Abstract
Background
Pain after arthroscopic shoulder surgery is often severe, and establishing a pain treatment regimen that does not delay discharge can be challenging. The reported ability of ketamine to prevent opioid-induced hyperalgesia has not been investigated in this particular setting.
Methods
300 adult patients scheduled for shoulder arthroscopy under general anesthesia were recruited for this observational clinical trial and were allotted to either receive 1mg/kg IV bolus of ketamine before surgery (ketamine group, KG) or to a control group (CG) without ketamine. NRS pain scores were obtained on the operative day and on postoperative days 1 and 2 and compared between groups. Secondary variables were blood pressure, heart rate, process times, satisfaction with the anesthetic and unwanted effects.
Results
Pain severity did not differ significantly between the groups at any time. Propofol injection rate and cumulative dose were higher in the KG. Heart rates and blood pressures were similar. Time to emergence and time in PACU were longer and vomiting was more frequent in patients given ketamine.
Conclusion
Preoperative low-dose ketamine added to a general anesthetic does not reduce perioperative pain after outpatient shoulder arthroscopy. It increases procedural times and the incidence of PONV.
Keywords: Ketamine, outpatient, day surgery, postoperative pain, general anaesthesia
1 Introduction
Arthroscopic shoulder surgery is a frequent orthopaedic procedure, with approximately 1.4 million performed annually worldwide according to the American Orthopaedic Society for Sports Medicine. It is often performed on an outpatient basis, and establishing satisfactory postoperative pain control can be a challenge, but is a key constituent of successful treatment and patient satisfaction. Although shoulder arthroscopy is often offered to the patients as a less painful procedure, pain intensity during the first 24 to 48 hours is actually comparable to that of open shoulder surgery. Inadequate analgesia leads to patient dissatisfaction, delayed postoperative rehabilitation, increased nausea and vomiting, and prolonged process times [1]. Where outpatient shoulder surgery is performed without regional anesthesia, establishing other adequate strategies for pain management is particularly important. Opioid-induced hyperalgesia (OIH) is now well recognized and has been shown to increase sensitivity to postoperative pain [2–4], and agents including ketamine have been studied for possible preemptive effects on OIH [5]. An outright recommendation of the use of ketamine to diminish OIH is still not possible due to the contradictory results of various clinical trials; with some affirming a preemptive analgesic effect [6–8] and others disputing it [9–12]. While trials using low-dose ketamine for analgesia have been carried out in a variety of surgical procedures, data investigating its use as an adjunct to outpatient arthroscopic shoulder surgery are not available.
Patients undergoing shoulder arthroscopy are usually positioned in the sitting or “beach chair” position, which increases the likelihood and severity of hypotensive episodes that require prompt treatment to maintain adequate cerebral perfusion [13–15]. As such we also investigated hemodynamic parameters to see if ketamine’s cardiostimulatory effects could be observed in patients under general anesthesia for shoulder arthroscopy [16].
The primary aim of this study was to investigate if the addition of ketamine to a total intravenous anesthetic with propofol and remifentanil for outpatient arthroscopic shoulder surgery, would reduce the incidence and severity of postoperative pain. The potential influences of ketamine on hemodynamic stability, process times (operating, emergence, PACU times) and patient satisfaction were also investigated.
2 Material and methods
This was a single-center observational clinical trial of adult patients undergoing elective outpatient shoulder arthroscopy. After local ethics committee approval and trial registration (Deutsches Register Klinischer Studien, DRKS-ID: DRKS00004907) a total of 300 male and female patients older than 18 years with informed written consent were recruited in the period January 2012 to January 2013. Exclusion criteria included consent refusal, NYHA classification ≥ III and/or ASA Score > III, body mass index (BMI) ≥ 35 and a contraindication for any of the medications used in the study. All patients completed the study and no patients were excluded after inclusion. The patients were pseudorandomized according to the day of the week of the operation into either a group given ketamine or a control group who were not given ketamine. This pseudorandomization, i.e. scheduling of the operation date was performed by personnel in the surgeon’s office who were not involved in and were unaware of the study. The patients were blinded to group allocation as were the investigator who performed the postoperative data collection interviews and data analysis.
