Abstract
Background
Cholinesterase inhibitors and glutamate blockers are commonly used for the treatment of cognitive impairment in Alzheimer’s disease. The aim was to evaluate the effects of rivastigmine and memantine alone or in combination in rats with scopolamine-impaired memory.
Method
5 groups of rats were used: control, scopolamine (model), model with rivastigmine, model with memantine, and model with both drugs. Active avoidance test was performed and the number of conditioned responses, unconditioned responses and intertrial crossing were recorded. Passive avoidance tests step-through with criteria latency of reaction 180 s in the light chamber and step-down with criteria latency of reaction 60 s on the platform were done.
Results
Control rats learned the task and kept it on memory tests. Scopolamine treated rats failed to perform it. The rivastigmine, memantine and its combination groups showed increased CRs during learning and memory retention tests. In both passive avoidance tests an increased latency of reaction was observed in the drug treated groups.
Conclusion
The combination of both drugs rivastigmine and memantine is more effective than the use of the single drug in cognitive impaired rats. Cholinesterase inhibitors and NMDA blockers may be combined in the treatment of different kind of dementias.
Keywords: rivastigmine, memantine, drug combination, scopolamine model, rats
1 Introduction
Of the five drugs that have been approved for symptomatic treatment of Alzheimer’s disease (AD), (tacrine, donepezil, rivastigmine, galantamine and memantine), most act via the inhibition of acetylcholinesterase (AChE) or butyrilcholinesterase (BuChE).
Rivastigmine, a carbamate derivative of physostigmine, is unique in blocking AChE. It induces the attachment of a carbamyl residue instead of performing the usual microsomal activity. Rivastigmine is therefore classified as a pseudo-irreversible inhibitor of AChE and BuChE [1].
The changes in acethylcholine (ACh) levels positively modulate glutamatergic function in animal models [2]. Memantine is a non-competitive NMDA glutamate receptor channel inhibitor that binds to the disocilpine site [3]. NMDA receptors play a unique and vital role in subcellular signaling. Calcium influx through NMDA receptors initiates signaling cascades important for both synaptic plasticity and cell survival; however, over-activation of these receptors leads to toxicity and cell death. More specifically, it has been found, that NMDA receptors located at the synapse stimulate cell survival pathways, while extra-synaptic receptors signal for cell death [4]. This interplay between synaptic and extra-synaptic NMDA receptors has been studied exclusively in cortical and hippocampal neurons [5]. A deficit in NMDA receptor-dependent neocortical plasticity has been found in patients with AD and transgenic rodents [6]. Synaptic scaling is a form of synaptic plasticity, that involves NMDA receptors and tends to restore neuronal activity to baseline levels was described by Watt et al, [7] and Turrigiano et al, [8]. Diffuse synaptic loss in AD should cause a compensatory increase (scaling) in the excitability of adjacent healthy neurons by increasing presynaptic glutamate release [9] and postsynaptic receptor density [10]. Memantine’s inhibitory ability has been shown to significantly improve behavioral symptoms of AD, and is currently used for treatment of moderate to severe AD [5].
The hypothesis that a combination of cholinergic and glutamanergic intervention could potentially provide a more effective therapy for AD. It has been suggested that the specific combination of an AChE inhibitor and the NMDA receptor inhibitor memantine might yield a synergistic effect [11].
The aim of this study was to evaluate and compare the effects of rivastigmine or memantine alone or in combination of the two, on rats with scopolamine-impaired memory.
2 Experimental procedures
All experiments were carried out according to the guidelines for the use of laboratory animals in EU and Bulgaria. Permission for the study was obtained by the Bulgarian Food Safety Agency No73/03.12.2012 and the Ethics Committee of the Medical University Plovdiv No3/05.07.2013.
