Abstract
Objectives
It has been well documented that the platelet to lymphocyte ratio (PLR) and the neutrophil to lymphocyte ratio (NLR) are associated with outcomes for patients with gastric cancer, non-small cell lung cancer and acute heart failure. Inflammation may be the hidden factor that explains the correlation between NLP, PLR, and these diseases. However, to date, the data concerning NLR, PLR, and its association with inflammation are lacking in patients with rheumatoid arthritis (RA), thus, our aim to discuss whether NLR and PLR are associated with RA.
Methods
Patients with RA and healthy individuals were included according to the determined criteria, and laboratory indicators were measured.
Results
PLR and NLR were significantly higher in RA patients compared with healthy controls (3.20±2.06 vs. 1.56±0.47, P<0.01; 192.85±101.78 vs. 103.49±28.68, P<0.01). When leukocytes, neutrophil percentage, neutrophil, lymphocyte, platelet, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and rheumatoid factor (RF) were considered as confounders (crude model), our results indicated that ESR and RF were correlated to RA. Of note, ESR, RF, and PLR were associated with RA after further adjustment based on crude model for PLR and NLR. Receiver operating characteristic (ROC) curves analysis showed that PLR values higher than >115.7 evaluated RA with a sensitivity of 82.5%, a specificity of 74.8% and area under the curve ( AUC ) of 0.847.
Conclusions
Our results suggest that PLR is associated with RA, and PLR may be an underlying indicator indicating the chronic subclinical inflammation in patients with RA.
Keywords: Rheumatoid arthritis, platelet to lymphocyte ratio, neutrophil to lymphocyte ratio, subclinical inflammation
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune diseases with inflammation of synovial joints; it results in the progressive damage of joint, especially when inflammation is persistent [1,2]. Clinically, presence of C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), and the rheumatoid factor (RF) have been considered to be diagnosic indicators and markers that estimate disease activity in patients with RA. The level of CRP is frequently increased and is associated with the disease activity of RA; ESR is a component of the American College of Rheumatology core set for measuring clinical response in RA trials [3]. Very recently, accumulating data have indicated that the neutrophil to lymphocyte ratio (NLR) is an independent predictor of mortality in patients with acute heart failure [4,5], and the NLR has been introduced as a role to determine inflammation in cardiac and non-cardiac disorders [6–8].
In fact, previous studies showed that platelet levels, as a routine test, was involved in the atherogenesis through secretion of proinflammatory cytokines [9]; it was found that activated platelets play a critical role in the development of atherosclerosis [10]. Several lines of evidence now attest that the platelet to lymphocytes ratio (PLR) is associated with outcomes of patients with ankylosing spondylitis, non-small cell lung cancer, and acute coronary syndrome [11–13]. Inflammation may be a hidden factor that explains the correlation between NLP, PLR, and those diseases. However, to date, data about NLR, PLR, and their association with inflammation are lacking for patients with RA. Therefore, our study focus on the association between NLR, PLR, and RA.
1 Patients and methods
This cross-sectional study involved 104 newly diagnosed RA patients who were admitted to First Affiliated Hospital of Xinjiang Medical University from 2013 to 2014. The diagnosis for RA was based on the 1987 diagnostic criteria of American Rheumatism Association in [14]. The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee. Informed consent has been obtained from all individuals included in this study. The patients with following diseases and/or situations were excluded from the study: cardia-cerebrovascular disease, hematologic disease (such as chronic myelogenous leukemia, thrombocytosis), known chronic liver and kidney diseases, infectious diseases, malignant tumor diseases, hypertension, diabetes, metabolic syndrome, and other autoimmune diseases (such as ankylosing spondylitis and systemic lupus erythematosus). The hematological and immunological determinations were performed in our clinical laboratory, and blood routine tests were evaluated using the Sysmex XN-1000 automatic blood instrument (Sysmex Inc., Japan); the reference range of platelets and neutrophils in our laboratory was 100–300 (109/L), and1.8–6.3 (109/L), respectively. 115 healthy individuals who were undergoing routine physical examination in our hospital were selected as the healthy controls.