General anesthesia was induced in both groups with a remifentanil bolus (1 μg/kg) followed by a propofol bolus injection (2 mg/kg over 2 minutes) and intubation was facilitated with vecuronium bromide (0.1 mg/kg). After intubation an anaesthesia nurse administered a 1 mg/kg bolus dose of racemic ketamine to the patients in the Ketamine Group (KG). Anesthesia was maintained with infusions of remifentanil (starting rate 0.2 μg/kg/min) and propofol (starting rate 6 mg/kg/h) that were titrated to maintain heart rate and mean arterial pressure within 20% of preoperative baseline values. Patients were positioned in the “beach chair” position. All procedures were carried out by the same experienced surgeon. All patients were given metamizole 2.5g, paracetamol 1g and parecoxib 40 mg intraoperatively for postoperative pain. Postoperatively, IV piritramide was given, if required, in 3 mg increments every 10 min in the post anaesthesia care unit (PACU) to achieve a numeric rating scale (NRS) score ≤ 3. Readiness for home discharge was assessed using the items of the Aldrete score: orientation to time and place, stable vital signs, no nausea or vomiting, tolerable pain with minor analgesics, ability to tolerate oral fluids, ability to stand and walk. The patients were given a prescription for ibuprofen (800 mg twice daily), metamizole (1 g four times daily) and tramadol (100 mg once daily). They were encouraged to resume normal activities as soon as possible and were reminded that they would be contacted by telephone on that evening as well as on the following two days and asked to answer questions regarding their recovery.
The patient characteristics were documented and compared to assess comparability of the two groups. Cumulative doses of ketamine, propofol, remifentanil and other administered medications were recorded. Pain intensity was assessed using the 11-point numerical rating scale (NRS) that ranges from 0 (no pain) to 10 (worst pain imaginable for the patient). The intraoperative courses of blood pressure and heart rate as well as process times were recorded. Pain scores and potential side effects, such as nausea and/or vomiting, postoperative shivering, sore throat, hoarseness and difficulties in swallowing were recorded in the PACU. The patients were then contacted by telephone at home on the evening after surgery (OP day) and on following two days (POD1 and POD2) by an investigator who was blinded to the patient’s anesthetic and questioned using a standardized interview. The patients were asked to describe their worst pain score in the period since the last telephone call, which was not necessarily the one that they were experiencing at that moment. They were asked to grade their satisfaction with the anesthetic on a six-point scale from 1 (very satisfied) to 6 (very unsatisfied).
3 Statistical analysis
Statistica10® (StatSoft Europe GmbH, Hamburg, Germany) software was used for all statistical calculations including sample size and power and determined that two groups of 80 patients would be required to detect a clinically relevant reduction of postoperative pain [6], with a power of 80% and a p-value < 0.05. To compensate for possible dropouts 150 patients were recruited for each group. Continuous data was tested for normal distribution with the Kolmogorov-Smirnov test. Normally distributed data were described by mean and standard deviation and compared by Student’s t-test for unpaired samples. Categorical data were given as absolute numbers and percentages, and analyzed by Pearson’s chi-squared or Fisher’s exact test depending on the number of categories. For all tests p < 0.05 was considered significant. Postoperative pain was considered the primary end point, secondary end point variables included; hemodynamic stability, process times and patient satisfaction.
4 Results
A total of 300 patients were enrolled and completed the study. The patients were divided equally into two groups. No patients were excluded after inclusion and all were available to answer the standardized interview. The two groups were similar in regard to demographic and morphometric characteristics (Table 1).
Table 1.