2.1 Chemicals
Memantine hydrochloride (Sigma)
Rivastigmine tartrate (Aldrich)
(−) Scopolamine hydrochloride (Sigma)
2.2 Animals
This study used 40 male Wistar rats with body weights of 180–220g, divided into 5 groups (n=8). The animals were kept under standard laboratory conditions in an 08.00 – 20.00 h light/dark cycle and were provided with food and water ad libitum. Groups were divided according to the administered treatments, as follows: 1) saline 0.1 ml/100g b.w. i.p. + saline 0.1 ml/100g b.w. p.o.; 2) scopolamine 1mg/kg i.p. + saline 0.1 ml/100g b.w. p.o.; 3) scopolamine 1mg/kg i.p. + rivastigmine 2 mg/kg p.o.; 4) scopolamine 1mg/kg i.p. + memantine 10 mg/kg p.o. and 5) scopolamine 1mg/kg i.p. + rivastigmine 2 mg/kg p.o. + memantine 10/mg/kg p.o. Substances were administered once daily for the duration of the experiment, 60 minutes before testing.
We used the effective doses of rivastigmine and memantine, improving effects on learning and memory processes in our preliminary experiments on naïve rats. Scopolamine was used as a model of impaired learning and memory in a dose, effective in our experiments studying other cholinesterase inhibitors.
2.3 Behavioral tests
2.3.1 Active avoidance testing
An automatic reflex conditioner for an active avoidance “shuttle box” (Ugo Basile, Italy) was used. The rats were placed into one of the chambers. Learning sessions were performed daily over 5 consecutive days. Each session consisted of 30 trials with the following parameters: 6s light and buzzer (670 Hz and 70 dB), 3s 0.4 mA foot shock, 12s pause. A memory retention session was performed 7 days after the last learning session (12th day) with the same parameters, but with the absence of a foot shock. The following parameters were recorded automatically: 1 – number of conditioned responses (CR; avoidances); 2 – number of unconditioned responses (UCR; escapes from foot shock); 3 - number of intertrial crossings.
2.3.2 Passive avoidance testing (two passive avoidance tests were used)
An automatic set-up for a passive avoidance “step-through” test (Ugo Basile, Italy) was used in a wire cage with 2 separate light and dark compartments. The rat was placed into the light chamber. The test parameters were as follows: door closed for 6s, door opened to allow entry into darkened chamber for 12s, 9 sec later 0.4mA foot shock and door closed. Learning sessions were performed over 2 consecutive days, a short memory retention session was performed 24 hours later (3rd day), and a long memory retention session was performed on the 10th day. Memory retention tests were performed using the same parameters, except for the absence of a foot shock. Sessions consisted of 3 trials separated by 30 minute intervals. The learning criterion used was a latency of reaction of 180 ± 2 seconds staying in the light chamber.
An automatic set-up for a passive avoidance “step-down” test (Ugo Basile, Italy) was used in a wire-floor single cage with a round central plastic platform. The rat was placed gently on the plastic platform. Learning sessions consisted of 2 trials separated by a 60 minute interval. During each trial, electrical stimulation (0.4mA) was delivered to the wire floor for a duration of 10 s when the animal step down from the platform. Learning sessions were performed over 2 consecutive days, a short memory retention session was performed 24 hours later (3rd day), and a long memory retention session was performed on the 7th day. Memory retention tests were performed using the same parameters, but with the absence of a foot shock. A latency of reaction of 60 s (the rat was required to remain with all 4 paws on the platform for more than 60 s) was used as a criterion for learning and retention.
2.4 Statistical analysis
All observed parameters were expressed as means ± S.E.M. for each group. The comparison between groups was made using the repetitive measures ANOVA followed by a Tukey post-hoc test for multiple comparison on the Excel and Instat computer programs. A P-value of P<0.05 was considered representative of a significant difference.
