Abstract
We compared the effects of 16-week-training on rest metabolic rate, aerobic power, and body fat, and the post-exercise effects upon rest oxygen uptake and respiratory exchange ratio in overweight middle-aged females. Twenty nine overweight women (BMI 29.9 ± 1.2 kg*m−2) participated in training (3 days a week). The subjects were divided onto groups of aerobic (AT) and strength (ST) training. The results showed that the total body mass decrease and VO2 max increase did not differ in both groups. Decrease in waist circumference after 16 weeks was higher in the ST group. In the ST group fat-free mass increased during the first 8 weeks. Rest metabolic rate was increased significantly at 16th week compared to initial value in ST group only. Significant increase in post-exercise resting VO2 and respiratory exchange ratio at 12 and 36 h was observed after the strength training session only. Increase in rest metabolic rate and post-exercise rest energy expenditure occurred after strength training but not after aerobic training despite the similar increase in aerobic power. The effect of 8–16 weeks of strength training on body mass decrease was higher in comparison to aerobic training.
Keywords: Aerobic power, middle-aged women, overweight, rest metabolic rate, strength training
1 Introduction
According to Central Statistical Office (GUS), women constituted 51% of Polish population in 2009. Almost 45% of them in 2009 were overweight or obese (29.4% and 15.2%, respectively). It was almost 3 percent-point-increase compared to data from 2004 [1]. Moreover, detailed statistical analyses revealed that among women aged 40–49 years, only 50.3 % was normal weight, and it was a decrease (of almost 5 percent-points) in that percentage compared to 2004 [1]. GUS states also that the amount of time spent on physical activity increases with the age but only to the range of 40–49 years, and after that it decreases. Almost 80% of women 40–49 years old declare walking as main physical activity type. They also state that they spend about an hour a day walking [1].
There is a decline in resting metabolic rate [2, 3], and energy expenditure of physical activity resulting in decline of total energy expenditure within women of advanced age [4, 5]. These age-related changes in total energy expenditure may lead to increased adiposity in pre- and postmenopausal women. The menopause transition may represent a risky period in a woman’s life, ‘triggering’ adverse metabolic and cardiovascular processes [5]. These observations have led to attempts to optimize exercise to maximize fat oxidation as a means to promote weight loss.
Reduced rates of fat oxidation, which is prevalent in insulin-resistant individuals [7] (i.e., increased respiratory exchange ratio, RER), have been shown to lead to weight gain [6]. In both pre- and postmenopausal women, increased glucose and insulin concentrations associated with decreased amount of sex hormones [8] and decreased basal fat oxidation [9] have been shown.
Because resting metabolic rate (RMR) and energy expenditure of physical activity account for about 90% of total energy expenditure, any intervention that can increase these two components of energy expenditure could be useful in restoring energy balance and preventing women becoming overweight. Naturally, the combination of calories restricted diet and physical loads provides the greatest effect on body fat reduction [9, 10]. On the other hand, the way of leisure time spending in young age can influence human behaviour as well as conditions and sports habits, which is crucial for the fitness level in the future [11]. Although general requirements concerning possible ways of excessive body mass correction are quite clear, their practical realization encounters many difficulties. In overweight middle-aged women (OMW) the probability of increasing energy expenditures using physical loads is rather restricted due to decreased physical fitness and everyday motor activity [12]. Physical inactivity tends to increase with the decline of endogenous estrogen [13]. These problems aggravate with age and become especially obvious after the age of 40 [14].
Until now, the most widely spread type of physical exercises for excessive body mass reduction has been aerobic exercises. An increase in their duration at about 40% of maximum workload can further activate fat oxidation [12, 15]. However, recent studies show that efficiency of this type of physical training is insufficient from the point of view of excessive body fat reduction [16]. Besides, some data indicate that in order to maintain the total effect that aerobic training has on fat oxidation activation its intensity should gradually increase [16, 17]. Therefore, there is a search for new forms and modes of health-related resistance training that take account of the age of the women.
Literature data demonstrate an increase in RMR, norepinephrine levels and reduction in intra-abdominal adipose tissue in healthy older women in response to strength training (ST) [18, 19]. In contrast, investigations in young women have found a consistent lack of change in RMR with ST [20]. Weight training increases FFM and strength in untrained young women [20]. Strength training of postmenopausal women increases FFM and maintains RMR despite of weight loss [19].
There are numerous reasons to consider ST as one of the most effective types of health-related training [21]. However, its influence upon energy expenditures and activation of fat losses at rest in OMW need further investigations. It is assumed that ST of OMW is a significant stimulating factor for self-regulation of body energy balance and body mass correction. This factor may be related to changes of RMR and fat oxidation. Although post ST increase of rest energy expenditure and body composition has already been demonstrated for men, the influence of ST on OMW remains to be studied.
2 Material and methods
2.1 Experimental Approach to the Problem
The aim of the present study was to compare the effects of 16 week (wk) training in groups of whole body strength training (ST) and aerobic training (AT) on resting metabolic rate, aerobic power, body fat, and also on post-exercise effects of single training sessions upon rest oxygen uptake and respiratory exchange ratio in OMW.
2.2 Participants
Twenty-nine 40–49 yrs old overweight premenopausal middle-aged women from Gdansk, Poland volunteered to participate in this study after screening of medical history, physical activity questionnaire, and a thorough physical examination to rule out any obvious signs of cardiovascular, musculoskeletal, and metabolic disorders. Selected women had never participated in special physical training and during the last 6 months did not follow any special calorie restricted diets. In the process of study the subjects were recommended to follow their ordinary diet typical to the last 6 months. Subjects were non-smokers, physically inactive for the last 6 months, and were not currently taking any cardiovascular, antihypertensive, or metabolic medications, with the exception of two women on oral contraceptives. About 70% of women had regular menstrual cycles 25–31 days. Others had irregularity in the cycle between 1 and 5 days. None of the participants were menopausal. Body mass variations during this period have not exceeded 3.0 kg. Participants were familiarized with content of studies and measurement procedures and gave consent for its execution. All procedures in the study were approved by the Local Institutional Ethical Committee at the Sniadecki University School of Physical Education and Sport in Gdansk.