2 Statistical Analysis
The data used SPSS16.0 (SPSS Inc, Chicago, IL, USA) statistical software for statistical analysis. P<0.05 was considered statistically significant. Continuous variables are given as means±standard deviation. Normal distribution was determined using the Kolmogorov-Smirnov test. The student’s t-test, Mann-Whitney U test, and chisquare test were used for the comparison between the groups. Multivariate logistic regression analysis was also used to identify factors associated with AS. Finally, the receiver operating characteristic (ROC) curves were evaluated to measure the performance of the variables.
3 Results
According to the criteria, a total of 104 eligible RA patients and 115 healthy individuals were included in this study, the baseline characteristics of patients with RA and healthy individuals was showed in Table 1. At baseline, NLR and PLR were significantly higher in RA patients compared with healthy controls (3.20±2.06 vs.1.56±0.47, P<0.01; 192.85±101.78 vs. 103.49±28.68, P<0.01). There were differences with respect to the following variables between RA patients and healthy controls: leukocytes; neutrophil percentage; neutrophils; lymphocytes; platelets; CRP; ESR; RF.
Table 1.
Participant characteristics
| RA patients N=104 |
Healthy Controls N=115 |
p-value | |
|---|---|---|---|
| Gender (male/female) | 36/69 | 36/79 | 0.638 |
| Age(y) | 54.01±15.84 | 53.77± 11.82 | 0.898 |
| Leukocyte (×109/L) | 7.45±2.44 | 6.04±1.17 | <0.01 |
| Neutrophil percent (%) | 64.70±10.92 | 55.05±6.63 | <0.01 |
| Absolute neutrophil count (×109/L) | 4.88±1.96 | 3.34±0.82 | <0.01 |
| Absolute lymphocyte count (×109/L) | 1.78±0.70 | 2.22±0.48 | <0.01 |
| Absolute platelet count (×109/L) | 300.63±118.60 | 222.15±46.76 | <0.01 |
| C-reactive protein (mg/L) | 32.01±41.80 | 2.24±1.48 | <0.01 |
| Erythrocyte sedimentation rate (mm/h) | 43.47±22.65 | 8.04±4.41 | <0.01 |
| Rheumatoid factor (IU/mL) | 463.69±734.82 | 33.35±53.85 | <0.01 |
| Neutrophil to Lymphocyte ratio | 3.20±2.06 | 1.56±0.47 | <0.01 |
| Platelet to Lymphocyte ratio | 192.85±101.78 | 103.49±28.68 | <0.01 |
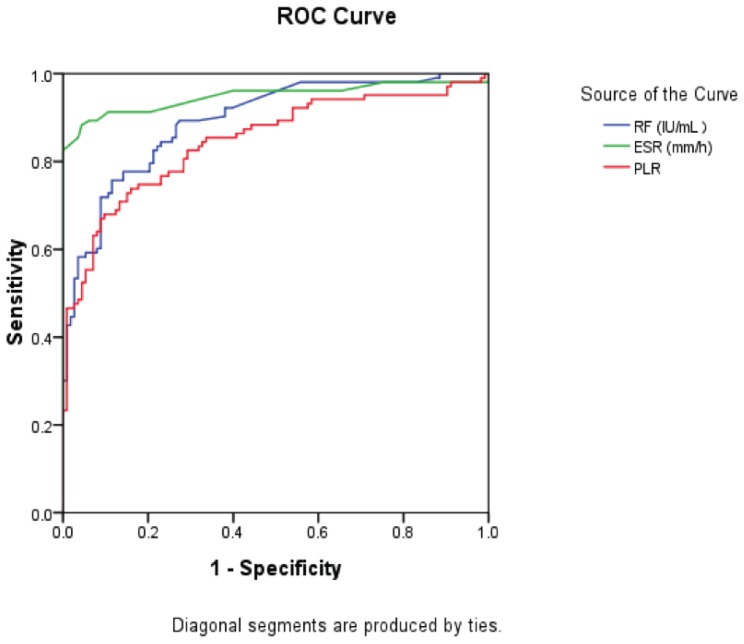
Stepwise logistic regression analysis was used to identify factors associated with RA, when leukocyte, neutrophil percentage, neutrophil, lymphocyte, platelet, ESR, CRP, and RF were considered as confounders (crude model); our results showed that ESR and RF were associated with RA. Of note, ESR, RF, and PLR were associated with RA after further adjustment based on the crude model for PLR and NLR (adjusted model), and CRP was not related to RA in the adjusting model, as shown in Table 2. Finally, receiver operating characteristic (ROC) curves were used to measure the performance of ESR, RF, and PLR, as shown in Table 3 and Figure 1.
