Abstract
Objective
The aim of this study was to investigate the effect of hypothermia (H) on skeletal ischemia-reperfusion (IR) injury in rats by measuring malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), nitric oxide (NO), and interleukin-1 beta (IL-1β) in muscle, and measureing immunohistochemical-inducible nitric oxide synthase (iNOS) staining of skeletal muscle.
Materials and Methods
Eighteen Wistar Albino rats were divided randomly into three groups (sham, IR, hypothermia) (n=6). The sham group had all procedures without the IR period. The lower right extremity of rats in the IR and hypothermia groups was subjected to 2 hours of ischemia and 22 hours of reperfusion by applying a clamp on the common iliac artery and a rubber-band at the level of the lesser trochanter under general anesthesia. Rats in the hypothermia group underwent 4 hours of hypothermia during the first four hours of reperfusion in addition to a 2-hour ischemia and 22-hour reperfusion period. All rats were sacrificed at end of the IR period using a high dose of anesthesia. The tibialis anterior muscles were preserved. Immunohistochemical iNOS staining was performed, and MDA, SOD, GSH-Px, NO, and IL-1β were measured in the muscle.
Results
The level of MDA, NO, and IL-1β in muscle was increased in the IR group compared with that in the sham group, but these parameters were decreased in the hypothermia group compared with the IR group. The activities of SOD and GSH-Px in muscle were decreased in the IR group; however, these parameters were increased in the hypothermia group. The score and intensity of iNOS staining of skeletal muscle was dens in IR group, mild in hypothermia group, and weak in sham group.
Conclusion
The present study has shown that hypothermia reduced IR injury in the skeletal muscle by decreasing the levels of MDA, NO, and IL-1β, and increasing the activities of SOD and GSH-Px. In addition, hypothermia attenuated the score and intensity of iNOS staining.
Keywords: IR injury, hypothermia, MDA, NO, IL-1β, SOD, GSH-Px, iNOS staining
1 Introduction
Ischemia-reperfusion (IR) injury means that after an ischemic period, oxidative and nitrosative substances emerge in local and distant organs during the reperfusion period. Skeletal muscle IR injury may result from fracture, joint dislocation, vascular trauma, amputation/replantation, transplantation free vascular flap, vascular thrombus-emboli, and tourniquet use during orthopedic surgery [1].
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced during the reperfusion period. Superoxide anions (O-2), molecules that initiate ROS, might be turned into more active and toxic forms of ROS, such as the hydroxyl radical (HO-) or hydrogen peroxide (H2O2), and peroxynitrite (ONOO-) in the presence of nitric oxide (NO) [2]. These molecules result in lipid peroxidation as measured by MDA, protein oxidation, and fragmentation of deoxyribonucleic acid. Therefore, structural integrity, transport capacity, and energy production are disrupted [3].
Antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) preserve the cells by scavenging the free oxygen radicals under conditions of oxidative stress. However, antioxidant enzymes of organ can not overcome the rapid-onset oxidative stress during IR injury [4].
Some studies have shown that local hypothermia during the ischemic period reduces the tissue inflammatory response and edema [5]. Wright et al reported that local hypothermia during reperfusion and controlled reperfusion decreased tissue edema and acidosis in IR injury [6]. Another study reported that hypothermia reduced oxygen consumption, muscle damage, and size of infarct in canine gracilis after 6 hours of ischemia [7]. Arian et al. found that hypothermia during early reperfusion was more effective than hypothermia in ischemia for decreasing neutrophil infiltration and CD11b expression, muscle edema, and nitrobluetetrazolium staining [8].
We hypothesized that hypothermia attenuates tissue damage during the ischemia-reperfusion period. The aim of the present study was to investigate the mechanism of effects of hypothermia on skeletal muscle IR injury by measuring antioxidant enzymes, MDA, NO, IL-1β, and iNOS staining of muscle.
2 Materials and methods
Animal experiments were approved by the Animal Ethics Committee of our hospital (GATA Ethics Committee). Eighteen Wistar albino rats (weight range 250–320 gr) were divided into three groups: sham, IR, and hypothermia (n=6). They were housed in standard laboratory conditions. All rats were fed a standard diet and water; saved in a frame house with same temperature and 12 hours of dark-light period.