3 Results
3.1 Effects on active avoidance test
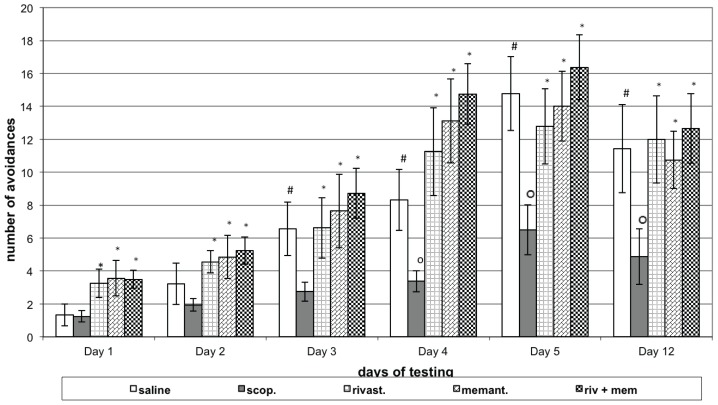
The control group of rats (saline) showed an increased number of conditioned responses (avoidances) on the 3rd, 4th, and 5th days of learning sessions and on the memory retention session of the 12th day (P<0.05), when compared to 1st day. The animals treated with scopolamine (model group) showed decreased number of avoidances (P<0.05) on the 3rd, 4th and 5th days’ learning sessions and during memory retention testing, compared to respective day control. Animals with model treated with rivastigmine showed increased number of avoidances on learning session (1st to 5th days) and on memory test (P<0.05) compared to the respective day scopolamine group. Rats with model treated with memantine also showed increased number of avoidances on learning session and on memory retention test ({<0.05) compared to the respective day of model group. Rats with model, treated with rivastigmine and memantine also showed increased number of avoidances on learning session and on memory test (P<0.05), compared with the respective day model group, but express only the tendency to increased it compared to rivastigmine or memantine alone groups (Figure 1A).
Figure 1A.
Effects of rivastigmine, memantine and combinatory treatment on active avoidance testing in rats with scopolamine-impaired memory.
Abscissa – days of testing; Ordinate – number of conditioned responses (CR; avoidances). OP<0.05 compared to respective day control group data; *P<0.05 compared to respective day scopolamine group data; #P<0.05 compared to Day 1 control group data.
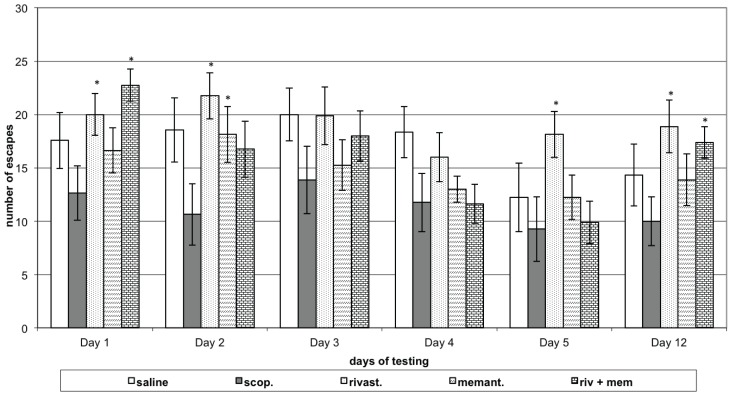
The saline group of rats did not show significant changes in the number of UCRs (escapes) during any of the five learning sessions or during retention testing compared to first day performance. Animals treated with scopolamine (model group) made relatively fewer escapes during all learning sessions and during retention testing, compared to respective day control group. Animals with scopolamine model, treated with rivastigmine showed a greater number of escapes on the 1st, 2nd and 5th days of learning sessions and on the retention test (P<0.05) compared to respective day model group. Rats with model, treated with memantine showed an increased number of escapes only on the 2nd day of learning sessions (P<0.05) when compared to the scopolamine group. Rats with model treated with rivastigmine and memantine in combination showed a greater number of escapes on the 1st day of learning sessions and on the memory retention test (P<0.05) when compared to respective day scopolamine group performance (Figure 1B).
Figure 1B.
Effects of rivastigmine, memantine and combinatory treatment on active avoidance testing in rats with scopolamine-impaired memory.
