Abstract
Objective
Gastrointestinal dysfunction or gut failure frequently occurs in seriously ill patients and can be responsible for multi-organ failure. Trefoil factor 3 (TFF3) was characterized for its role in reconstitution of an epithelial barrier after mucosal injury in the jejunum. The aims of our study was an analysis of TFF3 levels dynamics in patients with sepsis and the correlation of TFF3 with severity of sepsis and mortality.
Methods
Prospective observational study, a ten days evaluation period in children aged 0–19 years with systemic inflammatory response syndrome or septic state. Blood tests to determine levels of TFF3 were obtained as long as the patient met the criteria for systemic inflammatory response syndrome or sepsis.
Results
Analysis of dynamics revealed steady levels of TFF3 during the 10 day period evaluated. TFF3 levels could not differentiate between various septic conditions in patients until a marked organ dysfunction developed. Higher Area Under Curve was noticed between control group and patients with sepsis. We could not make any strong conclusions based on mortality model.
Conclusions
Levels of TFF3 are elevated in paediatric patients with sepsis through organ dysfunction.
Keywords: Trefoil factor, gut injury, sepsis, children, mortality
1 Introduction
Patients with systemic inflammatory response syndrome (SIRS) and sepsis have an increased risk of developing multiple organ failure (MOF) [1].
The gastrointestinal tract has various functions including digestion, the production of hormones with local and systemic effects, a major role in immunological function, and acting as a barrier against antigens within its lumen [2]. Gastrointestinal dysfunction or gut failure frequently occurs in seriously ill patients and is responsible for bacterial translocation. This may in turn cause sepsis, with the initiation of a systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), and / or death [3].
Numerous growth factors regulate the process of epithelial repair. Growth factors control a wide variety of activities, including stimulation of proliferation and migration, cell differentiation, acceleration of angiogenesis and extracellular matrix remodeling, as well as promotion of epithelial mucosal repair. These factors can ether be derived from the luminal environment as the result of intrinsic secretions from epithelial cells, or they can be produced by a wide variety of mucosal and submucosal cells [4].
1.1 Trefoil Factor Family
The trefoil factors have been shown to play an important role in the protection and repair of the gastrointestinal mucosa. These proteins are small, compact peptides containing one or two trefoil domains. A trefoil domain consists of 42–43 amino acid residues with six cysteine residues forming three disulfide bonds, thereby creating a characteristic three-leafed structure [5]. Trefoil factors are expressed in several tissues of the body but most pronouncedly in the gastrointestinal tract, where the individual trefoil factors are expressed in a tissue-specific manner.
Trefoil factor 3 (TFF3) has a quite different localization pattern when compared with other TFF because expression is very scarce in the human stomach. A major source of TFF3 are human intestinal goblet cells [6]. TFF3 was characterized for its role in reconstitution of an epithelial barrier after mucosal injury in the jejunum [7]. During the mucosal restitution process, TFF3 is required to maintain the integrity of the mucosal barrier to protect the epithelial layer against environmental insult, as well as, for promotion of wound repair by enhancing epithelial dispersion at sites of injury [8]. In addition, TFF3 has also been shown to function as an epithelial anti-apoptotic factor, and neural signaling peptide [9].
Should a new potential diagnostic marker be used in a clinical setting it is necessary to describe its behavior under given conditions. This means, in our study, describe blood levels changes over time and diagnostic characteristics. Thus, the first objective of our study was an analysis of TFF3 levels dynamics in patients with SIRS or septic condition during a 10-day period after admission. The second objective was to determine optimal cut-off value and quantify diagnostic characteristics of TFF3 protein between controls and patients with various septic conditions.