Table 2.
Stepwise Logistic regression analysis of inflammation factors associated rheumatoid arthritis
| Crude model OR(95%CI) | p-value | Adjusted Model OR (95%CI) | p-value | |
|---|---|---|---|---|
| Erythrocyte sedimentation rate (mm/h) | 1.278(1.173–1.393 ) | <0.01 | 1.213(1.096–1.344) | <0.01 |
| Rheumatoid factor (IU/mL) | 1.011(1.003–1.019 ) | 0.006 | 1.010(1.002–1.017) | 0.013 |
| Platelet to Lymphocyte ratio | 1.026 (1.007–1.045) | 0.007 |
Crude model: adjustment for leukocyte, neutrophil percentage, neutrophils, lymphocytes, platelet, erythrocyte sedimentation rate, C-reactive protein, and rheumatoid factor.
Adjusted Model: adjustment based on Crude model for Neutrophil to Lymphocyte ratio and Platelet to Lymphocyte ratio
Table 3.
The performance of ESR, RF, and PLR for RA
| AUC | Cutoff values | Sensitivity | Specificity | 95% CI | |
|---|---|---|---|---|---|
| Erythrocyte sedimentation rate (mm/h) | 0.948 | 17.00 | 88.3% | 95.6% | 0.914–0.983 |
| Rheumatoid factor (IU/mL) | 0.893 | 21.25 | 89.3% | 72.6% | 0.851–0.935 |
| Platelet to Lymphocyte ratio | 0.847 | 115.66 | 82.5% | 74.8% | 0.794–0.901 |
AUC: Areas under the curve
Figure 1.
Receiver operating characteristic (ROC) curves of ESR, RF, and PLR levels for patients with RA
4 Discussion
We found that PLR was associated with RA in our study; however, the association between NLR, CRP and RA was not observed in present study.
Over the past decades, in clinical practice white blood count has been used as a traditional inflammatory marker. In fact, a relationship between PLR and colorectal cancer, non-small cell lung cancer, and acute coronary syndrome has been reported [11–13], and PLR is considered to be a prognostic indicator of tumor diseases. Kultigin et al. [15] reported that PLR is better than NLR for the predictive value of end-stage renal disease. There is increasing evidence that activated platelets, especially for patients during chronic inflammation, could be an important part of increased atherogenesis [16]. Platelets can interact with various cell types, including endothelial cells, T-lymphocytes, neutrophils, and mononuclear phagocytes, and earlier investigations strongly suggested that chronic inflammation may contribute to the development of atherosclerosis [17–18]. In the present study, we confirmed that PLR was higher compared with healthy controls, and that it remained a convincing correlation with RA after adjusting various confounders. The association between NLR, CRP, and RA was not observed in this study. Although the mechanism between PLR and RA remains unknown, the mechanism to explain the increased levels of PLR in RA is the presence of a chronic inflammatory state that affects the progressive damage of joint. In an earlier trial, the systemic and chronic inflammation associated with thrombocytosis was demonstrated [19], wherein a number of pro-inflammatory cytokines promote megakaryocyte proliferation such as IL-1 and IL-6 [19], leading to a higher platelet count in patients with RA. It is then noteworthy that thrombocytosis induced by pro-inflammatory cytokines such as IL 6, IL 1, GM-CSF and G-CSF was also frequently observed in patients with malignant tumors [20]. Obviously, pro-inflammatory cytokines represent a potential cause for proliferation of megakaryocyte in patients with RA, contributing to increases in platelet levels. Further research is necessary to clarify the mechanism associated with PLR in RA patients.