2.1 The Ischemia-Reperfusion Model
The intramuscular rodent anesthetic mixture (dose, 85 mg/kg and 12.5 mg/kg) was injected for anesthesia in all rats (ketamine and xylazine 150:30 mg/mL). Anesthesia was continued with administration of extra doses of the mixture during the procedure. The inguinal region was then shaved and skin cleaned with a 10% solution of povidone-iodine (Betadine; Purdue Products, Stamford, CT). Body temperature was monitored with a rectal probe and maintained at approximately 31.2–34.5°C with a surgical lamp. The common iliac artery was clamped by an incision over medial site of the right hind extremity. In addition, a rubber arterial tourniquet was applied at the level of the trochanter lesser to block collateral blood flow. After the ischemia period, the clamp and tourniquet were removed, and the skin was sutured with 2.0 vicryl. All rats were exposed to 22 hours of reperfusion. After a total of 24 hours, the ischemia-reperfusion period was complete; rats were sacrificed using a high dose of anesthesia. The sham group underwent all procedures except the IR period. The IR group had 2 hours of ischemia and 22 hours reperfusion. The hypothermia group was exposed to 4 hours of local hypothermia in addition to 2 hours of ischemia and 22 hours of reperfusion.
2.2 Hypothermia Model
We used a wooden water tank for our hypothermia model. The upper surface of the water tank was covered by glass with a hole. The tank was filled up with water, with a 3-mm gap provided under the glass to avoid excess decrease in body temperature. The right extremities of the six rats in the hypothermia groups were put in a latex glove, and then the rats were laid on the glass surface of the tank. The right lower extremities were extended from the holes into the tank water, which was maintained at 12±2 °C by addition of ice. Room temperature was maintained at 20° C during hypothermia. After 4 hours of local hypothermia, rats were taken to the cage under standard conditions.
2.3 Tissue Sampling
At the end of the 24-h I/R period, the skin of the right limb was incised and the tibialis anterior muscle was removed immediately. The tibialis anterior muscle samples were washed with physiologic serum. The frozen skeletal muscle tissues were homogenized in phosphate buffer (pH7.4) by a homogenizator (HeidolphDiax 900; Heidolph Elektro GmbH, Kelhaim, Germany). Whereas one part of the tissue samples was kept in 10% formalin solution for immunohistochemical iNOS staining, the other part was stored at −80°C for oxidant parameter studies.
2.4 Biochemical Analysis
The protein content of skeletal muscle was assayed by the Lowry method [9] with bovine serum albumin. The level of lipid peroxidation was assayed with a thiobarbituric acid (TBA) reaction using the Ohkawa method [10]. This method was utilized to provide a spectrophotometric measurement of the color produced during the reaction to TBA with malonyldialdehyde (MDA) at 535 nm. Therefore, 2.5 ml of 100 g/l trichloroacetic acid solution was added to 0.5 ml homogenate in each centrifuge tube and put in a boiling water bath for 20 minutes. The mixture was cooled and centrifuged at 1000g for 10 minutes. Then, 2 ml of the supernatant was added to 1 ml of 6.7 g/l TBA solution in a tube and put in a boiling water bath for 15 min. The solution was then cooled, and its absorbance was measured with a spectrophotometer (Shimadzu UV-1601; Kyoto, Japan). MDA levels were expressed as mmol/g of protein.
SOD activity was measured using the Sun nitrobluetetrazolium (NBT) method [11]. The stock solution contained 10 mg of CuZn-SOD from bovine liver dissolved in 10 ml of isotonic saline then diluted to 600 μg/l with distilled water before use. The SOD assay reagent included these reagents: 80 ml of 0.3 mmol/l xanthine solution, 40 ml of 0.6 mmol/l ethylenediaminetetraacetic acid (EDTA) solution, 40 ml 150 μmol/l NBT solution, 24 ml 400 mmol/l Na2CO3 solution, and 12 ml bovine serum albumin. The supernatants were exposed to ethanol-chloroform (62.5/37.5%) extraction before the assay of enzymatic activity. Then, 400 μl of ice-cold ethanol/chloroform mixture was mixed thoroughly with 250 μl of sample. After vortexing for 30 s and centrifugation at 3000g at 4 °C for 5 min, the upper aqueous layer was gathered. The gathered samples were diluted by a factor of 100, and 0.5 ml of the diluted solution was used for the assay by adding to 2.5 ml of SOD assay reagent. NBT was reduced to blue formazan by O2-, which has a strong absorbance at 560 nm. One unit (U) of SOD is expressed as the amount of protein that inhibits the rate of NBT reduction by 50%. The measured SOD activity was defined as U/g of protein.
