Abstract
In the past decades, tumor necrosis factor alpha (TNF-a) antagonist has been a milestone in the treatment of many chronic inflammatory diseases. TNF antagonist can increase patients’ susceptibility to many different kinds of infections especially those requiring granuloma formations despite regular performance of Screening for latent tuberculosis infection (LTBI). We report 2 cases of patients who developed tuberculosis under treatment with adalimumab, which was discontinued after the diagnosis of tuberculosis. During the tuberculosis therapy they unexpectedly developed a prolonged paradoxical reaction. In both cases we were only able to manage the progress of the paradoxical reaction through high steroid doses. Patients undergoing therapy with TNF- alpha-blocker are prone to develop tuberculosis infection, which could in turn lead to severe prolonged paradoxical reaction during anti-tuberculous treatment. An increased steroid dose may be required and is sometimes necessary.
Keywords: Adalimumab, Paradoxical reaction, IRIS (immune reconstitution inflammatory syndrome), SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome, latent tuberculosis infection (LTBI), Tuberculosis (TB), IGRA (Interferon Gamma Release Assay), TNF (tumor necrosis factor)
1 Introduction
An immune reconstitution inflammatory syndrome (IRIS) has emerged as a major early complication of AIDS-associated Tuberculosis. Paradoxical reaction, a similar syndrome to IRIS, is described in Tuberculosis patients without HIV infection. IRIS and paradoxical reaction with clinical worsening of the general condition of the patients could both be seen after the initiation of tuberculosis therapy [1].
Paradoxical reaction is a clinical and radiological tuberculosis. Conditions worsen for at least four weeks after starting of tuberculosis therapy, in a patient whose clinical status initially improved. Especially the cerebral lesions of paradoxical reactions could be a life threatening situation for the patients. The conventional therapy of cerebral lesions include high dose of steroid and eventual neurosurgical intervention.
Despite appropriate LTBI (latent tuberculosis infection screening), patients can develop tuberculosis while treated with adalimumab. The standard treatment of paradoxical reaction is the initiation of systemic steroid with eventual elevation of the treatment dose.
2 Case report patient 1