When pooled together all subjects were characterized by excessive body mass which was ≥ 125% of standard weight for females of the given age determined as a mean value of body mass range based on standards given by McArdle et al. [4]. Average body mass of subjects constituted 78.7 ± 3.0 kg, whereas BMI was equal to 29.9 ± 1.2 kg·m−2. Average percentage of fat constituted 35.0% (within 68% of variations of 29.3–37.8). In order to realize different types of health-related training the subjects were randomly assigned to aerobic training group (AT, n = 14) aged 43.8 (40–49) years and strength training group (ST, n = 15) aged 43.1 (40–48) years. In the ST and AT groups there were 11 and 12 women, respectively, and all had regular menstrual cycles. In both groups there was one woman taking oral contraceptives. The subjects’ physical characteristics and VO2 max (baseline values) of both groups are presented in Table 1.
Table 1.
Physical characteristics and maximum oxygen uptake of overweight females participating in health related physical training (mean ± SD).
| Group of aerobic training (AT) | Group of strength training (ST) | |
|---|---|---|
| Age (yr) | 43.8 (40–49) | 43.1 (40–48) |
| Body mass (kg) | 78.4 ± 2.3 | 79.0 ± 2.4 |
| BMI (kg·m−2) | 30.2 ± 1.8 | 29.8 ± 1.7 |
| Body fat (%) | 35.1 ± 2.2 | 34.9 ± 2.1 |
| Lean body mass (kg) | 50.8 ± 1.8 | 49.8 ± 1.9 |
| Waist circumference (cm) | 89.9 ± 4.0 | 91.7 ± 4.2 |
| Number of participants with type of obesity in: | ||
| - the upper part of the body | 6 | 7 |
| - the lower part of the body | 8 | 8 |
| VO2 max (ml·min−1 per kg of body mass) | 29.1 ± 2.6 | 29.9 ± 2.8 |
Differences in the type of obesity (preferentially of the upper or the lower part of the body) relative to waist and hip circumference (waist-to-hip ratio, WHR) were evaluated. The criterion of van Aggel-Leijssen et al. [22] was applied according to which WHR ≥ 0.85 was indicative of the obesity of the lower part of the body. In accordance to this criterion there were similar number of women with obesity of the lower and upper part of the body in the AT and ST groups.
2.3 Anthropometrical data
The body mass was determined with a calibrated digital scale to nearest 0.1 kg after shoes, coats, and sweaters had been removed. Body height was obtained by a height measurement device integrated in scale to the nearest 0.005 m with subjects in standing position without shoes. On the test day the subjects were in post absorptive state, dehydrated and had not exercised for at least 24 h. Percentage body fat and fat free mass (FFM) were estimated from four skin folds (biceps, triceps, sub scapular and suprailiac) performing every measurement 3 times - before, after 8 and 16 wk of training, according to the method of Durnin and Womersley [23]. Waist circumference was measured at the smallest circumference between the ribs and iliac crest. Anthropometric measurements were conducted by the same individual, according to conventional criteria and measurements procedures.
2.4 Resting metabolic rate, oxygen uptake and respiratory exchange ratio
RMR was determined by a ventilated hood indirect calorimetry after an overnight fast. Oxygen uptake and carbon dioxide production were measured by respiratory gas analysis. Concentrations of CO2 and O2 were measured in the morning in the laboratory using metabolic system (Quark b2, COSMED, Italy) calibrated with standard gas mixtures. These gas concentrations were then used to determine 24h RMR energy expenditure using the Weir’s equation [24]. Subjects were instructed to wear comfortable clothing and drink only water for 6 h before testing. All data collections were performed under identical conditions relative to ambient temperature (23–26°C) and relative humidity (45–55%). Subjects were also instructed to keep physical activity to a minimum in the morning. They were instructed to lie on the bed for 30 min. After the first acclimatization of 10 min interval the measurement was made. Twenty-four-hour RMR was calculated from the average O2 and CO2 concentrations collected during the last 20 min. After single training sessions resting post-exercise oxygen uptake and RER were measured (using the same procedures) at 12 and 36 h after last session of the 1st and 16th week for evaluation of delayed effects of aerobic and strength sessions. The subjects followed their ordinary diet during the study. We assumed that a decrease in RER value may indicate increased portion of fat oxidation within total energy supply of the body.
2.5 Maximal oxygen uptake
Maximal oxygen uptake test was performed using incremental grade bicycle exercise protocol 7 days prior to onset of training sessions. All subjects were familiarized with bicycle exercise (Monark, Sweden) and gas analysis procedures prior to the study. The test was terminated when the subjects could no longer continue pedalling at rate of 50 cycles·min−1. VO2 max was determined from the measurement of the fractional concentrations of O2 and CO2 (Quark b2, Cosmed, Italy). To achieve a true VO2 max, subjects had to meet two of the following criteria: plateau in VO2 max and RER > 1.1, or HR within 10 beats of their age predicted HR max.
2.6 Training
Both groups of participants trained at the same University gym.
Group AT performed exercises of preferentially aerobic type (cyclic character) on training apparatus (bicycle and rowing ergometer). All recommendations concerning intensity, frequency and duration of exercises were thoroughly followed [12, 17]. Training sessions (80 min) were conducted 3 times a week. The duration of activity in one session at a heart rate of 136–156 beats/min varied from 35–42 min to 50–55 min; a definite alternation of activity types was envisaged. The intensity of training loads was gradually increased weekly as the level of females’ conditioning improved. The total energy cost of a session was in the range of about 400–600 kcal which corresponded to recommendations for such type of training for health-related physical training [17].
In group ST during 16 wk women performed an extended whole body strength training 3 times a week on standard strength devices according to “circuit” method of training in series of 8–12 repetitions (60–65% of maximum strength in one maximum repetition – 1 RM) in the first two weeks.