GSH-Px activity was assayed by the method of Paglia and Valentine [12] in which GSH-Px activity was connected with the oxidation of NADPH by glutathione reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37°C. The action mixture consisted of 50 mmol potassium phosphate buffer (pH 7), 1 mmol EDTA, 1 mmol NaN3, 0.2 mmol NADPH, 1 mmol glutathione, and 1 U/ml of glutathione reductase. The absorbance at 340 nm was recorded for 5 min. The activity was defined as the slope of the lines as mmol of NADPH oxidized per minute., and the activity of GSH-Px was defined as U/g of protein.
Tissue NO levels were determined with an ion chromatograph (Dionex ICS-1000, Sunnyvale, CA, USA). Prior to NO measurements, supernatants were transferred through 0.45-μm pore membrane nitrocellulose filters. Anion and guard columns (AS-9HC/AG-9HC, CS12A/CG12A, Sunnyvale, CA, USA) and automated suppression were utilized. NOx levels were quantified using separate standard solutions for each ion and presented as g/g of protein [13]
Muscle IL-1β contents were assayed in duplicate with a commercially available enzyme-linked immune sorbent assay kit (Biosource, USA) following the manufacturer’s instructions.
2.5 Immunohistochemical iNOS staining
Tissue sections were exposed to deparafinization, hydration, and endogenous peroxidase blocking. Antigen retrieval was performed by Dako Target Retrieval Solution, pH6 (Dako, Carpinteria, CA), in a pressure cooker set at 95°C for 22 minutes, followed by gradual cooling for 20 minutes. Tissues were incubated for 30 minutes at normal room temperature with an anti-iNOS polyclonal antibody (dilution 1:75). The staining reaction was determined with an enzyme-conjugated polymer complex adapted for automatic stainers (Ventana Medical Systems, Tucson, AZ).
2.6 Statistics
All numeric data were analyzed first using the Kruskal-Wallis test to determine differences between the groups; the Mann-Whitney U test was employed to analyze two groups consecutively. The results were expressed as the mean±SEM; p<0.05 was considered statistically significant.
3 Results
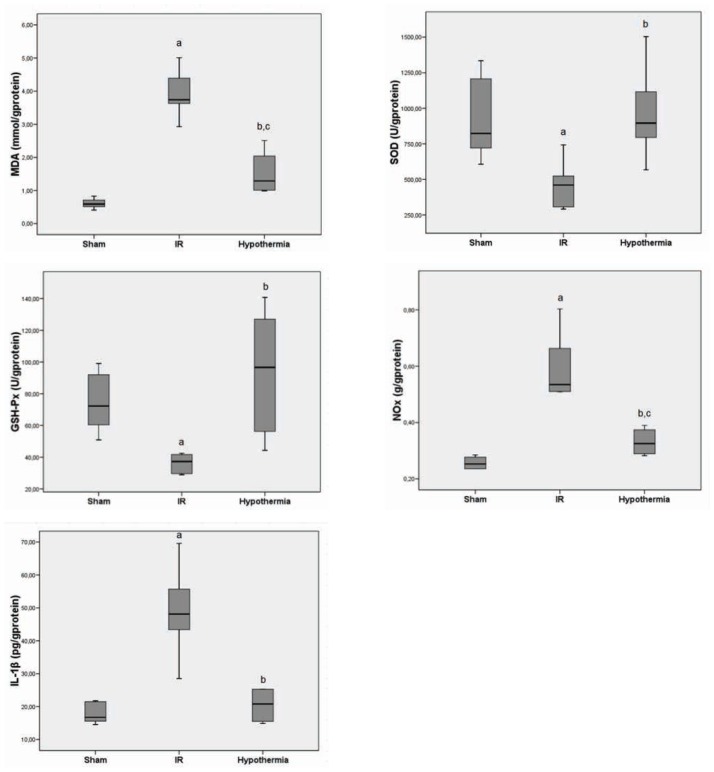
MDA indicated lipid peroxidation, NO, and IL-1β increased in the IR group compared with the sham group, but decreased in hypothermia group compared with the IR group (Figure 1).