A 17- year- old woman was regularly treated for years with TNF- a- blocker adalimumab based on her refractory SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome. TB- Screening with IGRA (Interferon Gamma Release Assay) before the therapy with TNF- a-blocker tested negative.
Due to increasing dyspnea, somnolence and septic shock, she was treated in the emergency room of a community hospital. Through sputum, blood culture and cerebrospinal puncture, the diagnosis of severe tuberculosis with meningitis and pulmonary infiltrates was rendered. After the diagnosis of tuberculosis, her regular therapy with adalimumab was discontinued. The standard treatment of four antibiotics (INH isonicotinic acid hydracide 300 mg daily, RIFA rifampicin 600 mg daily, PZA pyrazinamide 2000 mg daily and SM streptomycin 1000 mg daily) was initiated.
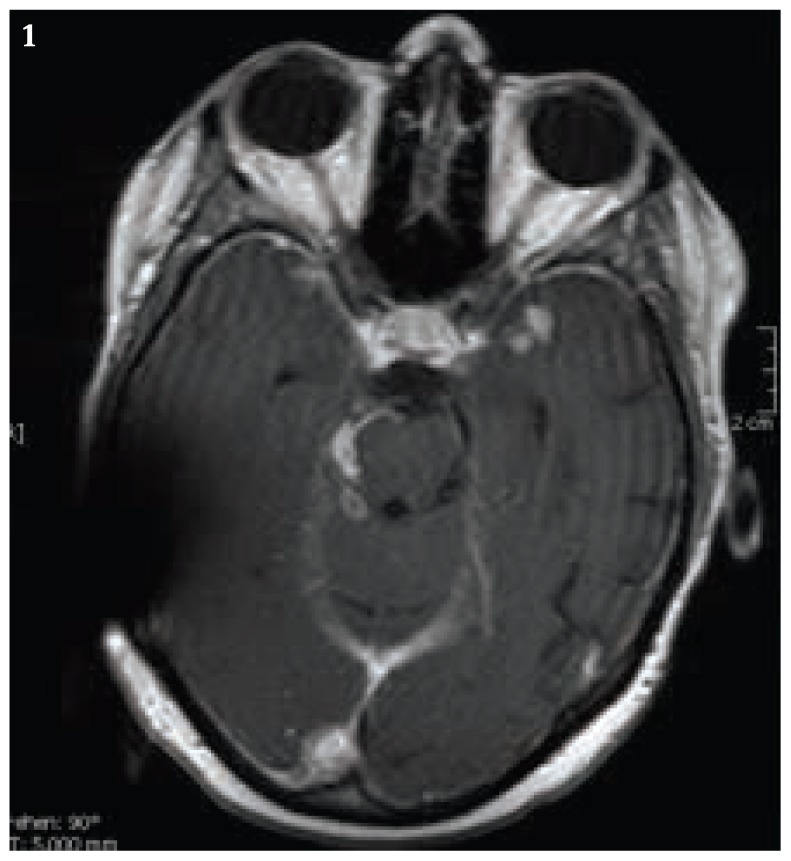
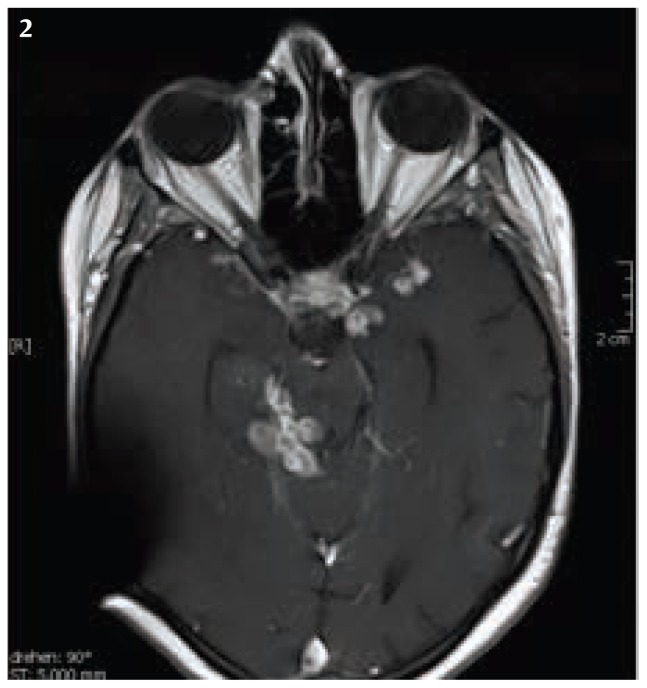
Five months after the discontinuation of the adalimumab therapy, we observed cerebral lesions in the basal meningeal region and in the follow- up cerebral MRI- Scan (Fig.1 and Fig 2). Due to cerebral progression and consequential tonic- clonic convulsive seizure, the initiation of antiepileptic drugs with levetiracetam has been initiated. Because of the raising intracranial pressure and ventricular distension, a ventriculo- peritoneal shunt was also installed after the first tonic-clonic convulsive seizure.
Figure 1.
Cerebral MRI Scan with the follow up survey, it showed the essential progression of intracranial lesions. Artifact of V-P-Shunt system.
Figure 2.
Cerebral MRI Scan with the follow up survey, it showed the essential progression of intracranial lesions. Artifact of V-P- Shunt system.
A neurosurgical intervention was not considered based on location of those cerebral lesions. The oral steroid prednisolone dose was elevated to 80 mg per day. Under the oral steroid therapy clinical conditions improved in patient. Steroid therapy was continued for overall 6 months with stepwise reduction of the dose until the maintenance dose of 7.5 mg per day. The patient still has a slightly vertigo distraction, but she stays employable at a limited level.
3 Case report patient 2
A 37- year- old Indian man with diagnosed Psoriasis arthritis was treated with adalimumab on a regular basis. TB-Screening with IGRA (Interferon Gamma Release Assay) before the therapy with TNF- a- blocker tested negative.