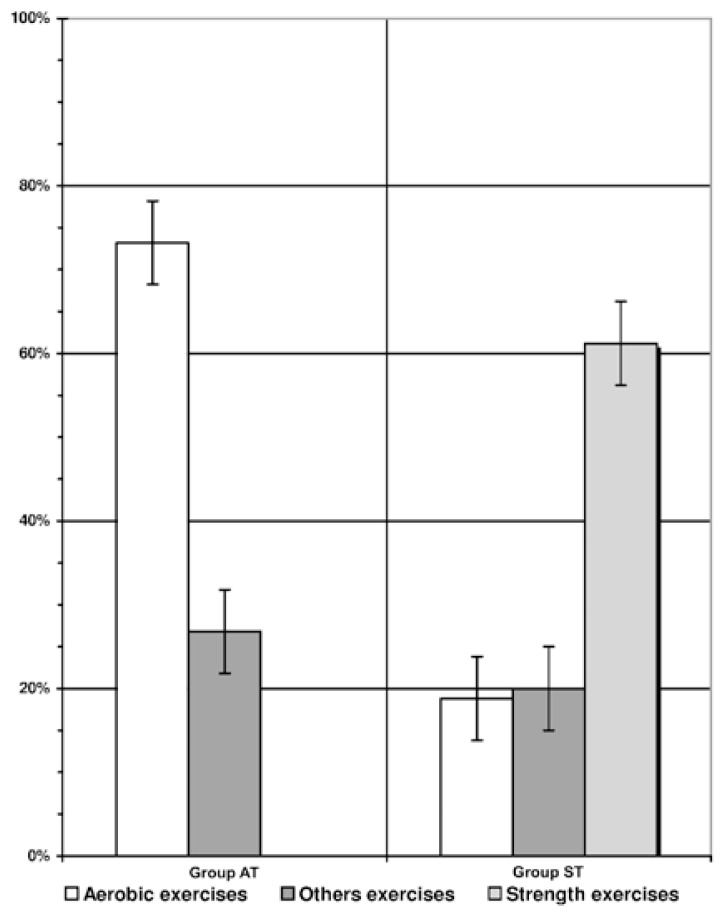
All subjects were given 2–3 min of rest between sets throughout the entire strength training program. Training included exercises that trained all the major muscle groups of the body (whole body strength training) [21]. Lower body exercises included unilateral leg press, leg curl, and leg extension, whereas the upper body muscle groups were trained using the chest press, alternating pull-down, military press, upper back, and triceps machines. The biceps were trained unilaterally using free weight dumbbells with biceps curls, whereas the abdominal muscles were exercised with both the Keiser abdominal machine and abdominal crunches. The concentric phase of each repetition was performed in about 1.5–2 s and the eccentric in about 2–3 s. Principles of progressive strength training with orientation of person 1 RM were applied. Subjects gradually increased the resistance, after a warm-up at 50% of their 1 RM, until failure to complete a repetition occurred. This resulted in 12–14 RM for the 14–16 wks. Strength training loads was gradually increased weekly as the level of females’ 1 RM improved. On this basis, the degree of increase of resistance and exercise sessions load were determined [25]. Alternated exercises of standard type for muscles of the upper and the lower part of the body were performed. Subjects performed one set on all the upper body exercises and two sets of the lower body exercises. The strength exercises were aimed at both maximum strength development and strength endurance enhancement [25]. Total energy cost of such training session constituted about 400–480 kcal. All aerobic and strength sessions were subjectively heavy and monitored by at least two fitness coaches. Figure 1 represents the specific weight of physical exercises mainly aerobic or strength type and other exercises for health-related training sessions in the group of OMW.
Figure 1.
The parts (in percent) of whole time of training sessions mainly aerobic, strength type and other exercises in groups of overweight females with aerobic (AT) and strength (ST) training.
2.7 Statistical analyses
Statistical analyses were performed using a statistical software package (SYSTAT). Data were checked for the normal distribution by Kolmogorov-Smirnov test. All obtained data showed normal distributions. Responses to aerobic and strength training were assessed using a repeated measures’ Students t-test because all variables were assessed before and after the period of training. Values were presented by means ± standard deviations, and significance level was set at α = 0.05.
3 Results
3.1 Body composition
Changes of total body mass and body mass index in AT and ST groups after 8 and 16 wks are presented in Table 2. The data showed that both types of training resulted in significant reduction of body mass and BMI. It took place after both the first 8 and the following 8 wks of training. The comparison of changes (decrease in percent) in body mass (from initial level to level at 8th wk, and from level at 8th wk to level at 16th wk) shows that after 8 and 16 wks of training reduction of body mass in the AT group constituted 2.7 kg and 5.0 kg (6.3%), respectively, as compared to initial values. In the ST group the above reduction constituted 0.6 kg and 4.1 kg, respectively (5.4 %). There were significant differences in the decrease (in percent) in body mass between initial value and value at 8th week of training in the AT group. This reduction was higher in the AT group as compared to ST one. At the next stage of training (from 8th to 16th wk) we observed significant decrease in body mass in both AT and ST groups. Slightly higher decrease of total body mass was observed in females engaged in ST group from 8th to 16th wk.
Table 2.
Values of body mass and body mass index (BMI) before and after 8 and 16 wks of aerobic (AT) and strength (ST) training of overweight females (mean ± SD).
| Before | 8th wk | 16th wk | Significance of differences (P values) | |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Group AT (n = 14) | ||||
| Body mass (kg) | 78.4 ± 2.3 | 75.7 ± 1.8 | 73.4 ± 1.7 | 1–2 (0.0019); 2–3 (0.0018); |
| BMI (kg·m−2) | 30.2 ± 1.8 | 29.2 ± 1.6 | 28.3 ± 1.5 | 1–2 (0.0454); 2–3 (0.0458) |
| Group ST (n = 15) | ||||
| Body mass (kg) | 77.9 ± 2.4 | 77.3 ± 2.0 | 73.8 ± 1.8 | 2–3 (0.0000) |
| BMI (kg·m−2) | 29.8 ± 1.7 | 29.6 ± 1.5 | 28.1 ± 1.4 | 2–3 (0.0085) |
The data of changes in body fat and FFM are presented in Table 3. The results showed that during 16 wks of training percent of body fat has gradually decreased. The degree of body fat (%) decrease in the ST group at 16 weeks of training was significantly higher than in the AT group. During 16 wks of training percent of body fat gradually decreased from 35.1% to 33.0%, and from 36.6% to 30.5% in the AT and ST groups, respectively. A significant increase of FFM has been observed during the first 8 weeks in the ST group. Total increase in FFM after 16 wks of strength training was 1.6 kg (1.2 kg for the first 8 wks). Data presented in Table 3 show that both types of training led to significant decrease in waist circumference. Total decrease in waist circumference after 16 weeks of training was higher in the ST group. The decrease in the AT group constituted 5.5 cm (−6.3%), whereas that in the ST group was more evident and constituted 9.6 cm (−10.7%).