Abscissa – days of testing; Ordinate – number of unconditioned responses (UCR; escapes). *P<0.05 compared to respective day scopolamine group data;
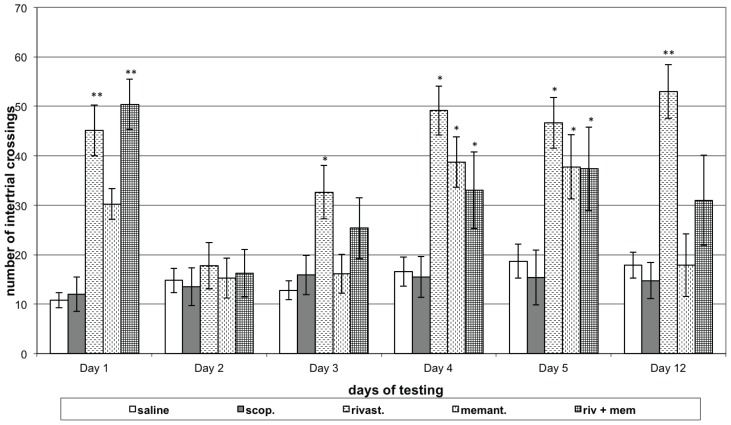
Neither the control nor the scopolamine (model) groups showed significant changes in the number of intertrial crossings during either the learning or memory retention test sessions, compared to 1st day of respective group. Rats with model, treated with rivastigmine showed an increased number of intertrial crossings on the 1st, 3rd, 4th and 5th days of learning session (P<0.05) and on the memory retention test (P<0.01), compared to respective day of model group. Rats with model, treated memantine showed an increased number of intertrial crossings on the 4th and 5th days of learning session (P<0.05), compared to respective day model group. Animals with model, treated with rivastigmine and memantine in combination made a greater number of intertrial crossings on the 1st (P<0.01), 4th and 5th days (P<0.05) of learning session, compared to the respective day of model group (Figure 1C).
Figure 1C.
Effects of rivastigmine, memantine and combinatory treatment on active avoidance testing in rats with scopolamine-impaired memory.
Abscissa – days of testing; Ordinate – number of intertrial crossings. *P<0.05 and **P<0.01 compared to respective day scopolamine group data;
3.2 Effects on step-through passive avoidance tests
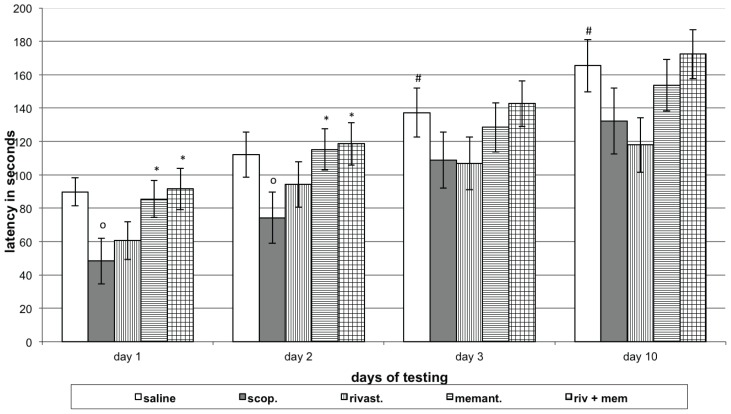
The control group of rats showed a prolonged latency of reaction (P<0.05) during both short- and long-term memory tests, compared to 1st day learning session. Animals in the scopolamine group (model group) showed a shortened latency of reaction (P<0.05) on the 1st and 2nd learning sessions, compared to respective day of controls. Rats with model, treated with rivastigmine do not change the latency of reaction on learning and memory retention, compared to the respective day of model group. Rats with model, treated with memantine, and rats with model, treated with the combination of the two (rivastigmine and memantine) showed a prolonged latency of reaction during the 1st and 2nd days of learning sessions (P<0.05), compared to respective day of model group (Figure 2).