2 Materials and Methods
Prospective observational study occurred during the period from June 2009 to March 2011. The study protocol and informed consent approach were approved by the Ethics committee of the University Hospital, Brno. Parents provided informed written consent for their children to participate in this trial. Data were collected and analyzed from sixty-three consecutive patients with SIRS or septic state who was admitted to the Department of Anesthesia and Intensive Care of the University Children’s Hospital Brno, Czech Republic. The most common sources of infection that led to sepsis were the lungs – bacterial and viral infections, and central nervous system – bacterial infections of the brain. Infections, sepsis, severe sepsis, septic shock and multiple organ dysfunction syndromes (MODS) were defined according to commonly used criteria– by International pediatric sepsis consensus conference [10]. The criteria for adult SIRS were modified for pediatric use. Age-specific norms of vital signs and laboratory data were incorporated into the definitions of SIRS. Patients were categorized into five groups according to their clinical data and to the described definitions: a) SIRS, b) sepsis, c) severe sepsis, d) septic shock, e) MODS. In these groups, we compared the difference in the levels of TFF3. The samples from 70 children undergoing elective surgery were used as controls (strabismus surgery, umbilical and inguinal hernia repair), i.e. samples from patients without signs of infection. Patient data were recorded at the time of diagnosis of SIRS or septic state and consisted of age, sex, Paediatric logistic organ dysfunction score (PELOD score), length of hospitalization. PELOD score is a tool which is used to characterize severity of organ dysfunction in critically ill child. Score which is given to each organ will increase according the severity of organ dysfunction so PELOD score can be used to predict severity of organ dysfunction. The PELOD scoring system consists of physical and laboratory variables representing 6 organs, namely nervous, cardiovascular, renal, respiratory, hematologic, and hepatic system [11]. Specimens for the diagnosis of infection were obtained as early as possible. Complete medical history and clinical examination, laboratory parameters, and disease-specific examinations were evaluated.
Blood samples were obtained from a central venous catheter during the first 12 h after the diagnosis of SIRS or septic state, or at the beginning of surgery in the control group. Samples were allowed to clot at room temperature and were centrifuged at 3000rpm for 10 min. Separated serum was stored at −80 °C until further analysis.
Samples were measured by quantitative enzyme immunoassay (BioVendor, Laboratorní medicína a.s., Czech Republic). Samples were incubated in microtitration plate wells pre-coated with polyclonal anti-human TFF3 antibody. After 60 minutes of incubation and washing, polyclonal anti-human TFF3 antibody, conjugated with horseradish peroxidase (HRP) was added to the wells and incubated for 60 minutes with captured TFF3. After another washing step, the remaining HRP conjugate was allowed to react with the substrate solution (TMB). The reaction was stopped by addition of acidic solution and absorbance of the resulting yellow product was measured. The laboratory technicians performing the assays were completely blinded to the clinical information.
2.1 Statistical Analysis
Sample descriptives were given for baseline data. TFF3 data were logarithmically transformed in order to achieve aproximately normal distribution. Histograms of the data were inspected for normality evaluation. Parametric analyses were done on transformed data.
First, TFF3 dynamics in various groups of patients during a 10 day period of time after admission was evaluated using repeated mesure ANOVA models. Significance of main and interaction effects of the model was assessed and group model means were graphed to ease the results interpretation. Sensitivity analyses on restricted sample of data were done where apropriate in ordred to validate conclusions.
Second, ROC analysis was performed on selected time point based on the previous analysis of dynamics. Comparison was performed between analyzed groups of patients where significant difference was found in the former analysis. Moreover, all patients were compared with control group of surgical patients. Cut-off values for optimal, highly sensitive, and highly specific test characteristics were given for above comparisons, all of which might be of clinical importance depending of the diagnostic purpose.
Analysis of surviving versus non-surviving patients was performed despite highly unbalanced data for that was of most clinical importance. Analyses were done using R version 2.10.1 and level of significance was considered alpha=0.05.
3 Results
Total of 63 patients were enrolled into the study and analyzed. Sample baseline characteristics are summarized in Table 1 and summary of TFF3 levels during the 10 day evaluated period are given in Table 2.
Table 1.