Figure 1.
Muscle level of MDA, NOx, IL-1B, SOD, and GSH-Px in all groups (median±standart deviation).
aP< 0.05 compared between sham and I/R groups,
bP< 0.05 compared between I/R and hypothermia groups,
cP< 0.05 compared between sham and hypothermia groups.
Antioxidant enzymes, including SOD and GSH-Px, decreased in the IR compared with the sham group, but these enzymes increased in the hypothermia group compared with the IR group (Figure 1).
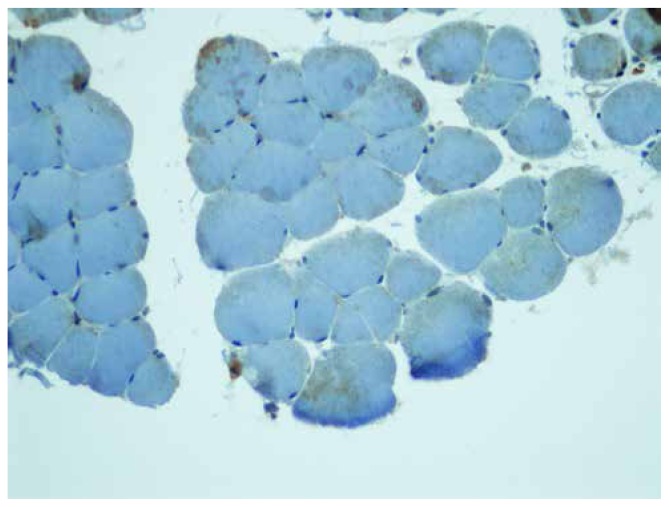
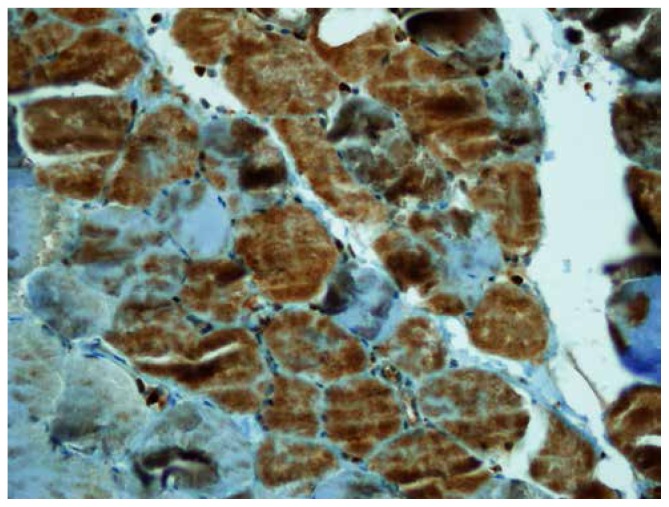
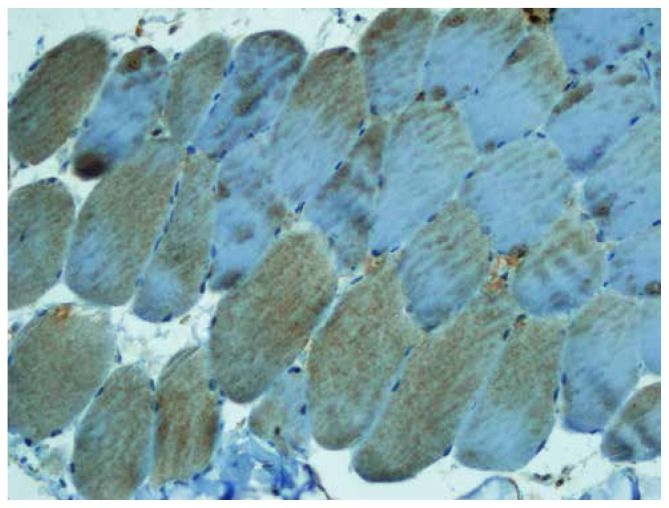
The mean values of iNOS staining scores and intensity in the IR group were much higher than those in the sham and hypothermia groups (Figure 2–3). Whereas the intensity of iNOS staining in the IR was very dense, its intensity in the hypothermia group was mild, and its intensity in the sham group was very weak (Figure 3–4).