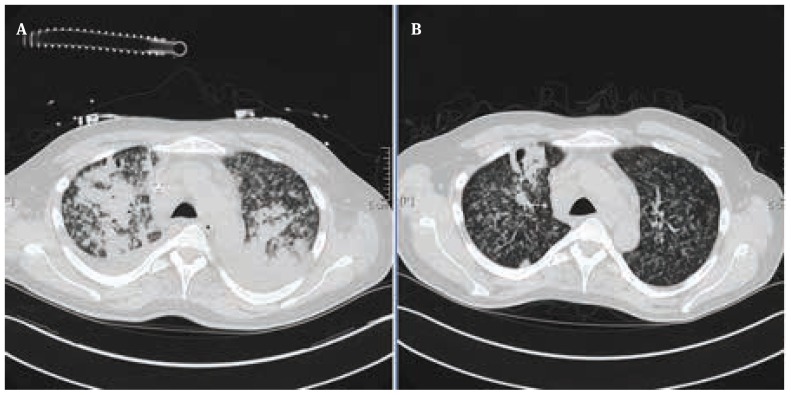
He was diagnosed with severe disseminated miliary tuberculosis with extensive acute respiratory distress syndrome under treatment with adalimumab. Laboratory examination revealed initial CRP 10.3 mg/dl, LDH 489 U/L and Leukocyte with 14.0 GIGA/L. The initial contrast enhanced CT- Scan revealed severe progressive bilateral pulmonary infiltrates (Fig.3a). During that period, intensive care was required. After the first treatment course of two weeks antituberculosis treatment with four antibiotics (INH isonicotinic acid hydracide 300 mg daily, RIFA rifampicin 600 mg daily, PZA pyrazinamide 2000 mg daily and SM streptomycin 1000 mg daily), the contrast enhanced CT scan showed regressive bilateral pulmonary infiltrates (Fig.3b). Under the tuberculosis therapy, the laboratory parameters were also improved after 2 weeks of treatment; CRP 2.8 mg/dl, LDH 203 U/L and Leukocyte was 12.1 GIGA/L.
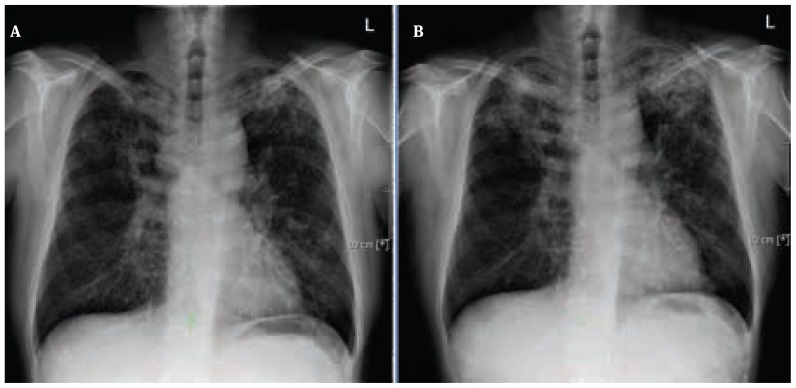
Figure 3.
A Contrast enhanced scan showed severe bilateral pulmonary infiltrates. B: Contrast enhanced scan revealed regressive pulmonary infiltrates on both sides after the first course of 2 weeks tuberculosis treatment.
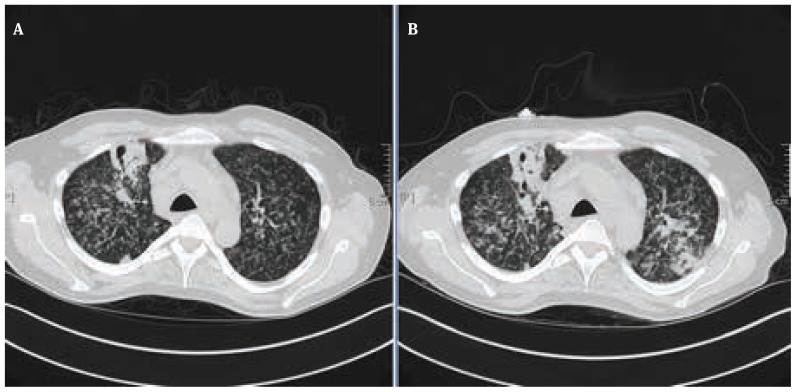
During the treatment of tuberculosis therapy, therapy with TNF- alpha- blocker adalimumab was discontinued. Six weeks after the discontinuation of the adalimumab therapy, he developed a prolonged paradoxical reaction with reverse episodes of fever, elevation of CRP 7.1 mg/dl; Leukocyte of 16.0 GIGA/L and LDH of 276 U/L. and progressive bilateral pulmonary infiltration which were revealed in both contrast enhanced CT-scan and chest x- ray (Fig. 4, 5).