Patient Characteristics
| Ketamine group n=150 |
Control group n=150 |
p-value | ||
|---|---|---|---|---|
| Age | years | 52 ± 11 | 50 ± 11 | 0.29 |
| Sex | 0.24 | |||
| Male | n (%) | 82 (55) | 92 (61) | 0.24 |
| Female | n (%) | 68 (45) | 58 (39) | |
| Weight | kg | 82 ± 15 | 82 ± 14 | 0.68 |
| Height | cm | 173 ± 0 | 174 ± 0 | 0.25 |
| BMI | kg/m2 | 27 ± 4 | 27 ± 4 | 0.79 |
| PONV predictors | ||||
| PONV | n (%) | 48 (32) | 45 (30) | 0.71 |
| Travel sickness | n (%) | 20 (13) | 14 (9) | 0.27 |
| Smoker | n (%) | 43 (29) | 52 (35) | 0.26 |
| ASA classification | 0.21 | |||
| I | n (%) | 104 (69) | 112 (75) | |
| II | n (%) | 41 (27) | 37 (25) | |
| III | n (%) | 5 (4) | 1 (0) | |
Continuous variables are presented as mean ± standard deviation (SD) and categorical variables are presented as counts (percentage). ASA classification=American Society of Anesthesiologists physical status classification system: 1=Healthy person, 2=Mild systemic disease, 3=Severe systemic disease, BMI=body mass index, PONV=postoperative nausea and vomiting.
4.1 Perioperative medication
Infusion rates and total doses of the drugs administered perioperatively are given in Table 2. The infusion rate of propofol and the total dose were significantly higher in the ketamine group. The infusion rate of remifentail was the same in both groups but a higher total dose was administered in the ketamine group due to the longer duration of surgery (see below). The infused volume of crystalloid solutions did not differ between the two groups. A similar number of patients required the intraoperative administration of theodrenaline/cafedrine (Akrinor®) to treat hypotension and the required doses did not differ significantly. The number of patients requiring opioid medication for postoperative pain and the administered doses were the same in both groups (Table 2).
Table 2.
Perioperative Drugs and Fluids
| Ketamine group n=150 |
Control group n=150 |
p-value | ||
|---|---|---|---|---|
| Ketamine | ||||
| Dose | mg | 80 ± 18 | 0 | |
| Propofol | ||||
| Infusion rate | mg/kg/h | 8.8 ± 6.6 | 6.6 ± 5.9 | 0.002 |
| Total dose | mg | 518 ± 344 | 467 ± 169 | 0.01 |
| Remifentail | ||||
| Infusion rate | μg/kg/min | 0.15 ± 0.03 | 0.15 ± 0.03 | 0.13 |
| Total dose | μg | 828 ± 344 | 727 ± 342 | 0.01 |
| Theodrenaline/cafedrine (Akrinor®) | ||||
| Patients requiring | n (%) | 99 (67) | 96 (64) | 0.72 |
| Administered dose | ml | 1.3 ± 0.8 | 1.4 ± 0.9 | 0.49 |
| Piritramide | ||||
| Patients receiving | n (%) | 65 (43) | 61 (41) | 0.64 |
| Administered dose | mg | 10.1 ± 6.9 | 9.8 ± 4.7 | 0.38 |
| Infused fluids | ||||
| Crystalloids | ml | 500 ± 0 | 498 ± 20 | 0.32 |
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as counts (percentage).
4.2 Perioperative pain incidence and intensity
The data regarding pre-existing and postoperative pain are given in Table 3. The majority of patients reported having shoulder pain prior to surgery. The incidence as well as the reported severity of this pain did not differ between the groups. Approximately one third of patients in both groups reported having taken analgesic medication prior to surgery on a regular basis ( KG: n=54 patients (36%), CG: n=46 (31%); p=0.30). and the majority (90%) of patients in both groups followed the pain treatment schedule prescribed at discharge (KG: 134 patients (89%), CG: 135 patients (90%), p=0.85). The incidence of perioperative pain did not differ significantly between the two groups, either at PACU admission or during the course of the day of surgery (POD O). Eighty-eight patients (59%) in the KG and 79 patients (53%) in the CG reported pain (p=0.30). The intensity did not differ between the groups (KG: 5.1 ± 2.5 points vs. CG: 4.8 ± 2.3; p=0.45; Table 3).
Table 3.