Figure 2.
iNOS staing of sham group was very weak
Figure 3.
iNOS staing of IR was very dense
Figure 4.
iNOS staining of hypothermia group was mild
4 Discussion
The current study investigated and evaluated the effect of hypothermia on IR injury in skeletal muscle of rats. The results showed that hypothermia treatment during the early reperfusion period decreased tissue MDA, NO, and IL-1β levels, indicating a reduction in IR injury. Hypothermia also decreased the score, and the intensity of iNOS staining, indicating reduced peroxinitrite formation and thereby nitrosative injury.
Hypothermia has been routinely used to prolong the critical ischemia time during the ischemic period. However, the application of hypothermia during the reperfusion period was avoided, especially to prevent vasoconstriction. Recently, some studies have shown that hypothermia during the reperfusion period exerted a protective effect against IR injury [8,14]. Hypothermic reperfusion has been demonstrated to decrease the release of vasoactive neurotransmitter peptides and thus to decrease the hyperperfusion that is associated with membrane dysfunction, edema, increased metabolic activity, and excessive production of free radicals in brain tissue [15,16]. Wright et al. reported that hypothermic reperfusion reduced tissue edema formation and acidosis, oxygen consumption, and muscle infarct in skeletal muscle IR injury [7]. Mowlavi et al. demonstrated that hypothermic reperfusion in skeletal muscle decreased CD11b expression, neutrophil infiltration, local edema, and muscle damage by evaluating nitrobluetetrazolium staining [8]. Hypothermia achieves these effects through vasoconstriction of vessels and decreased vascular flow [7].
Previous studies recommended a temperature of local hypothermia of about 10°C [17]. Under these conditions, the body temperature should be between 30°C to 35°C [18]. If local hypothermia is lower than 10°C or the body temperature is lower than 30°C, hypothermia may increase the cell damage [15]. Local hypothermic exposure is effective between 30 minutes to 4 hours, according to the literature [7,19]. Therefore, we chose 10°C and 4 hours of hypothermia in early reperfusion period.
Oxidative stress related to IR injury results in lipid peroxidation of the plasma membrane, causing disruption of the cell membrane and finally leads to cell death. MDA is the main indicator and final product of lipid peroxidation. Therefore, increasing MDA in IR injury is well known in the literature [1]. Lei B et al [20]. reported that moderate hypothermia initiated after resuscitation reduced accumulation of lipid peroxidation (MDA) and increased antioxidant enzymes of SOD and GSH-Px in brain tissue. Dos Santos et al [21]. showed that hypothermia in reperfusion decreased the level of MDA, as indicated by thiobarbituric acid reactive species (TBARS) and increased catalase (CAT). However, they found no difference in levels of SOD, NO3 (nitrite), or NO2 (nitrate) between hypothermic or normothermic reperfusion in IR kidney injury. Grezzana Filho [22] indicated that hypothermia during reperfusion decreased TBARS levels and increased SOD and CAT in IR liver injury. In our study, the decrease in MDA levels in the hypothermia group compared with that in the IR group was consistent with the above studies that showed hypothermia reduced reperfusion injury. Different levels of SOD and GSH-Px during hypothermic reperfusion are reported in the literature. In our study, SOD and GSH-Px levels were increased during hypothermic reperfusion, similar to studies of Lei B et al. and Grezzana Filho, but in contrast to Dos Santos.
It was also reported that NO-induced cellular damage is attributable to a powerful and cytotoxic oxidant, ONOO−, that is generated by interaction of NO with the superoxide radical. Production of ONOO− (nitrosative stress) occurs almost instantaneously and causes the nitration of cellular proteins, with subsequent damage in tissues [23]. We determined the level of tissue NO to evaluate whether nitrosative stress had contributed IR injury on muscle tissue. Because NO and ONOO− are eventually converted to NO as measured with nitrite (NO2) and/or nitrate (NO3), NO levels are used as an indirect, but reliable, indicator for NO and ONOO− formation in vivo [24]. We found that tissue NO levels were significantly higher in muscle tissue subjected to an IR procedure. However, NO levels decreased in hypothermia group compared with IR group. In parallel with the NO levels, the extent and intensity of iNOS staining in the hypothermia group was lower than that in the IR group. It is more likely that hypothermia reduced the amount of superoxide by slowing down metabolism and thus reduced excessive formation of peroxynitrite by preventing the interaction of NO with superoxide radicals. Eventually, IR injury in muscle tissue may be attenuated through this mechanism. These results were similar to other studies that showed that hypothermia reduced NO and increased antioxidant enzymes in different tissues [20,25].