Table 3.
Characteristics of overweight females’ body fat before and after 8 and 16 wks of aerobic (AT) and strength (ST) training (mean ± SD).
| Before | 8th wk | 16th wk | Significance of differences (P values) | |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Group AT (n = 14) | ||||
| Body fat (%) | 35.1 ± 2.0 | 34.1 ± 1.9 | 33.0 ± 1.6 | – |
| Fat-free mass (kg) | 50.8 ± 1.8 | 49.9 ± 1.9 | 49.4 ± 1.7 | – |
| Abdominal circumference (cm) | 89.9 ± 4.0 | 87.7 ± 3.2 | 84.4 ± 3.0 | 2–3 (0.0092) |
| Group ST (n = 15) | ||||
| Body fat (%) | 36.0 ± 2.1 | 33.9 ± 1.8 | 30.5 ± 1.7* | 1–2 (0.0065); 2–3 (0.0000) |
| Fat-free mass (kg) | 49.9 ± 1.9 | 51.1 ± 1.7 | 51.5 ± 1.5 | 1–3 (0.0162) |
| Abdominal circumference (cm) | 91.7 ± 4.2 | 87.0 ± 3.1 | 82.1 ± 2.8 | 1–2 (0.0016); 2–3 (0.0001) |
– significant difference between AT and ST group, P = 0.0004.
3.2 Resting metabolic rate and oxygen uptake changes
The RMR and oxygen uptake significantly increased (P = 0.0014 and P = 0.0085, respectively) at 16th week compared to initial value in the ST group (Table 4). There were no statistically significant changes in the VO2 and RMR in the AT group.
Table 4.
Resting oxygen uptake and rest metabolic rate changes related to 16 wks of aerobic (АT group) and strength (ST group) training of overweight females (mean ± SD).
| Before | 16th wk | Significance of differences (P vaules) | Increase (%) | |
|---|---|---|---|---|
| Group AT (n = 14) | ||||
| O2 uptake (ml·min−1) | 184.1 ± 15 | 193.8 ± 18 | – | – |
| RMR (kJ·d−1) | 5527 ± 827 | 5821 ± 898 | – | 5.30 |
| Group ST (n = 15) | ||||
| O2 uptake (ml·min−1) | 185.8 ± 18 | 203.4 ± 16 | 0.0085 | – |
| RMR (kJ·d−1) | 5540 ± 875 | 6688 ± 901 | 0.0014 | 9.52 |
3.3 Maximal oxygen uptake
VO2 max per kg of body mass significantly increased at 16th wk compared to initial value in the AT and ST groups (P = 0.0354; P = 0.0140, respectively). However, VO2 max per kg of FFM increase did not reach the significant level (Table 5).
Table 5.
Maximal oxygen uptake changes related to 16 wks of aerobic (AT group) and strength (ST group) training of overweight females (mean ± SD).
| Before | 16th wk | Significance of differences (P values) | |
|---|---|---|---|
| Group AT (n = 14) | |||
| VO2 max (l·min−1) | 2281 ± 187 | 2305 ± 197 | – |
| VO2 max (ml·min−1 per kg of body mass) | 29.09 ± 2.6 | 31.40 ± 2.9 | 0.0354 |
| VO2 max (ml·min−1 per kg of FFM) | 44.90 ± 2.8 | 46.66 ± 2.4 | – |
| Group ST (n = 15) | |||
| VO2 max (l·min−1) | 2330 ± 191 | 2395 ± 201 | – |
| VO2 max (ml·min−1 per kg of body mass) | 29.91 ± 2.8 | 32.45 ± 2.5 | 0.0140 |
| VO2 max (ml·min−1 per kg of FFM) | 46.69 ± 2.4 | 48.48 ± 2.6 | – |
3.4 Post-exercise effects of single training session
Results of resting oxygen uptake and RER measurements for 12 and 36 h after aerobic training session (in the AT group) at the beginning (1st wk) and at the end (16th wk) training program are presented in Table 6. No significant differences were observed between VO2 and RER of pre-exercise, 12 and 36 h after the AT session. There were no statistically significant changes between the 1st and the 16th wk of training. Results of resting VO2 and RER measurements for 12 and 36 h after single strength training session (in the ST group) at the beginning (1st wk) and at the end (16th wk) training showed an increase at 12 and 36 h vs. pre-exercise at 16th wk only (data not presented). Comparison of RER values at 1st and 16th wk also showed an increase (data not presented).
Table 6.