Sample characteristics
| Parametr | Non-survivals | Survivals | Total |
|---|---|---|---|
| N | 6 | 57 | 63 |
| Age m±SD | 96.8±76.1 | 66.0±72.6 | 68.9±72.8 |
| Weight m±SD | 22.2±21.8 | 21.3±27.0 | 21.4±26.4 |
| Gender n (%) | |||
| F | 4 (66.7%) | 22 (38.6%) | 26 (41.3%) |
| M | 2 (33.3%) | 35 (61.4%) | 37 (58.7%) |
| Sepsis condition (%) | |||
| 1) SIRS | 1 (16.7%) | 13 (22.8%) | 14 (22.2%) |
| 2) Sepsis | 1 (16.7%) | 6 (10.6%) | 7 (11.1%) |
| 3) Severe sepsis | 0 (0%) | 19 (33.3%) | 19 (30.2%) |
| 4) Septic shock | 4 (66.7%) | 19 (33.3%) | 23 (36.5%) |
| MODS n (%) | |||
| yes | 5 (83.3%) | 25 (43.9%) | 30 (47.6%) |
| no | 1 (16.7%) | 32 (56.1%) | 33 (52.4%) |
| PELOD median (min–max) | 26.0 (11.0–41.0) | 11.0 (1.0–61.0) | 11.0 (1.0–61.0) |
| Hospital stay median (min–max) | 7.5 (1.0–19.0) | 10.0 (1.0–53.0) | 10.0 (1.0–53.0) |
Abbreviations: SIRS – systemic inflammatory response syndrome; MODS – multiple organ dysfunction syndrom; PELOD – pediatric logistic organ dysfunction score
Table 2.
Summary of TFF3 levels during a 10 day period
| Day | N | G. mean | Mean±SD | Median (min–max) |
|---|---|---|---|---|
| 0 | 32 | 2.06 | 4.35±11.830 | 1.60 (0.55 – 68.64) |
| 1 | 50 | 2.15 | 3.98±7.236 | 1.86 (0.40 – 47.52) |
| 2 | 55 | 2.10 | 3.78±6.600 | 2.03 (0.46 – 35.85) |
| 3 | 48 | 2.01 | 3.58±6.835 | 1.93 (0.46 – 36.06) |
| 4 | 43 | 2.03 | 3.93±8.193 | 1.82 (0.52 – 40.25) |
| 5 | 39 | 2.15 | 4.33±9.028 | 1.94 (0.52 – 43.68) |
| 6 | 34 | 2.37 | 4.60±8.766 | 1.91 (0.52 – 39.10) |
| 7 | 27 | 2.63 | 5.23±10.018 | 2.06 (0.99 – 41.15) |
| 8 | 26 | 2.55 | 4.91±8.445 | 2.03 (0.87 – 35.40) |
| 9 | 24 | 2.79 | 5.87±10.611 | 1.89 (0.82 – 40.95) |
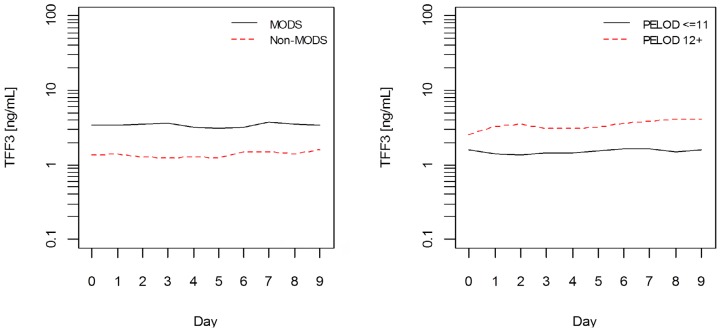
Analysis of dynamics revealed steady levels of TFF3 during the 10 day period evaluated, which was pointed out by time effect non-significance. Although significant effect of time was identified in one of the models (with MODS effect), variations over the period within analyzed groups were small. Its significance disapeared when sample was restricted by day 7 behind which bias could be introduced due to unbalance. No difference in TFF3 level dynamics could be identified in any of the analyzed groups (non-significant interaction effects). However, patients with MODS, higher PELOD score, or those longer hospitalized had significantly higher TFF3 levels (significant main effects) (Figure 1). Sensitivity analysis on restricted sample resulted in loss of significance in PELOD and hospital stay models. TFF3 levels varied in non-survivals. We could not make any strong conclusions based on mortality model due to highly unbalanced data. P-values of all ANOVA models analyzed are summarized in Table 3.
Figure 1.
Mean models of MODS (left panel) and PELOD (right panel) groups of patinets
Table 3.
Summary of p-values of ANOVA model effects
| Effect | Main | Time | Interaction |
|---|---|---|---|
| Sepsis category | 0.285 | 0.965 | 0.802 |
| Septic shock | 0.118 | 0.064 | 0.635 |
| MODS | 0.001 | 0.014 | 0.501 |
| PELOD | 0.005 | 0.814 | 0.618 |
| Hosp. stay | 0.011 | 0.669 | 0.954 |
| Mortality | 0.088 | 0.089 | 0.322 |
Abbreviations: MODS – multiple organ dysfunction syndrom; PELOD – pediatric logistic organ dysfunction score
Based on above results, ROC analysis was performed on data from day 1 when sufficient data were available and was of most clinical importance. TFF3 could significantly discriminate all patients from controls, patients with MODS, or patients with higher PELOD score. Optimal cut-off values with best discriminative performance ranged from 1.2 to 2.5 nmol/mL. Results from ROC analysis is summarized in Table 4.