Figure 2.
Effects of rivastigmine, memantine and combinatory treatment on step-through passive avoidance testing in rats with scopolamine- impaired memory.
Abscissa – days of testing; Ordinate – latency of reaction in seconds. OP<0.05 compared to respective day control group data; *P<0.05 compared to the respective day scopolamine group data; #P<0.05 compared to the Day 1 control group data.
3.3 Effects on step-down passive avoidance testing
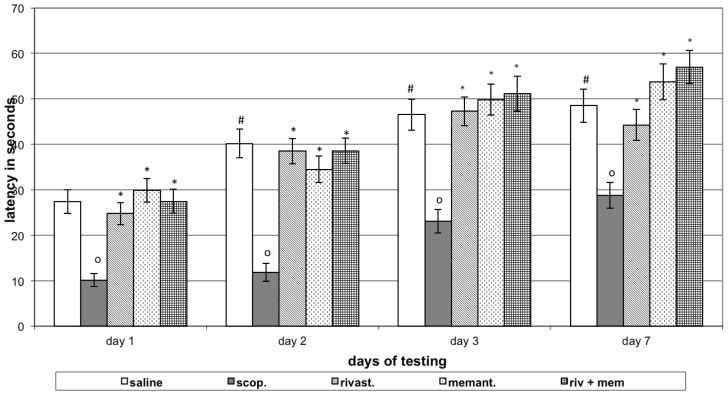
The control group of rats showed a prolonged latency of reaction (P<0.05) on the 2nd day of learning and on both short- and long-term memory retention tests, compared to 1st day. Animals with scopolamine (model group) demonstrated a shortened latency of reaction on two days learning, short- and long-term memory retention tests (P<0.05), compared to those days controls. Animals with model treated with rivastigmine showed a prolonged latency of reaction on the 1st and 2nd days of learning sessions and during short- and long-term memory retention test (P<0.05), compared to the same days model group. Rats with model treated with memantine showed prolonged latency of reaction (P<0.05) on learning and short and long memory retention tests, compared to the respective days of model group. Rats with model treated with the combination showed prolonged latency (P<0.05) on learning and on short and long memory tests, compared to the respective days of model group (Figure 3).
Figure 3.
Effects of rivastigmine, memantine and combinatory treatment on step-down passive avoidance testing in rats with scopolamine-impaired memory.
Abscissa – days of testing; Ordinate – latency of reaction in seconds. OP<0.05 compared to the respective day control group data; *P<0.05 compared to the respective day scopolamine group data; #P<0.05 compared to the Day 1 control group data.
4 Discussion
Our experiments suggest that control rats learned the task and keep it during memory tests in both active and passive avoidance tests. Rats with scopolamine-induced memory impairment failed to perform during learning and memory retention sessions of active avoidance test and during step-through and step-down passive avoidance tests.
Rivastigmine improved learning in active avoidance testing and performance during memory retention test of rats with scopolamine-impaired memory, as evidenced by the increases in observed CR and UCR values (Figure 1A, B and C). Rivastigmine also resulted in enhanced performance of rats with model of impaired memory in the step-down passive avoidance test, but do not change it in step-through test (Figure 2 and Figure 3).
Generally, rivastigmine enhances cholinergic function by prolonging the activity of endogenously released acetylcholine [1, 12]. There is evidence that an abundance of ACh will positively modulate glutamatergic function in animal models [2]. In acting on muscarinic receptors, ACh enhances the NMDA-mediated component of excitatory postsynaptic potentials [13]. Multiple lines of evidence suggest, that ACh might influence the excitability of cortical and hippocampal glutamatergic networks [14] and plays a prominent role on neocortical excitability through complex interactions with glutamatergic pathways.
In the present study, administration of memantine on rats with model of amnesia, improved performance during learning and memory retention sessions in active avoidance testing, as evidenced by the increase in CR values in the data (Figure 1A, B and C). Memantine administration also improved the latency of reaction during learning (step-through) and memory (both step-through and step-down) sessions (Figure 2 and Figure 3).