Figure 4.
A. Contrast enhanced CT- Scan of follow- up control 2 weeks after the first course of anti- tuberculous therapy. B: Contrast CT- Scan of follow- up control 6 weeks after the discontinuation of adalimumab therapy showed progressive pulmonary infiltrates on both sides in term of paradoxical reaction.
Figure 5.
A: Chest X- ray 2 weeks after the initiation of the tuberculosis therapy. B: Chest X- ray 6 weeks after the discontinuation of adalimumab therapy, it revealed progressive pulmonary infiltrates in term of paradoxical reaction.
Multiple blood cultures rendered no clear evidence of pathogen agents. HIV- test was again tested negative. Not until the initiation of steroid prednisolone therapy with a daily dose of 1.5 mg/kg could we improve his general condition. After two weeks of prednisolone therapy, his laboratory findings were also improved; CRP 4.2 mg/dl, Leukocyte of 10.0 GIGA/L and LDH of 252 U/L. We continued the steroid therapy for overall 6 months with stepwise reduction of the dose until the maintenance dose of 7.5 mg per day.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent
Informed consent has been obtained from all individuals included in this study.
4 Discussion
Immune-mediated Tuberculosis exacerbation can occur under several conditions. Immune reconstitution inflammatory syndrome has emerged as a major early complication of AIDS-associated Tuberculosis. Paradoxical reaction, a similar syndrome, is described in Tuberculosis patients without HIV infection. In paradoxical reaction, worsening of the patient’s condition is attributed to the reversal of disease-associated immunosuppressive mechanisms. Manifestations of both syndromes include worsened fever, infiltrates, hypoxia, and lymphadenopathy and the appearance of new lesions in previously uninvolved organs, even cerebral lesions. Neither biomarkers nor a consensus definition exist to facilitate the diagnosis of paradoxical reaction, hindering research [1,2].
Despite appropriate LTBI (latent tuberculosis infection) screening, patients can develop tuberculosis while treated with adalimumab. HIV- negative patients could develop a severe prolonged paradoxical reaction under the ongoing tuberculosis therapy. Based on the review of the literature, patients with discontinuation of the regular treatment with TNF- alpha–blockers do have a higher risk to develop an unexpected prolonged paradoxical reaction with a severe and also fatal outcome during tuberculosis therapy [3–5].
HIV- positive patients are prone to immune reconstitution inflammatory syndrome IRIS with a severe and also fatal outcome during tuberculosis therapy [3–5]. In literature, cases in which patients develop prolonged paradoxical reaction under tuberculosis therapy remain extremely rare [3–5].
Due to the central role of TNF- alpha in host defense mechanisms, TNF antagonist can increase patients’ susceptibility to various kinds of infections, especially those requiring granuloma formations [6,7]. Several studies have shown that all currently available TNF blockers carry an increased risk for the development of active tuberculosis [8–11].
Paradoxical reaction and IRIS are due to awakening of cellular immune response toward somatic antigens released from dying or dead mycobacteria. Steroids are often the first choice treatment and they have been evaluated in a randomized controlled trial [12].
Withdrawal of anti-TNF therapy is a recognized precipitating cause of TB paradoxical reaction [13]. There are also reports in which TNF antagonists were actually used to treat severe paradoxical reactions in adults, both with positive results [13,14].
Tumor necrosis factor could enhance immune reactions as well as autoimmune reactions. TNF- alpha- blocker adalimumab was often used for autoimmune disease like SAPHO- Syndrome in order to suppress the immune reactions, but a suppressed immune system makes patients vulnerable to tuberculosis infection. After the discontinuation of TNF- alpha- blocker adalimumab, the paradoxical reaction of the immune system occurred in both patients.