IL-1β is a proinflammatory cytokine that is increased during the ischemic-reperfusion period [26]. Zhang H et al [27] reported that post-ischemic mild hypothermia decreased the level of IL-1β in brain tissue. In this study, the increased level of IL-1β in the IR group was reduced with hypothermia during reperfusion in muscle tissue. This result was consistent with the above studies.
In conclusion, hypothermia during early reperfusion is safe and reduces IR injury by decreasing MDA, NO, and IL-1β, while increasing SOD, GSH-Px and diminishing the score and intensity of iNOS staining. This study presents strong evidence for potential clinical benefits of local hyperthermia during the early reperfusion period in tissues exposed the IR injury. Thus, local hypothermia can be used in orthopedic and reconstructive surgery, and routinely used in tourniquet- and trauma-associated IR injury.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Koca K, Yurttas Y, Bilgic S, Cayci T, Topal T, Durusu M, Kaldirim U, Akgul EO, Ozkan H, Yanmis I, Oguz E, Tunay S, Korkmaz A, Basbozkurt M. Effect of Preconditioned hyperbaric oxygen and ozone on ischemia-reperfusion induced tourniquet in skeletal bone of rats. J SurgRes. 2010 Nov;164(1):e83–89. doi: 10.1016/j.jss.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Taha MO, Simões MJ, Noguerol EC, Mendonça FP, Pascoalick HMA, Alves RAM, Vivian MEM, Morales FP, Campos ACA, Magalhães KG, Venerando PS, Tersariol ILS, Monteiro HP, Oliveira I, Jr, Jurkiewicz A, Caricati-Neto A. Effects of allopurinol on ischemia and reperfusion in rabbit livers. Transplant Proc. 2009 Apr;41(3):820–823. doi: 10.1016/j.transproceed.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 3.Scarfiotti C, Fabris F, Cestaro B, Giuliani A. Freeradicals, atherosclerosis, ageing, and related dysmetabolic pathologies: pathological and clinical aspects. Eur J Cancer Prev Suppl. 1997;1:31–36. doi: 10.1097/00008469-199703001-00007. [DOI] [PubMed] [Google Scholar]
- 4.Tunç T, Öter S, Güven A, Topal T, Kul M, Korkmaz A, et al. Protectiveeffect of sulfhydryl-containing antioxidants against ischemia/reperfusioninjury of prepubertal rat intestine. J Gastroenterol Hepatol. 2009;24:681–687. doi: 10.1111/j.1440-1746.2008.05673.x. [DOI] [PubMed] [Google Scholar]
- 5.Beris AE, Soucacos PN, Seaber AV, Urbaniak JR. Effects of cold ischemia on reflow patterns in the rat cremaster muscle microcirculation. Int Angiol. 1995 Sep;14(3):248–252. [PubMed] [Google Scholar]
- 6.Wright JG, Araki CT, Belkin M, Hobson RW. Postischemic hypothermia diminishes skeletal muscle reperfusion edema. J SurgRes. 1989;47(5):389–396. doi: 10.1016/0022-4804(89)90089-9. Now. [DOI] [PubMed] [Google Scholar]
- 7.Wright JG, Belkin M, Hobson RW. Hypothermia and controlled reperfusion: Twonon-pharmacological methods which diminish ischemia-reperfusion injury in skeletal muscle. Microcirc Endothelium Lymphatics. 1989 Jun-Oct;5(3–5):315–334. [PubMed] [Google Scholar]
- 8.Mowlavi A, Neumeister MW, Wilhelmi BJ, Song YH, Suchy H, Russell RC. Local Hypotermia during early reperfusion protects skeletal muscle from ischemia-reperfusion injury. Plast Recontr Surg. 2003 Jan;111(1):242–250. doi: 10.1097/01.PRS.0000034936.25458.98. [DOI] [PubMed] [Google Scholar]
- 9.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin-phenol reagent. J BiolChem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.Okhawa H, Ohshi N, Yagi K. Assay or lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Oberley LW, Ying L. A simple method for clinical assay of superoxidedismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 12.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 13.Koca K, Yurttas Y, Yıldız C, Çaycı T, Uysal B, Korkmaz A. Effect of hyperbaric oxygen and ozone preconditioning on oxidative/nitrosative stress induced by tourniquet ischemia/reperfusion in rat skeletal muscle. Acta Orthop Traumatol Turc. 2010;44(6):476–483. doi: 10.3944/AOTT.2010.2327. [DOI] [PubMed] [Google Scholar]