It is well known that amyloid-beta peptides (Aβ) observed in AD can trigger apoptotic cascades in neurons. Miguel-Hidalgo JJ et al, [15] found that memantine, an uncompetitive antagonist of NMDA receptors approved for the treatment of moderate to severe Alzheimer’s disease, can prevent neurodegeneration induced by intracranial Aβ (1–40) injection. The study hypothesized that memantine would prevent Aβ (1–40) induced cognitive impairment, neurodegeneration and apoptosis in hippocampal neurons of rats and improve performance in active avoidance testing. Based on their results, the authors concluded, that memantine can prevent amyloid-triggered expression of apoptosis-related markers and concomitant cognitive deficits. Others [16] found, that memantine prevents memory consolidation failure induced by soluble beta amyloid in rats.
Our results showed that the administration of rivastigmine and memantine in combination on rats with model of impaired memory, improved learning and the formation of memory in rats during active avoidance tests. The combination treatment also improved performance during the learning sessions of step-through testing and during both learning and memory retention sessions of step-down passive avoidance testing.
Jung JY et al, [17] studied the effects of cholinesterase inhibitors donepezil and galanthamine in combination with the NMDA receptor blocker memantine on sleep-wake architecture in rats. The study found that the combination of donepezil and memantine induced a significant increase in total wake duration as well as a decrease in total slow-wave sleep, REM sleep, and delta activity. Additionally, it was found that memantine administration alone increased sleep latency and motor activity. Based on these results, it was concluded that cholinesterase inhibitors might be useful as anti-dementia drugs that do not cause sleep disturbances. Others [18] establish that acetylcholinesterase inhibitors galantamine and donepezil, used in small doses fully reversed both methyllycaconitine- and scopolamine-induced cognitive dysfunction in mice.
Alzheimer’s disease is characterized by imbalances of various neurotransmitter systems long before the onset of symptoms. It has been proposed that a treatment plan which enhances both cholinergic and glutamatergic systems, implemented at the earliest stages of the disease, would be advantageous in counteracting the decline of cortical synaptic plasticity [19]. Our results are in favour of such hypothesis. The mechanism underlying the synergistic interaction between the two drugs is unclear. There are multiple neural substrates that might be involved in this interaction. One notable is the hippocampal excitatory circuit. This circuit receives tonic excitatory cholinergic input from the medial septum and the diagonal band of Broca. Glutamate, on the other hand, acts on NMDA receptors located on inhibitory GABAergic interneurons within the septum to inhibit the activity of cholinergic neurons that project to the hippocampus [12]. Consistent with this localization, behaviour and elecrophysiological studies have shown that memantine increases cholinergic signalling and excitability in the mouse hippocampus and that this action is blocked by the muscarinic antagonist scopolamine [20]. Therefore, the memory potentiation observed and documented in our study may be due to a cholinergic activation resulting from concomitant treatment with memantine and rivastigmine, acting through N and M cholinergic pathways in accordance with the hypothesis of Busquet et al [21].
Further investigation of the neurochemical alterations involved in these key cognitive areas of the brain may lead to complex implications about the mechanisms of learning and memory. One such implication is that the combination of a cholinesterase inhibitor with a glutamate antagonist may be a beneficial option for the treatment of AD [22]. Perhaps the use of small doses of rivastigmine and memantine in a multitargeted approach to treatment could overcome some of the major limitations of current drug therapies and provide such benefits as increased efficacy and a reduced incidence of adverse drug reactions.
Acknowledgement
The paper is part of project HO-03/2012 of MU-Plovdiv.