The activity of the NK cells is determined by the balance between activation and inhibitory receptor molecules expressed on the surface of NK cells. However, several cytokines and chemokines can significantly modulate their activity, inducing increase of NK cell activity. Immunomodulation mediated by NK cells is very important mechanism in tumor immunity, as well as in other immunodepressions of the immune system. TNF- alpha induces cell apoptosis and immune reactions [15].
We report two rare cases of patients with severe paradoxical reaction of different manifestations during ongoing tuberculosis therapy after the discontinuation of TNF- alpha blocker adalimumab, which could be handled only with a high dose of steroids over months.
In conclusion, HIV- negative patients under therapy with TNF- alpha- blocker are prone to develop tuberculosis infection, which could in turn lead to severe prolonged paradoxical reaction during tuberculosis therapy. The elevation of the steroid dose may be required and is on occasions essential.
Footnotes
Authors’ disclosures of potential conflicts of interest: The author(s) indicated no potential conflicts of interest. We do not have any financial supports of any firms of pharmacies. This case report has only involved two hospitalized patients with severe diseases. There was not any form of studies or experiments included. We did not plan any studies for our treatment.
Contributor Information
Roger Fei Falkenstern-Ge, Email: rogerfalkenstern@yahoo.de.
Kim Husemann, Email: kim.husemann@klinik-schillerhoehe.de.
Martin Kohlhäufl, Email: martin.kohlhaeufl@klinik-schillerhoehe.de.
References
- 1.Lawn SD, Wilkinson RJ, Lipman MCI, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008 Apr;Jan;177(7):680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng VCC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PCY, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002 Nov;21(11):803–809. doi: 10.1007/s10096-002-0821-2. [DOI] [PubMed] [Google Scholar]
- 3.Melboucy-Belkhir S, Flexor G, Stirnemann J, Morin A-S, Boukari L, Polliand C, et al. Prolonged paradoxical response to anti-tuberculous treatment after infliximab. Int J Infect Dis. 2010 Sep;14(Suppl 3):e333–334. doi: 10.1016/j.ijid.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Yoon YK, Kim JY, Sohn JW, Kim MJ, Koo JS, Choi JH, et al. Paradoxical response during antituberculous therapy in a patient discontinuing infliximab: a case report. J Med Case Reports. 2009;3:6673. doi: 10.1186/1752-1947-3-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia Vidal C, Rodríguez Fernández S, Martínez Lacasa J, Salavert M, Vidal R, Rodríguez Carballeira M, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis. 2005 Mar;Jan;40(5):756–759. doi: 10.1086/427941. [DOI] [PubMed] [Google Scholar]
- 6.Hess S, Hospach T, Nossal R, et al. Life- threatening disseminated tuberculosis as a complication of TNF- a blockade in an adolescent. Eur J Pediatr. 170(10):1337–1342. doi: 10.1007/s00431-011-1501-y. [DOI] [PubMed] [Google Scholar]
- 7.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 8.Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43:717–722. doi: 10.1086/506935. [DOI] [PubMed] [Google Scholar]
- 9.Wallis RS. Infectious complications of tumor necrosis factor blockade. Curr Opin Infect Dis. 2009;22:403–409. doi: 10.1097/QCO.0b013e32832dda55. [DOI] [PubMed] [Google Scholar]
- 10.Wallis RS, Broder MS, Wong JY, et al. Granulomatous infectious diseases associated with TNF antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 11.Wallis RS, Broder MS, Wong JY, et al. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–1256. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 12.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo- controlled trial of prednisone for paradoxical tuberculosis- associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis RS, Van Vuuren C, Potgieter S. Adalimumab treatment of life- threatening tuberculosis. Clin Infect Dis. 2009 May 15;48(10):1429–1432. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]
- 14.Blackmore TK, Manning L, Taylor WJ, et al. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008 Nov 15;47(10):e 83–85. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 15.Jurisic V, Stojacic-Djenic S, Colovic N, Konjevic G. The role of cytokine in regulation of the natural killer cell activity. Srp Arh Celok Lek. 2008 Jul-Aug;136(7–8):423–429. doi: 10.2298/sarh0808423j. [DOI] [PubMed] [Google Scholar]