In most of the previous trials, RF, CRP, and ESR have been considered to be indicators associated with RA; however, none of CRP concentrations remained significantly associated with RA in the present study when associated inflammatory indicators were considered as covariates. Contradictory results have been reported on the association between CRP concentrations and RA. The discrepancy may be explained by the possibility that CRP is an acute-phase protein that is produced by the liver and that it is very sensitive to short-term changes in inflammation [21]. That could indicate that CRP concentrations may not increase in patients with RA during the stage of long-term and chronic inflammation. In contrast, the level of ESR is an indirect measure of acute-phase protein and has a slow response after inflammatory stimulation or resolution [21]. ESR is also sensitive to non-acute-phase proteins such as RF, and may be a better indicator for estimation of the subclinical inflammation than is CRP [22]; further, based on the present results, PLR may be superior to CRP to estimate the chronic subclinical inflammation in patients with RA.
There may be some limitations in the present study. In fact, the variation of PLR, as a cross-sectional study, was not observed dynamically after undergoing effective treatment for patients with RA. Moreover, the association between PLR and the severity of RA should be discussed to clarify whether PLR is an indicator estimating the severity for patients with RA. Finally, our samples were measured from a single laboratory; because PLR values may be slightly different in the various populations and regions. However, our results suggest that PLR is associated with RA, and PLR, but not NLR and CRP, may be an underlying indicator indicating the chronic subclinical inflammation in patients with RA.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 4.Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: A community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudiger A, Burckhardt OA, Harpes P, Muller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451–454. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Nunez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;46:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Huo Y, Ley KF. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004;14:18–22. doi: 10.1016/j.tcm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Boyraz İsmail, Koç Bünyamin, Boyacı Ahmet, Tutoğlu Ahmet, Sarman Hakan, Özkan Hilal. Ratio of neutrophil/ lymphocyte and platelet/lymphocyte in patient with ankylosing spondylitis that are treating with anti-TNF. Int J Clin Exp Med. 2014;7:2912–2915. [PMC free article] [PubMed] [Google Scholar]
- 12.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. doi: 10.7314/apjcp.2013.14.9.5237. [DOI] [PubMed] [Google Scholar]
- 13.Akkaya E, Gul M, Ugur M. Platelet to lymphocyte ratio: A simple and valuable prognostic marker for acute coronary syndrome. Int J Cardiol. 2014;28:13–15. doi: 10.1016/j.ijcard.2014.08.143. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P. Malnutrition and chronic inflmmation as risk factors for cardiovascular disease in chronic renal failure. Blood Purif. 2001;19:143–151. doi: 10.1159/000046932. [DOI] [PubMed] [Google Scholar]
- 16.Koyama H, Maeno T, Fukumoto S, et al. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation. 2003;108:524–529. doi: 10.1161/01.CIR.0000081765.88440.51. [DOI] [PubMed] [Google Scholar]
- 17.Borissoff JI, Spronk HM, Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2013;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 18.Turkmen K, Erdur FM, Ozcicek F, et al. Platelet-to-lymphocyte ratio better predicts inflmmation than neutrophil-tolymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17:391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 19.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 20.Sierko E, Wojtukiewicz MZ. Inhibition of platelet function: does it offer a chance of better cancer progression control? Seminars in thrombosis and hemostasis. 2007;33:712–721. doi: 10.1055/s-2007-991540. [DOI] [PubMed] [Google Scholar]
- 21.Kushner I. C-reactive protein in rheumatology. Arthritis Rheum. 1991;35:982–984. doi: 10.1002/art.1780340819. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J Rheumatol. 1997;24:1477–1485. [PubMed] [Google Scholar]