Table 4.
Summary of ROC analysis and diagnostic characteristics of TFF3 on D1
| Parameter | Control/patients | MODS/non-MODS | PELOD 11/12+ | Hosp. stay 10/11+ days | Survivals/non-survivals |
|---|---|---|---|---|---|
| Cut-off (SE>95%) | 0.57 | 1.08 | 0.81 | 0.8 | 1.08 |
| Cut-off (ROC optimum) | 1.24 | 1.25 | 2.45 | 1.24 | 4.88 |
| Cut-off (SP>95%) | 3.335 | 6.66 | 5.28 | 10.45 | 10.45 |
| AUC | 0.768 | 0.774 | 0.746 | 0.667 | 0.652 |
| p (logistic regression) | <0.001 | 0.004 | 0.004 | 0.073 | 0.211 |
Abbreviations: AUC – area under curve; MODS – multiple organ dysfunction syndrom; PELOD – pediatric logistic organ dysfunction score
4 Discussion
The development of sepsis and multiple organ failure (MOF) are important determinants of the outcome in critically ill patients. Hepatosplanchnic hypoperfusion and resulting intestinal and hepatic cell damage have been implicated as central events in the development of sepsis and MOF [1].
Our aim was to study the relation between intestinal cell damage in an early phase of sepsis using serum TFF3 levels and the correlation of these levels with severity of sepsis. Our results imply that TFF3 levels could not differentiate between various septic conditions in patients until a marked organ dysfunction developed. This conclusion could be made considering significantly higher and discriminating TFF3 levels in patients with MODS on one hand and high PELOD score on the other. It is obvious that gut damage is to some extent present during sepsis with multiple organ dysfunctions. These conclusions are supported by the finding that higher AUC (better discriminant value) was noticed in comparison of controls with patients on Day 1. Organ dysfunction comprising gut damage can develop or worsen during further course of sepsis.
Difference between conclusions made with respect to ANOVA results and those from ROC analysis could be noticed. Significant difference of TFF3 between MODS and non-MODS patients was similar. A noticed difference in TFF3 levels dynamics in survivors and non-survivors was consistent with other findings. This finding could be biased due to early drop-out of non-survivors. These data indicate a strong relationship between gut mucosal damage, measured by TFF3, and severity of disease (represented by the PELOD). Further conformation is however needed to proof the hypothesis.
The role of intestinal compromise in sepsis has been studied using markers for plasma levels of endotoxin and gut wall integrity. TFF3 has been previously studied in preterm infants as biomarkers for differentiating necrotizing enterokolitis (NEC) from septicemic/control infants. Plasma concentrations of gut barrier biomarkers TFF3 were significantly higher in the NEC than in the septicemia or control group [12]. It has been shown that plasma levels of TFF3 are elevated in adult patients with ulceration or inflammation of the GI tract [13]. The TFF3 peptide may potentially serve a protective role against complement activation through the induction of Decay-Accelerating Factor (DAF) in intestinal epithelial cells. DAF prevents the assembly, and accelerates the dissociation, of autologous C3/C5 convertases, and thereby prevents complement activation on the cell surface protecting host tissues from autologous complement injury [14]. DAF is a negative modulator of T cell (adaptive) immunity and suppresses T cells through mechanisms involving complement regulation [15].
One of the other biomarkers may be intestinal-type fatty acid-binding protein (I-FABP). Derikx et al. investigated the association of hepatosplanchnic hypoperfusion, objectivated by gastric mucosal PiCO2, with intestinal and hepatic cell damage by measurement of plasma I-FABP and L-FABP in critically ill patients. They reported that splanchnic hypoperfusion (gastric mucosal PiCO2) in the early phase of abdominal sepsis correlates strongly with intestinal mucosal damage (plasma I-FABP) [1].