Pain course
| Ketamine group n=150 |
Control group n=150 |
p-value | ||
|---|---|---|---|---|
| Patients with pain before surgery | n (%) | 137 (91) | 143 (95) | 0.16 |
| Intensity of this pain | NRS | 7.0 ± 1.8 | 7.1 ± 1.6 | 0.80 |
| Regular use of pain medication prior to surgery | n (%) | 54 (36) | 46 (31) | 0.31 |
| Patients with pain on evening after surgery | n (%) | 88 (59) | 79 (53) | 0.30 |
| Intensity POD 0 | NRS | 5.1 ± 2.5 | 4.8 ± 2.3 | 0.45 |
| Pain on POD 1 | n (%) | 102 (68) | 91 (61) | 0.28 |
| Intensity | NRS | 5.0 ± 2.4 | 4.6 ± 2.4 | 0.24 |
| Pain on POD 2 | n (%) | 106 (71) | 88 (59) | 0.07 |
| Intensity | NRS | 4.5 ± 1.8 | 4.5 ± 1.6 | 0.80 |
| Complied with prescribed postoperative analgesic regimen | n (%) | 134 (89) | 135 (90) | 0.85 |
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as counts (percentage). NRS: Numeric rating scale - 0 = no pain, 1–3 = Mild Pain (nagging, annoying, interfering little with activities of daily living), 4–6 = Moderate Pain (interferes significantly with activities of daily living, 7–10 = Severe Pain (disabling; unable to perform activities of daily living) POD: post operative day
On postoperative day 1 similar numbers of patients in each group reported pain of a comparable maximum severity. There was a statistically non-significant tendency toward a larger number of patients reporting pain in the ketamine group on the second day (POD2) after surgery (71% vs. 59%, p = 0.07) (Table 3).
4.3 Hemodynamics
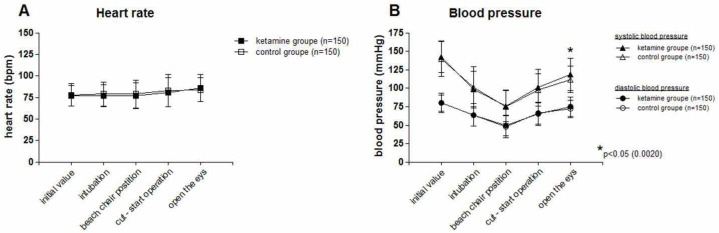
Heart rates were nearly identical in both groups (Figure 1). Blood pressures behaved in a similar fashion except that they were slightly higher in the ketamine group at emergence from anesthesia (Figure 1).
Figure 1A and B.
Blood pressure and heart rate.
A: Heart rate. No statistically significant difference
B: Blood pressure. No significant difference in systolic and diastolic blood pressures between groups until emergence. Systolic blood pressure then slightly but significantly higher in the KG.
4.4 Process times
The time from the end of anesthesia induction until skin incision was the same in both groups. However, operative time (skin incision to closure) and consequently total anesthesia time, drug infusion time as well as time from end of surgery to patient extubation were significantly longer in the KG (Table 4). Patients of the ketamine group remained longer in the PACU (Table 4).
Table 4.
Process times
| Ketamine group n=150 |
Control group n=150 |
p-value | ||
|---|---|---|---|---|
| Surgical preparation time | min | 15 ± 5 | 15 ± 5 | 0.98 |
| Surgical time | min | 52 ± 28 | 42 ± 25 | 0.002 |
| Theater emergence time | min | 10 ± 5 | 8 ± 5 | 0.001 |
| Total anesthesia time | min | 92 ± 30 | 80 ± 28 | 0.0003 |
| Propofol/remifentanil infusion time | min | 72 ± 30 | 60 ± 28 | 0.0003 |
| PACU time | min | 129 ± 62 | 109 ± 52 | 0.003 |
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as counts (percentage).
“Ready for surgical preparation time”: time from end of anesthesia until beginning of surgical treatment;
“Surgical time”: time from incision to skin closure and dressing;
“Theater emergence time”: time from end of surgery until leaving the operating theatre;
“Total anaesthesia time”: anaesthesia control time;
“Propofol/remifentanil infusion time”: time from start to end propofol/remifentanil infusion
“PACU time”: time from arrival in the PACU to the eligibility for discharge
4.5 Patient satisfaction
The cumulative incidence of nausea on the day of surgery or the following days and the number of patients given ondansetron was the same in both groups (Table 5). Significantly more patients of the ketamine group vomited during this period than in the control group (Table 5). The incidence of postoperative shivering, urinary retention, sore throat, hoarseness, and difficulties in swallowing was the same in both groups (Table 5).