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- 1.Wilkinson D. Pharmacotherapy of Alzheimer’s disease. Alzheimer’s disease Psychiatry. 2005;4:43–47. [Google Scholar]
- 2.Kozhemyakin M, Rajasekaran K, Kapur J. Central cholinesterase inhibition enhances glutamate synaptic transmission. J Neurophysiology. 2010;103:1748–1757. doi: 10.1152/jn.00949.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Evola M, Young AM. Memantine and dizocilpine interactions with antinociceptive or discriminative stimulus effects of morphine in rats after acute or chronic treatment with morphine. Psychopharmacology. 2013;225(1):187–199. doi: 10.1007/s00213-012-2807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L. Opposing roles of synaptic and extrasynaptic NMDA receptor signalling in cultured striatal and cortical neurons. J Neurosci. 2012;21(12):3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tariot PN, Farlow MR, Grossenberg GT. Memantine treatment in patients with moderate to severe Alzheimer’s disease. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 6.Battaglia F, Wang HY, Ghilardi MF, Gahi E, Quartarone A, Friedman E. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Watt AJ, Syöström PJ, Häusser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- 8.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 9.Small GW, Bookheimer SY, Thompson PM, Cole GM, Huang SC, Kepe V, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161–172. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 11.Enz A, Gentsch C. C-administration of memantine has no effect in the in vitro or ex vivo determined acetylcholinesterase inhibition of rivastigmine in the rat brain. Neuropharmacology. 2004;47:408–413. doi: 10.1016/j.neuropharm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Csermansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H. Cholinesterase inhibitors ameliorate behavioural deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markram H, Segal M. Acetylcholine potentiates responses to NMDA receptors in the rat brain hippocampus. Neurosci Letters. 1990;113:62–65. doi: 10.1016/0304-3940(90)90495-u. [DOI] [PubMed] [Google Scholar]
- 14.Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miguel-Hidalgo JJ, Paul IA, Wanzo V, Banerjee PK. Memantine prevents cognitive impairment and reduces Bcl-2 and capsase 8 immunoreactivity in rats injected with amyloid β1–40. Eur J Pharmacol. 2012;692(5):38–45. doi: 10.1016/j.ejphar.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Tucci P, Mhillaj E, Morgese MG, Colaianna M, Zotti M, Schiavone S, et al. Memantine prevents memory consolidation failure induced by soluble beta amyloid in rats. Front Behav Neurosci. 2014;19(8):1–11. doi: 10.3389/fnbeh.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JY, Roh M, Ko KK, Jang HS, Lee SR, Ha JH. Effects of single treatment of anti-dementia drugs on sleep-wake patterns in rats. Korean J Physiol Pharmacol. 2012;16(4):231–236. doi: 10.4196/kjpp.2012.16.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderiambeloson E, Huyard B, Poirand E, Wagner S. Methyllycacoinite- and scopolamine-induced cognitive dysfunction: differential reversal effect by conginition-enhancing drugs. Pharmacol Res Perspect. 2014;2(4):1–8. doi: 10.1002/prp2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trebbastoni A, Gilio F, D’Antonio F, Cambieri C, Ceccanti M, de Lena C. Chronic treatment with rivastigmine in patients with Alzheimer’s disease: A study of primary motor cortex excitability tested by 5-Hz repetitive transcranial magnetic stimulation. Clinical Neurophysiology. 2012;123:902–909. doi: 10.1016/j.clinph.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Drever BD, Anderson WG, Johnson H, O’Callaghan M, Seo S, Choi DY. Memantine acts as a cholinergic stimulant in the mouse hippocampus. J Alzheimer’s Dis. 2005;2:319–333. doi: 10.3233/jad-2007-12405. [DOI] [PubMed] [Google Scholar]
- 21.Busquet P, Capurro V, Cavalli A, Piomelli D, Reggiani A, Bertolelli R. Synergistic effects of galantamine and memantine in attenuating scopolamine-induced amnesia in mice. J Pharm Sci. 2012;20:305–309. doi: 10.1254/jphs.12166sc. [DOI] [PubMed] [Google Scholar]
- 22.Wallace TL, Ballard TM, Pouzet B, Rieder WJ, Wettstein JG. Drug targets for cognitive enhacement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]