Oxygen uptake and resting respiratory exchange ratio at 12 and 36 h after aerobic training session (n = 8).
| Parameters | Pre-exercise | 12 h | 36 h |
|---|---|---|---|
| 1 | 2 | 3 | |
| 1st week | |||
| O2 uptake (ml·min−1) | 183.4 ± 15 | 196.2 ± 17 | 187.6 ± 19 |
| Respiratory exchange ratio | 0.881 ± 0.05 | 0.862 ± 0.03 | 0.884 ± 0.01 |
| 16th week | |||
| O2 uptake (ml·min−1) | 193.0 ± 18 | 205.5 ± 19 | 190.1 ± 17 |
| Respiratory exchange ratio | 0.863 ± 0.003 | 0.856 ± 0.01 | 0.861 ± 0.01 |
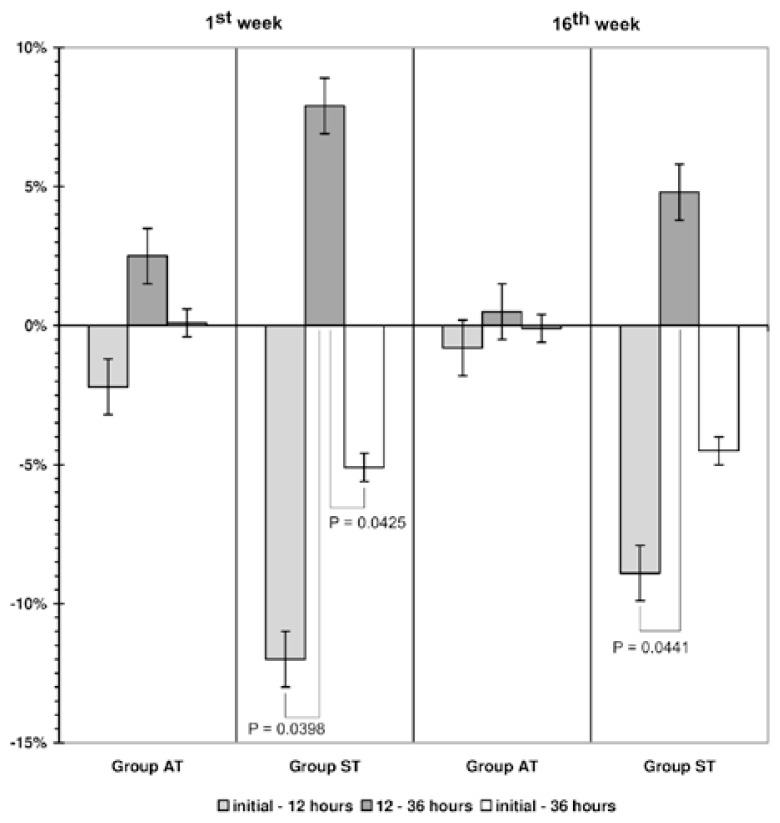
The study of RER values changes (in %) after training sessions of different type showed that significant changes took place after single strength training session only (Figure 2). For the first week after the strength training session, a significant decrease in RER was noted between the initial basal level and the level at 12 h, and between the level at 12 h and the level at 36 h. For the 16th wk a significant decrease was noted between the initial basal level and the level at 12 h.
Figure 2.
Post-exercise changes of resting respiratory exchange ratio (as % of initial basal level) at 12 and 36 hours after training session in groups of aerobic (AT) and strength (ST) training. Data for the 1st and the 16th week of training are presented.
4 Discussion
The increase in RMR and energy expenditure of physical activity with strength training could explain the decrease in fat mass that has been reported in response to this type of training [26]. The resulting effects of ST is induced by increases in muscle mass and RMR [10]. Escalating decline of basal fat oxidation with aging is a high gender specific characteristic of women [2, 27, 28]. In middle-aged (pre-menopausal) women this may be more distinct [2]. Presented data demonstrate border-line level of excessive body mass of studied females. Most frequently the degree of excessive body mass is classified as the initial stage of obesity when BMI exceeds 30 kg*m−2. BMI standard has a tendency to shift towards higher indices with age.
The stimulation effect of health related physical training on body composition for women can be explained by differential responses in RMR or energy expenditure of physical exercise. Some studies have demonstrated that strength training may increase resting energy expenditures in elderly males [18, 29], whereas single training session with concentric resistance exercises enhances RMR within 48 hours after load in healthy 59–77 yrs old men [30, 31]. At the same time increase in fat oxidation (on the basis of RER changes) has not been noted in these studies. Melanson et al. [32] showed that moderate-duration single exercise (≤ 1 h) has little impact on 24-h fat oxidation. In contrast, 48 hours single bout of strenuous resistance exercise of young men resulted in elevation of RER with concomitant increase in fat oxidation. However, the possibilities of correction of excessive body mass by using prompt effects of strength exercises for women of different age groups were not experienced. For that reason, studies of effects of strength training in middle-aged women may help to better understand this type of training’s effect factors that influence body composition. Thus the present study deals with the examination of health training in overweight women, which relates to determining the role of strength training as one of the adequate means for excessive fat reduction. We assumed that in pre-menopausal (middle-aged) women the results of strength training may be more pronounced then in young female. The effects of strength- and aerobic training on body mass, body fat decrease and on aerobic power and RMR were controlled in the study. Attention was focused on augmentation of resting metabolism under the influence of aerobic and strength exercises rather than on energy expenditure during training sessions. It is known that RMR may be the dominating factor of increase daily energy expenditure and decrease of excessive body mass [4, 12]. Prerequisites for such examination arise from logical analysis of known general effects of strength training. General and particular programs of strength training including those for females both for sport and health-related purposes have been elaborated [17, 21, 25]. The present study investigates the role of aerobic and extended program of strength exercises in body mass correction of OMW independently. Aerobic training included cyclic type of exercises. It represents widely used type of physical training for correction of excessive body mass by increasing the energy expenditure. Sixteen weeks of training induces the increase in VO2 max per kg of body mass and of fat-free body mass only. In AT and ST groups the increase in VO2 max per kg of body mass did not differ significantly and reached 7.9 and 8.4%, respectively. In the present study, the training effects in both groups were related mainly to the decrease in whole body mass but not to the increase in VO2 max of FFM. The physical fitness level of studied females (estimated by VO2 max values) was comparable for both groups and corresponded to usual values for the age groups of women who did not participated in a special program of physical training [2, 4]. Therefore, this type of 16-wks physical training of OMW had no specific effects for VO2 max increase.