Table 5.
Patient satisfaction
| Ketamine group n=150 |
Control group n=150 |
p-value | ||
|---|---|---|---|---|
| Dreaming during surgery | n (%) | 11 (7) | 6 (4) | 0.27 |
| If YES, was it unpleasant? | n (%) | 1 (9) | 1 (17) | 0.64 |
| Nausea on day of surgery or on first postoperative day | n (%) | 36 (24) | 36 (24) | 1.00 |
| Vomiting on day of surgery or on first postoperative day | n (%) | 22 (15) | 11 (7) | 0.04 |
| Shivering directly after surgery | n (%) | 24 (16) | 23 (15) | 0.87 |
| Urinary retention on day of surgery or on following days | n (%) | 17 (11) | 12 (8) | 0.33 |
| Sore throat | n (%) | 20 (13) | 22 (15) | 0.74 |
| Duration | d | 1.4±0.8 | 1.4±0.6 | 0.95 |
| Hoarseness | n (%) | 36 (24) | 32 (21) | 0.58 |
| Duration | d | 1.6±0.8 | 1.4±0.7 | 0.35 |
| Difficulty swallowing | n (%) | 15 (10) | 17 (11) | 0.71 |
| Duration | d | 1.7±0.8 | 1.6±0.7 | 0.71 |
| Overall satisfaction with the anesthetic procedure | Scale of 1 to 6 | 1.8±0.8 | 1.8±0.9 | 0.55 |
| Would recommend this procedure to others | n (%) | 143 (95) | 142 (95) | 0.79 |
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as counts (percentage).
In the final interview the patients graded their satisfaction with the overall anesthetic procedure and stated if they would recommend it to others. No statistically significant difference was found between the groups, and both groups gave their anesthesia experience an average score of 1.8 on a scale of 1 (very satisfactory) to 6 (very unsatisfactory) (Table 5).
5 Discussion
The objective of this study was to investigate if ketamine injected preoperatively as part of general anesthesia incurred any benefits regarding perioperative pain incidence and intensity, hemodynamic parameters, process times and patient satisfaction for outpatient arthroscopic shoulder surgery.
Our results showed that perioperative pain did not differ significantly at any time between KG and CG. The propofol infusion rate and the total dose were higher in KG than in CG. Hemodynamics did not differ significantly. The operative times, emergence times, and PACU times were longer in KG and the incidence of vomiting was more frequent in this group as well.
The analgesic potential of ketamine (an NMDA antagonist) has received a significant amount of attention, with a possible role in preventing opioid-induced hyperalgesia (OIH) being put forward as a key analgesic mechanism [17]. Withdrawal of opioids at the end of surgery can precipitate OIH and although remifentanil is often favored in the outpatient setting it has been shown to increase OIH to a greater degree than other opioids [18]. OIH research in animals has shown that a sudden reduction in the plasma levels of μ-receptor-agonists can lead to a long-lasting enhancement of signal transmission (long-term potentiation or LTP) at the first synapse of the tractus spinothalamicus (main pain pathway) and to central sensitization [19]. As activation of NMDA receptors plays a key role in LTP [19] ketamine could theoretically interrupt this pathway. However reviews of clinical trials have been unable to substantiate this claim. Alain et al. concluded that insufficient evidence exists to recommend ketamine as a preemptive analgesic agent [20] and similarly Oliveira et al. stated that its preemptive analgesic role cannot be confirmed. He suggested that differences in statistical analysis may have led to the contradictory outcomes in the various studies [21]. We found that there was not only no reduction in the incidence of severity of perioperative pain incidence but that potentially a larger number of patients might have late postoperative pain following intraoperative administration of ketamine. However, the observed 12 percent point difference was not statistically significant with a p-value of 0.07. One reason that ketamine did not decrease pain scores in our study could have been that ketamine is not a useful analgesic as other trials have found, or that the actual severity of pain was too great. Our patients reported high average maximum pre-operative NRS scores of about 7 (i.e. severe pain, unable to perform activities of daily living). Ketamine may not be able to offer any benefits in the face of such severe pain and may only be suited for mild or moderate pain. Average postoperative maximum NRS scores of over 7 show that pain after shoulder arthroscopy should not be underestimated [14].