Primarily animal studies have provided evidence that the gut plays an important role in the transition of systemic inflammatory response (SIRS) into sepsis and multiple organ failure [16]. SIRS potentially leads to splanchnic hypoperfusion, because blood is shifted away from the splanchnic organs towards the more vital organs (heart, brain). Especially the mature enterocytes are susceptible to hypoperfusion and will be damaged, which leads to loss of the intestinal barrier and translocation of microbiota and microbial products [17]. These danger signals possibly amplify the systemic inflammation by further release of cytokines, eventually resulting in a deregulated inflammatory response and MOF [16]. This is indeed confirmed by studies in adults and children with non-intestinal origin of sepsis, showing that increased intestinal permeability and low values of antibodies directed against the core of endotoxin (EndoCAb) are associated with the development of sepsis, multiple organ dysfunction syndrome and poor outcome [18]. Furthermore, primarily experimental animal studies have shown that gut wall integrity loss is involved in the development of various inflammatory syndromes, including post-operative or post-traumatic systemic inflammatory response syndrome, sepsis and multiple organ failure.
Footnotes
Conflict of interest: The authors report no conflict of interest.
References
- 1.Derikx JP, Poeze M, van Bijnen AA, Buurman WA, Heineman E. Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock. 2007;28:544–548. doi: 10.1097/shk.0b013e3180644e32. [DOI] [PubMed] [Google Scholar]
- 2.Azzopardi N, Fenech M, Piscopo T. Sepsis–An Ongoing and Significant Challenge. InTech; 2012. Sepsis, the Liver and the Gut. [Google Scholar]
- 3.Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Semin Respir Crit Care Med. 2011;32:626–638. doi: 10.1055/s-0031-1287871. [DOI] [PubMed] [Google Scholar]
- 4.Mammen JMVM, Matthews JBM. Mucosal repair in the gastrointestinal tract. Critical Care Medicine Healing Responses in Critical Illness. 2003;31:S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 5.Thim L. A new family of growth factor-like peptides: ‘trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP) and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 6.Hauser F, Poulsom R, Chinery R, Rogers LA, Hanby AM, Wright NA, et al. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci USA. 1993;90:6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xian CJ, Howarth GS, Mardell CE, Cool JC, Familari M, Read LC, et al. Temporal changes in TFF3 expression and jejunal morphology during methotrexate-induced damage and repair. Am J Physiol. 1999;277:G785–G795. doi: 10.1152/ajpgi.1999.277.4.G785. [DOI] [PubMed] [Google Scholar]
- 8.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzberg H, Kalbacher H, Hoffmann W. Differential behavioral effects of TFF peptides: Injections of synthetic TFF3 into the ratamygdala. Pharmacol BiochemBehav. 1999;62:173–178. doi: 10.1016/s0091-3057(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 11.Leteurtre S, Martinot A, Duhamel A, Gauvin F, Grandbastien B, Nam TV, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19:399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 12.Ng EW, Poon TC, Lam HS, Cheung HM, Ma TP, Chan KY, et al. Gut-Associated Biomarkers L-FABP, I-FABP, and TFF3 and LIT Score for Diagnosis of Surgical Necrotizing Enterocolitis in Preterm Infants. Ann Surg. 2013;258:1111–1118. doi: 10.1097/SLA.0b013e318288ea96. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard EM, Poulsen SS, Grønbaek H, Larsen R, Nielsen AM, Ejskjaer K, et al. Development and evaluation of an ELISA for human trefoil factor 3. Clin Chem. 2002;48:1689–1695. [PubMed] [Google Scholar]
- 14.Moran P, Beasley H, Gorrell A, Martin E, Gribling P, Fuchs H, et al. Human recombinant soluble decay accelerating factor inhibits complement activation in vitro and in vivo. J Immunol. 1992;149:1736–1743. [PubMed] [Google Scholar]
- 15.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;2:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill–evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741–757. doi: 10.1111/j.1365-2036.2006.03174.x. [DOI] [PubMed] [Google Scholar]
- 18.Stephens RC, Fidler K, Wilson P, Barclay GR, Mythen MG, Dixon GL, et al. Endotoxin immunity and the development of the systemic inflammatory response syndrome in critically ill children. Intensive Care Med. 2006;32:286–294. doi: 10.1007/s00134-005-0019-z. [DOI] [PubMed] [Google Scholar]