The presented data demonstrate that RMR increased from 1st to 16th wk of training. In the ST group a significant increase was observed in 12 out of 15 women. However, in the AT group only a tendency (statistically not significant) was observed in the increase in RMR and oxygen uptake at rest. Some studies on women solely to investigate the effect of exercise on RMR were provided. Lemmer et al. [33] showed that changes in RMR in response to strength training were affected by gender and not by age. When young and older men (20–30 and 60–75 yrs) were pooled as a group, there was a significant increase in RMR within 24 wks of strength training (total RMR significantly increased by 7%), whereas young and older women showed no change in response to this type of training [20, 33]. The lack of a change in RMR for women is consistent with previous studies in both young [20] and older women but is in contrast to other studies in women [19]. In the present study total RMR significantly increased by 9.5% vs. initial value. It may be related specifically to pre-menopausal women with more distinct age-related decrease in RMR [3]. Furthermore, one of the essential reasons of being overweight is the distinctive RMR decrease in women aged 40–50 yrs and the decrease in basal fat oxidation in aging women [28]. In both pre- and postmenopausal women, several studies have shown that increased glucose and insulin concentrations are associated with increased free testosterone and decreased sex hormone binding globulin [8]. It is known that female sex hormones may affect several enzymes involved in the metabolism of triglyceride and may also affect lipolysis [27]. The temporal direction of this relationship in women is not clear, however. Studies of young females showed no significant effects of aerobic and strength training when RMR was corrected for FFM [33]. The single study showed no change in responses of RMR to strength training for either young (age = 26 yr) or older (age = 70 yr) individuals [20]. However, this could be related to the fact that the subjects did not increase FFM in response to strength training. In the other studies the increase in RMR in response to strength training in older men and women of 60–70 yrs old was shown, when pooled together [19, 33]. Lemmer et al. [33] concluded that changes in absolute and relative RMR in response to strength training are influenced by gender but not age. In contrast to what has been suggested previously, changes in body composition in response to strength training are not due to the changes in physical activity besides training. These studies, however, cannot be compared with-strength training-induced changes in RMR in OMW.
Our results showed that the degree of body mass reduction for 16 wks was quite similar during aerobic and strength training. The comparable levels of decreasing body mass for 12–14 wks of training were obtained in other studies [20]. Significant differences in the extent of body mass reduction (%) were noted during the first stage (8 wks) of training. This reduction was higher in the aerobic group as compared to the strength one. The further decrease in body mass in ST group from 8th to 16th wks was however higher (P = 0.0000). Nevertheless, the main part is the increase in FFM and was noted for the first 8 wks of strength training. The same amount of BMI reduction at each training stage was observed. Differences of waist circumference reduction between groups were already evident at the first stage of training (the first 8 wks). Body fat from the abdominal region is recognized as being much more lipolitic than adipose tissue from the gluteal-femoral region [30]. It is noteworthy that the most obvious changes (decrease) in waist circumference in ST group took place for the second stage of training (from 8th to the 16th wk). Thus, the data resulted in higher degree (in %) of total body fat decrease in ST group during the whole period of training (−15.6%) as compared to AT group (−6.1%) which occurred mostly at the second stage of training.
The data presented showed that a single strength training session induced (unlike aerobic type one) significant increase in resting oxygen uptake after 12 h both during the first and the 16th week. During the 16th week even after 36 h it remained on the level significantly higher than the initial one. These findings are indicative of the fact that strength training (single training session) produces higher post-exercise effect on resting oxygen uptake than aerobic training. In other words, an increase in energy expenditures during strength training is taking place not only during the session but for about 24 h after one. In contrast, results of Jamurtas et al. [34] showed that post-exercise increase in RMR and fat oxidation (for at least 24 h post-exercise) of either strength or aerobic types of similar intensity and duration was comparable. It is noteworthy that in the ST group the initial (pre-exercise) level of RER during the 16th wk was significantly lower than during the 1st wk, that is, in comparison to the pre-exercise state. Analysis of RER changes (its decline) may indicate enhanced fat oxidation 12 h after training session. The decrease in RER within 16 wks of strength training was maintained 36 h later (−5.1% and −4.5%, respectively at 1st and 16th wk). During 16 wks of aerobic training these changes in RER were not observed. It was shown earlier that low-intensity aerobic training (40% VO2 max) did not change fat oxidation at rest in obese 32–42 yrs women [21]. In the ST group a decrease in gas exchange ratio 12 h after the session was observed during the first week of training (−12.0%) and the 16th wk (−8.9%). In the AT group RER did not change by more than 2.5% on the average after single training session. These data may indirectly indicate that strength training of OMW produces significant post-exercise effect which consists in daily increase in fat oxidation, or possibly even longer. The degree of this increase (up to 12%) may represent an important factor for excessive body mass reduction. The results showed that aerobic training had no effect on RMR and post-exercise effect. In the AT group increase in RMR did not reach the level of significance. It confirms an earlier study concerning the determination of RMR during 20 wks of endurance training of 17–63 yrs men and women [6]. There were no changes in post-training RMR after the last exercise bout and in chronic adaptation in the presence of small changes in body composition and a large increase in VO2 max (17.9%). Maximal fat oxidation rates seem to be higher in women compared with men. Fat oxidation rates are higher at a given exercise intensity in female subjects and maximal fat oxidation rates occur at a higher exercise intensity [26]. Although the mechanism is not clear, the ability of female subjects to use more fat during exercise may be due to differences in levels of circulating hormones and catecholamine, a more oxidative muscle fiber type distribution, an increased sensitivity to catecholamine-stimulated lipolysis, or increased activity of hormone-sensitive lipase [35]. The reason of the low effect of aerobic training in women may be related to its relatively low intensity. There were findings that show a relatively heavy work rate (at the ventilatory threshold) elicits the highest rate of fat oxidation in the female population [16]. Thus, strength training creates a predisposition for additional increase of daily energy expenditures at rest both as a result of chronic adaptation as well as by increasing the rest energy expenditure for 12–36 h after training bouts. Interestingly, the level of daily energy expenditure in the ST group for 12 h period after training sessions was higher (7206 kJ·d−1) than in the AT group (5963 kJ·d−1). The increase observed in the ST group after 12 h was 13.4% and 11.6% (during the 1st and the 16th wk, respectively).