Since ketamine inhibits presynaptic noradrenaline reuptake (increasing plasma catecholamine concentrations) we also studied hemodynamic parameters (blood pressure and heart rate), particularly because patients were “beach-chaired”. Timm et al. demonstrated that combining ketamine with propofol at induction of general anesthesia led to greater hemodynamic stability but that this effect was lost when the propofol infusion rate exceeded 3 mg/kg/h [22]. The total propofol infusion rates used in our study were up to more than 8 mg/kg/h (i.e. markedly greater than the cut-off point defined by Timm et al.) and we did not observe any hemodynamic benefits of ketamine; a similar number of patients in both groups required pharmacological circulatory support to treat hypotension. We also studied the effect of ketamine on overall process times (i.e. operating, emergence and PACU times) which are particularly pertinent for outpatient surgery. We found that although the surgical team was always the same, the surgical time was significantly longer in the ketamine group. The two anesthetics were consistently performed on different days of the week but any connection between the duration of surgery and the day of the week is most highly unlikely.
Emergence was slightly longer in the patients of the ketamine group. This may be a direct ketamine effect or be due to the longer infusion times and higher infusion rates for propofol and remifentanil The latter may have been due to ketamine’s tendency to increase blood pressure and heart rate, which would have forced the anesthesiologist to increase the infusion rate of propofol and/or remifentanil.
Patients of the ketamine group required longer to achieve “home readiness” than controls. This may have been due to the greater cumulative doses of propofol and remifentanil received by KG and to the greater incidence of postoperative vomiting compared to patients of the control group. In addition, patients recovering from an anesthetic employing ketamine are often more agitated, which can prolong PACU stay.
6 Limitations
For institutional reasons, this study was conducted as a pseudorandomized study. The group assignment was made by the day of the week (KG: surgery on Tuesday and CG: surgery on Friday). The dates for surgery were assigned to the patients by personnel in the surgeon’s office who were not involved in the study. This is a less robust method than other means of randomization, but we had no influence on which patient was given which anesthetic, which precludes bias, and the patients as well as the investigator who conducted the postoperative telephone interviews and analyzed the data were blinded to group allocation.
7 Conclusions
In summary, our study demonstrated that the preoperative administration of low-dose ketamine to propofol-remifentanil anesthesia does not improve postoperative pain relief in patients undergoing outpatient arthroscopic shoulder surgery. Ketamine was associated with a higher incidence of postoperative vomiting. We do not recommend administering low-dose ketamine to patients undergoing outpatient arthroscopic shoulder surgery.
Acknowledgements
Competing interests: The study was financed by departmental funds, including the purchase of all devices and materials used in the study. During the past five years none of the authors have received any form of reimbursement or financial or non-financial support from a company that could gain or lose financially from the publication of this manuscript. None of the authors hold any stocks or shares in a company that would gain or lose financially from the publication of this manuscript. None of the authors are applying for any patents related to the content of the manuscript. There are no other competing financial or non-financial interests.
We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Footnotes
Conflict of interest: The authors assure that they have no connections to any company whose products are mentioned in the article or to any company producing competing articles.