- 14.Canevari L, Console A, Tendi EA, Clarck JB, Bates TE. Effect of postischemia hypothermia on the mitochondrial damage induced by ischemia and reperfusion in the gerbil. Brain Res. 1999 Jan 30;817(1–2):241–245. doi: 10.1016/s0006-8993(98)01278-5. [DOI] [PubMed] [Google Scholar]
- 15.Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992 Oct;23(10):1454–1462. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- 16.Armstead WM, Mirro R, Busija DW, Leffler CW. Postischemic generation of superoxide anion by newborn pig brain. Am J Physiol. 1988 Aug;255(2 Pt 2):H401–3. doi: 10.1152/ajpheart.1988.255.2.H401. [DOI] [PubMed] [Google Scholar]
- 17.Sapega AA, Heppenstall RB, Sokolow DP, Graham TJ, Maris JM, Ghosh AK, Chance B, Lai Osterman. The bioenergetics of preservation in the limbs before reimplantation. J Bone Joint Surg Am. 1988 Dec;70(10):1500–1513. [PubMed] [Google Scholar]
- 18.Zhang JX, Wolf MB. Effect of cold on ischemia-reperfusion induced microvascular permeability increase in cat skeletal muscle. Cryobiology. 1994 Feb;31(1):94–100. doi: 10.1006/cryo.1994.1012. [DOI] [PubMed] [Google Scholar]
- 19.Laptook AR, Corbett RJ, Sterett R, Burns DK, Garcia D, Tollefsbol G. Modest Hypotermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res. 1997 Jul;42(1):17–23. doi: 10.1203/00006450-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lei B, Tan X, Cai H, Xu Q, Guo Q. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25:147–152. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 21.Dos Santos EB, Koff WJ, Grezzana Filho Tde J, De Rossi SD, Treis L, Bona SR, Pegas KL, Katz P, Meyer FS, Marroni NA, Corso CO. Oxidative stress evaluation of ischemia and reperfusion in kidneys under various degrees of hypothermia in rats. Acta Cir Bra. 2013 Aug;28(8):568–573. doi: 10.1590/s0102-86502013000800003. [DOI] [PubMed] [Google Scholar]
- 22.Grezzana Filho Tde J, Mendonça TB, Gabiatti G, Rodriques G, Marroni NA, Treis L, De Rossi SD, Corso CO. Topic liver hypothermia and ischemic preconditioning: a new model of ischemia and reperfusion in rats. Acta Cir Bras. 2011 Jun;26(3):194–201. [Google Scholar]
- 23.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996 Nov;271(5 Pt 1):C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Guven A, Gundogdu G, Uysal B, Cermik H, Kul M, Demirbag S, Ozturk H, Oter S. Hyperbaric oxygen therapy reduces the severity of necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2009 Mar;44(3):534–540. doi: 10.1016/j.jpedsurg.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons H, Sato TA, Draqunow M. Hypothermia suppresses inducible nitric oxide synthase and stimulates cyclooxygenase-2 in lipopolysaccharide stimulated BV-2 cells. Brain Res Mol Brain Res. 2003 Jan 31;110(1):63–75. doi: 10.1016/s0169-328x(02)00585-5. [DOI] [PubMed] [Google Scholar]
- 26.Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, Muratli SK. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. 2012 Jan;172(1):e39–46. doi: 10.1016/j.jss.2011.08.021. Epub 2011 Sep 13. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Zhou M, Zhang J, Mei Y, Sun S, Tong E. Therapeutic effect of post-ischemic hypothermia duration on cerebral ischemic injury. Neurol Res. 2008 May;30(4):332–6. doi: 10.1179/174313208X300279. [DOI] [PubMed] [Google Scholar]