One may assume that some specific effects of strength exercises in pre-menopausal women are related to hormonal background and resting metabolism change. It is evident that increase in muscular mass, strength and strength endurance would be the normalizing factor with respect to excessive body fat. There are data demonstrating that strength training results in increased resting blood epinephrine content, which is hardly observed during regular endurance training [14, 15]. Acute bouts of resistance exercise of young males elicited a greater response of growth hormone compared to continuous aerobic session [36]. One possible explanation for the training effect of strength training on RMR and post exercise fat oxidation rising in women is the increase in sympathetic nervous system activity [5, 33, 37]. Pratley et al. [18] attributed the increase in RMR over that which could be accounted for by increased FFM to an increase in sympathetic nervous system activity, which conversely was not demonstrated by Ryan et al. [19]. Unfortunately, sympathetic nervous system activity was not measured in the current study. In one case it was shown that body fat and body lipids in postmenopausal women are related to resting autonomic nervous system activity measured by power spectral analysis of heart rate variability [38]. This study further implies that autonomic depression could be a crucial risk factor in being overweight and in undermining the health of pre- and post-menopausal women [39]. Perhaps this was the reason of strength training (unlike the aerobic one) inducing post-exercise (after training bout) fat oxidation increase within 36 h. This effect was not only maintained but even reinforced under the influence of 16 wks of strength training. Thus, in the given work the above mentioned hypothesis has been convincingly confirmed. This fact acquires especially great importance taking into consideration that the degree of resting metabolism enhancement in studied OMW has correlated with the degree of post-exercise RER decrease (r = 0.59, P = 0.0432). Increased energy expenditures within relatively a long period of time after the session along with simultaneous enhancement of fat oxidation may explain the significant differences in adipose tissue loss in AT and ST groups. Differences in approximately 1250 kJ constitutes to about 65–70% of energy expenditures during the training session. As a result, the total effect (according to energy expenditures) of strength type bout along with its post-exercise effect exceeds that aerobic type sessions. Thus, in contrast to what has been suggested previously for young and old women, in middle-aged females changes in RMR and body fat composition in response to whole body strength training were shown. Previously, the treatment effect on body fat of twice-weekly strength training in 164 overweight women 25–44 yrs for 2 yrs was shown [29]. A comprehensive comparison of relevant data in the literature concerning the effects of strength training on resting energy expenditure and body composition changes in OMW is not possible due to the lack of such studies.
5 Possible limitations of the study
It remains unclear, how different intensities of strength training can influence RMR and body composition specifically in relation to hormone and resting autonomic nervous system activity changes in middle-aged women and the role of large individual differences in the responsiveness to endurance and resistance training [37]. It is determined by several factors including difference of hormone response to the characteristics of strength training: type of strength exercise, the frequency, intensity, volume, type of recovery. It may be suggested that a number of series are determinants of the level of activation of the endocrine and autonomic nervous systems.
6 Conclusion
The results of this study indicate an apparent effect of strength training on body fat and resting energy expenditure in OMW in the presence of increasing in VO2max (both per kg body mass and FFM) and decrease in BMI. These findings show that strength training can have a positive effect on body composition in OMW. Strength training-induced reductions in fat mass may be related to increases in fat oxidation. It is expressed in the increase in RMR and in more intensive post-exercise fat oxidation during 16 wks of strength training compared to equivalence severity of aerobic training. After aerobic training sessions the above post-exercise and post-training effects was not observed. The effect of the first 8 wks of aerobic training on body mass decrease was higher in comparison with strength training. However, for the 8–16 wks this effect was higher in ST group.
The results obtained can have application in exercise prescription in health-related training of OMW. By using available data on OMW body mass correction studies prescriptions may be based on the natural mechanism of self-regulated body energy expenditure.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Jakóbik K, editor. Zdrowie kobiet w Polsce w latach 2004–2009. Główny Urząd Statystyczny, Urząd Statystyczny w Krakowie, Kraków. 2012. http://www.stat.gov.pl/cps/rde/xbcr/gus/ZO_Zdrowie_kobiet_w_Polsce_w_latach_2004-2009.pdf (in Polish)
- 2.Arciero P, Goran MI, Poehlman ET. Rest metabolic rate is lower in women than in men. J Appl Physiol. 1993;75:2514–2520. doi: 10.1152/jappl.1993.75.6.2514. [DOI] [PubMed] [Google Scholar]
- 3.Poehlman ET. Regulation of energy expenditure in aging humans. J Am Geriatr Soc. 1993;41:552–559. doi: 10.1111/j.1532-5415.1993.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 4.McArdle WD, Katch FI, Katch VL. Exercise Physiology. Baltimore: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 5.Poehlman ET, Toth MJ, Ades PA, Calles-Escandon J. Gender differences in resting metabolic rate and noradrenaline kinetics in older individuals. Eur J Clin Invest. 1997;27:23–28. doi: 10.1046/j.1365-2362.1996.640620.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilmore JH, Stanforth PS, Hudspeth LA, Gagnon J, Daw EW, Leon AS, Rao DC, Skinner JS, Bouchard C. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: HERITAGE Family Study. Am J Clin Nutr. 1998;68:66–71. doi: 10.1093/ajcn/68.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, Valdez RA. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med. 1995;98:40–47. doi: 10.1016/s0002-9343(99)80058-8. [DOI] [PubMed] [Google Scholar]