References
- 1.Rawal N. Postdischarge complications and rehabilitation after ambulatory surgery. Curr Opin Anaesthesiol. 2008;21:736–742. doi: 10.1097/ACO.0b013e328316c152. [DOI] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 4.Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21:65–83. doi: 10.1016/j.bpa.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kohrs R, Durieux ME. Ketamine: Teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 6.Menigaux C, Guignard B, Fletcher D, Sessler DI, Dupont X, Chauvin M. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–612. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Ozyalcin NS, Yucel A, Camlica H, Dereli N, Andersen OK, Arendt-Nielsen L. Effect of pre-emptive ketamine on sensory changes and postoperative pain after thoracotomy: Comparison of epidural and intramuscular routes. Br J Anaesth. 2004;93:356–361. doi: 10.1093/bja/aeh220. [DOI] [PubMed] [Google Scholar]
- 8.Sen H, Sizlan A, Yanarates O, Emirkadi H, Ozkan S, Dagli G, et al. Comparison of gabapentin and ketamine in acute and chronic pain after hysterectomy. Anesth Analg. 2009;109:1645–1650. doi: 10.1213/ANE.0b013e3181b65ea0. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann KA, Klaschik M. Lack of pre-emptive analgesic effect of low-dose ketamine in postoperative patients. A prospective, randomised double-blind study. Schmerz. 2001;15:248–253. doi: 10.1007/s004820100059. [DOI] [PubMed] [Google Scholar]
- 10.Van Elstraete AC, Lebrun T, Sandefo I, Polin B. Ketamine does not decrease postoperative pain after remifentanil-based anaesthesia for tonsillectomy in adults. Acta Anaesthesiol Scand. 2004;48:756–760. doi: 10.1111/j.1399-6576.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 11.Lux EA, Haack T, Hinrichs K, Mathejka E, Wilhelm W. Ketamine racemate and fast track anaesthesia. Influence on recovery times and postoperative opioid needs. Anaesthesist. 2009;58:1027–1034. doi: 10.1007/s00101-009-1607-z. [DOI] [PubMed] [Google Scholar]
- 12.Reza FM, Zahra F, Esmaeel F, Hossein A. Preemptive analgesic effect of ketamine in patients undergoing elective cesarean section. Clin J Pain. 2010;26:223–226. doi: 10.1097/AJP.0b013e3181bff86d. [DOI] [PubMed] [Google Scholar]
- 13.Jeong H, Jeong S, Lim HJ, Lee J, Yoo KY. Cerebral oxygen saturation measured by near-infrared spectroscopy and jugular venous bulb oxygen saturation during arthroscopic shoulder surgery in beach chair position under sevoflurane-nitrous oxide or propofol-remifentanil anesthesia. Anesthesiology. 2012;116:1047–1056. doi: 10.1097/ALN.0b013e31825154d2. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27:889–894. doi: 10.1016/j.arthro.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Yadeau JT, Liu SS, Bang H, Shaw PM, Wilfred SE, Shetty T, et al. Cerebral oximetry desaturation during shoulder surgery performed in a sitting position under regional anesthesia. Can J Anaesth. 2011;58:986–992. doi: 10.1007/s12630-011-9574-7. [DOI] [PubMed] [Google Scholar]
- 16.Tweed WA, Minuck M, Mymin D. Circulatory responses to ketamine anesthesia. Anesthesiology. 1972;37:613–619. doi: 10.1097/00000542-197212000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–159. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: A systematic review. Anaesthesia. 2007;62:1266–1280. doi: 10.1111/j.1365-2044.2007.05221.x. [DOI] [PubMed] [Google Scholar]
- 19.Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- 20.Van Elstraete AC, Lebrun T, Sandefo I, Polin B. Are preemptive analgesic effects of ketamine linked to inadequate perioperative analgesia? Anesth Analg. 2004;99:1576. doi: 10.1213/01.ANE.0000137441.79168.C5. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira CM, Sakata RK, Issy AM, Garcia JB. Ketamine and preemptive analgesia. Rev Bras Anestesiol. 2004;54:739–752. doi: 10.1590/s0034-70942004000500016. [DOI] [PubMed] [Google Scholar]
- 22.Timm C, Linstedt U, Weiss T, Zenz M, Maier C. Sympathomimetic effects of low-dose S-(+)-ketamine. Effect of propofol dosage. Anaesthesist. 2008;57:338–346. doi: 10.1007/s00101-008-1331-0. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta S, Ghosh S, Rudra A, Kumar P, Maitra G, Das T. Effect of ketamine on bispectral index during propofol--fentanyl anesthesia: A randomized controlled study. Middle East J Anesthesiol. 2011;21:391–395. [PubMed] [Google Scholar]
- 24.Haas DA, Harper DG, Ketamine A review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–68. [PMC free article] [PubMed] [Google Scholar]