 13.Audouin J, Comperat E, Le Tourneau A, Camilleri-Broët S, Adida C, Molina T, et al. Myeloid sarcoma: clinical and morphologic criteria useful for diagnosis. Int J Surg Pathol. 2003;11(4):271–282. doi: 10.1177/106689690301100404. [DOI] [PubMed] [Google Scholar]
- 14.Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH, et al. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;2:42. doi: 10.1186/1746-1596-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986;58(12):2697–2709. doi: 10.1002/1097-0142(19861215)58:12<2697::aid-cncr2820581225>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: A Clinical Review. J Clin Oncol. 1995;13(7):1800–1816. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 17.Majhi U, Murhekar K, Sundersingh S. Primary myeloid sarcoma with megakaryocytic differentiation in lymph nodes and skin. Indian J Pathol Microbiol. 2011;54(3):660–1. doi: 10.4103/0377-4929.85146. [DOI] [PubMed] [Google Scholar]
- 18.Elyamany G, Khan M, El Hag I, El-Zimaity M, Albalawi M, Al Abdulaaly A. Generalized Lymphadenopathy as the First Presentation of Granulocytic Sarcoma: A Diagnostic Challenge. Case Rep Med. 2013;2013:483291. doi: 10.1155/2013/483291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima M, Nakamura S, Shimizu K, Yamane Y, Itoh H, Masawa N. Granulocytic sarcoma presenting with lymph node infarction at disease onset. APMIS. 2003;111(12):1133–6. doi: 10.1111/j.1600-0463.2003.apm1111209.x. [DOI] [PubMed] [Google Scholar]
- 20.Breccia M, Mandelli F, Petti MC, D’Andrea M, Pescarmona E, Pileri SA, et al. Clinicopathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res. 2004;28(11):1165–1169. doi: 10.1016/j.leukres.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsed cases. Cancer. 1981;48:1426–1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94(6):1739–1746. doi: 10.1002/cncr.10399. [DOI] [PubMed] [Google Scholar]
- 23.Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O’Brien S, Brandt M, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113(6):1370–1378. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 25.Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122(4):238–246. doi: 10.1159/000253592. [DOI] [PubMed] [Google Scholar]
- 26.Chevallier P, Labopin M, Cornelissen J, Socié G, Rocha V, Mohty M ALWP of EBMT. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haemat ologica. 2011;96(9):1391–1394. doi: 10.3324/haematol.2011.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antic D, Milic N. systemic versus localized therapeutic approach for isolated myeloid sarcoma: meta-analysis of individual patient data. Haematologica. 2013;98(s1):281. abstract n. 0667. [Google Scholar]