- 9.Ballor DL, Harvey-Berino JR, Ades PA, Cryan J, Calles-Escandon J. Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metabolism. 1996;45:179–183. doi: 10.1016/s0026-0495(96)90050-5. [DOI] [PubMed] [Google Scholar]
- 10.Melby CL, Commerford SR, Hill JO. Perspectives in Exercise Science and Sports Medicine. Exercise, Nutrition, and Weight Control, Carmel. Vol. 11. Cooper Publishing Group; 1998. Exercise versus dieting for weight loss. Exercise, macronutrient balance, and weight regulation; pp. 56–65. 1998. [Google Scholar]
- 11.Görner K, Jurczak A, Spieszny M, Mucha D, Makuch R. Chosen individual factors of adolescents’ physical development in their leisure time. Centr Eur J Sport Sci Med. 2013;1:31–37. [Google Scholar]
- 12.Bouchard C, Despres JP, Tremblay A. Exercise and obesity. Obes Res. 1993;1:133–147. doi: 10.1002/j.1550-8528.1993.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 13.Dubnov G, Brzezinski A, Berry EM. Weight control and the management of obesity after menopause: the role of physical activity. Maturities. 2003;44:89–101. doi: 10.1016/s0378-5122(02)00328-6. [DOI] [PubMed] [Google Scholar]
- 14.Hakkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Ger Biol Sci Med Sci. 2000;55:95–105. doi: 10.1093/gerona/55.2.b95. [DOI] [PubMed] [Google Scholar]
- 15.Knuttgen HG. Strength training and aerobic exercise: comparison and contrast. J Strength Cond Res. 2007;21:973–978. doi: 10.1519/R-505011.1. [DOI] [PubMed] [Google Scholar]
- 16.Astorino TA. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women? J Sports Med Phys Fitness. 2000;40:209–216. [PubMed] [Google Scholar]
- 17.Kieser W. Training für Frauen. München: Knauer Ratgeber Verlag; 2003. [Google Scholar]
- 18.Pratley R, Nicklas B, Rubin M, Miller J, Smith A, Smith M, Hurley B, Goldberg A. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;76:133–137. doi: 10.1152/jappl.1994.76.1.133. [DOI] [PubMed] [Google Scholar]
- 19.Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains resting metabolic rate despite weight loss in postmenopausal women. J Appl Physiol. 1995;79:818–823. doi: 10.1152/jappl.1995.79.3.818. [DOI] [PubMed] [Google Scholar]
- 20.Cullinen K, Caldwell M. Weight training increases fat-free mass and strength in untrained young women. J Am Diet Assoc. 1998;98:414–418. doi: 10.1016/S0002-8223(98)00094-7. [DOI] [PubMed] [Google Scholar]
- 21.Carpinelli RN, Otto RM, Winett RA. A critical analysis of the ACSM position stand on resistance training: insufficient evidence to support recommended training protocols. JEP online. 2004;7:1–60. [Google Scholar]
- 22.van Aggel-Leijssen D, Saris WH, Wagenmakers AJ, Hul GB, van Baak MA. The effect of low intensity exercise training on fat metabolism of obese women. Obes Res. 2001;9:86–96. doi: 10.1038/oby.2001.11. [DOI] [PubMed] [Google Scholar]
- 23.Durnin J, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 24.Weir JB. New methods for calculating metabolic rate with special references to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson JA, Bryant CX, Peterson SL. Strength training for women. Champaign IL: Human Kinetics; 1995. [Google Scholar]
- 26.Melanson EL, MacLean PS, Hill JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc Sport Sci Rev. 2009;37:93–101. doi: 10.1097/JES.0b013e31819c2f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashley CD, Bishop P, Smith JF, Reneau P, Perkins C. Menstrual phase effects on fat and carbohydrate oxidation during prolonged exercise in active females. JEP online. 2000;3:67–73. [Google Scholar]
- 28.Calles-Escandón JP, Arciero J, Gardner AW, Bauman C, Poehlman ET. Basal fat oxidation decreases with aging in women. J Appl Physiol. 1995;78:266–271. doi: 10.1152/jappl.1995.78.1.266. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen MD. Strength training and adiposity in premenopausal women: strong, health, and empowered study. Am J Clin Nutr. 2007;86:566–572. doi: 10.1093/ajcn/86.3.566. [DOI] [PubMed] [Google Scholar]
- 30.Arner P, Kriegholm E, Engfeldt P. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990;85:893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willamson DL, Kirwan JP. A single bout of concentric resistance exercise increases basal metabolism rate 48 hours after exercise in healthy 59–77-years old men. J Ger Med Sci. 1997;52:352–355. doi: 10.1093/gerona/52a.6.m352. [DOI] [PubMed] [Google Scholar]
- 32.Melanson EL, Cornier MA, Bessesen DH, Grunwald GK, MacLean PS, Hill JO. 24 H metabolic responses to low-and high-intensity exercise in lean and obese humans. Obes Res. 2006;14:180–182. [Google Scholar]
- 33.Lemmer JT, Ivey FM, Ryan AS, Martel GF, Hurlbut DE, Metter JE, Fozard JL, Fleg JL, Hurley BF. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001;33:532–541. doi: 10.1097/00005768-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Jamurtas A, Koutedakis Y, Paschalis V, Tofas T, Yfanti C, Tsiokanos A, Koukoulis G, Kouretas D, Loupos D. The effects of a single bout of exercise on resting energy expenditure and respiratory exchange ratio. Eur J Appl Physiol. 2004;92:393–396. doi: 10.1007/s00421-004-1156-8. [DOI] [PubMed] [Google Scholar]
- 35.Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Consitt LA, Bloomer RJ, Wideman L. The effect of exercise type on immunofunctional and traditional growth hormone. Eur J Appl Physiol. 2007;100:321–330. doi: 10.1007/s00421-007-0431-x. [DOI] [PubMed] [Google Scholar]
- 37.Hautala AJ, Kiviniemi AM, Makikallio TH, Kinnunen H, Nissila S, Huikuri HV, Tulppo MP. Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol. 2005;21:13–16. doi: 10.1007/s00421-005-0116-2. [DOI] [PubMed] [Google Scholar]
- 38.Kimura T, Matsumoto T, Akiyoshi M, Owa Y, Miyasaka N, Aso T, Moritani T. Body fat and blood lipid postmenopausal women are related to resting autonomic nervous system activity. Eur J Appl Physiol. 2006;97:542–547. doi: 10.1007/s00421-006-0207-8. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich DF, Ackermann-Liebrich U, Schindler C, Barthélémy JC, Brändli O, Gold DR, Knöpfli B, Probst-Hensch NM, Roche F, Tschopp JM, von Eckardstein A, Gaspoz JM Sapaldia team. Effect of physical activity on heart rate variability in normal weight, overweight and obese subjects: results from the SAPALDIA study. Eur J Appl Physiol. 2008;104:557–565. doi: 10.1007/s00421-008-0800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]