Abstract
Radiofrequency catheter ablation (RFA) has evolved as an effective treatment modality in patients with atrial fibrillation (AF). However, complication rates associated with RFA of AF have been cited in the range of 3.5 – 5% with the majority consisting of cardiac tamponade, vascular complications, and thromboembolic phenomena. In this review, the complications of AF ablation will be discussed along with associated clinical management strategies.
Introduction
Radiofrequency catheter ablation (RFA) has evolved as an effective treatment modality in patients with atrial fibrillation (AF). Despite advances in RFA techniques and technology, and attempts to increase awareness and optimize management of procedural complications, there has not been a substantial decrease in complication rates over the last decade. A recent survey conducted by Cappato et al that included 20,000 atrial fibrillation ablation procedures performed between 2003 and 2006 identified major complications in 4.5% of procedures;[1] this is not significantly decreased from the complication rate of 4.0% reported on a survey conducted by the same group between 1995 and 2002.[2] This lack of significant improvement is at least partly due to the higher proportion of older patients and those with significant comorbidities such as cardiomyopathy.[1] Overall, complication rates associated with RFA of AF have been cited in the range of 3.5 – 5%.[3,1,4] The distribution of complications has also remained similar over the last decade, with the majority consisting of cardiac tamponade, vascular complications, and thromboembolic phenomena.[3,1,4] In this report, the incidences and outcomes of major complications associated with catheter ablation of AF as well as potential strategies for their management will be reviewed.
Cardiac Tamponade
Perhaps the most significant of the bleeding complications associated with catheter ablation of AF in terms of frequency and morbidity is pericardial tamponade. This major complication has been reported in 0.5-2.4%[3,5,6] of cases. Although outcomes associated with pericardial tamponade when recognized and treated promptly are generally favorable, it remains the leading cause of mortality during catheter ablation of AF.,[7,3] Predictors of cardiac tamponade are not clear; however, prior left atrial catheter ablation may be an independent risk factor.[3]
With a recent shift in periprocedural anticoagulation strategy toward performing procedures with a therapeutic INR, there has been a growing concern for a potential increase in rates of tamponade. However, the incidence of tamponade has not been shown to be elevated with this anticoagulation strategy.[8,9] Preliminary data also suggests that the severity of this complication and outcomes when it does manifest are also not heightened in the presence of therapeutic anticoagulation.[10]
The hallmarks to management of tamponade are prompt recognition and treatment. Hypotension is often the initial finding suggesting a possible significant perforation. This can be quickly verified looking at the lack of excursion of the cardiac
silhouette in the left anterior oblique view fluoroscopically. An echocardiogram is helpful to confirm the effusion and guide emergent pericardiocentesis. Once a significant effusion is recognized, heparin is usually reversed using protamine. Patients therapeutic on warfarin may be considered for reversal with fresh frozen plasma or Factor VII complex, although it is not clear whether reversal of warfarin is associated with an improvement in clinical outcome. The majority of patients are successfully treated with percutaneous drainage and close inpatient monitoring. Patients with persistent accumulation of effusion or hemodynamic compromise not responding to initial drainage may require surgery.[5] Blood transfusion may also be required and autotransfusion has been reported as a safe option.[6]
A pericardial drain is usually left to dependent drainage with the patient monitored in an intensive care setting until drain output has decreased <30 cc in 24 hours. Repeat echocardiographic evaluation is often performed after temporarily occluding the drain for[6-12] hours. Due to the unpredictable level of anticoagulation, LMWH may not be preferable during reinitiation of anticoagulation in these patients. One approach is restarting warfarin prior to discharge. Since late recurrence of tamponade can occur, clinical follow-up with a repeat echocardiogram in 1-2 weeks may be appropriate.
Peripheral Vascular Complications
The most common peripheral vascular complications associated with catheter ablation are hematomas, pseudoaneurysms, and arteriovenous fistulas which are cumulatively reported in about 1-2% of cases.[3,11,1,4] Several studies have revealed that risk of vascular complications may be increased with female patients, older age, use of clopidogrel, and less experienced operators.[3,11,8,4] About half of pseudoaneurysms and fistulas ultimately require surgical intervention.[3]
Recent changes in anticoagulation protocols to perform RFA with a therapeutic INR have shown no increase in overall vascular complication incidence. In fact, minor bleeding complications may actually be decreased when the need for bridging with heparin or LMWH are avoided.[8]
Cerebrovascular Events
Recent studies have shown an overall incidence of cerebrovascular events including stroke and transient ischemic attack (TIA) ranging from 0.20% to 0.94%.[3,11,1,12] Several factors including chronicity of AF, left atrial size ≥ 4.5 cm, dislodgment of existing thrombus, and ablation-related char formation and tissue destruction can promote thrombogenicity.[13,14] Patients with CHADS2 scores ≥ 2 are five times more likely to experience a thromboembolic event when compared to those with a score of 0.13
Prior to the ablation procedure, patients should be maintained on anticoagulation if indicated by the American College of Cardiology/ American Heart Association/ European Society of Cardiology 2006 guidelines.[15] It is imperative that all patients receive anticoagulation for at least a period of 8 weeks post procedure regardless of their CHADS2 score due to the increased risk of thrombogenicity secondary to disruption of endothelial integrity during RF ablation and periprocedural cardioversion if performed.
During the ablation procedure, patients must be periodically assessed for evidence of neurologic abnormalities including asymmetric facial expression, slurred speech, and an inability to move extremities upon command. Asymptomatic “silent” cerebral embolism has been reported in >10% of patients after left atrial catheter ablation using diffusion-weighted magnetic resonance imaging despite rigorous periprocedural anticoagulation regimens.[16,17,18] The mechanism and clinical significance of these silent emboli and how best to prevent them remain to be determined. Maintenance of ACT > 300 seconds and continuous infusion through the sheaths during the procedure have been suggested to reduce the incidence of thromboembolic events.[19]
Di Biase et al. performed a prospective trial to evaluate whether anticoagulation strategy had an effect on the overall rate of cerebrovascular events.[8] Patients who had a therapeutic INR during the ablation procedure had a lower rate of periprocedural stroke compared to patients who had discontinued warfarin prior to the procedure (0.9% vs 0.0%) despite having a higher proportion of chronic AF and increased CHADS2 scores. No increase in pericardial effusions or other bleeding complications were noted.
Those patients who show evidence of cerebrovascular complications should immediately be evaluated to determine if tissue plasminogen activator (TPA) is appropriate. If TPA is administered, clinicians should be aware that the risk of vascular complications is significantly increased.
Although the overall risk of cerebrovascular events is low, additional modification of ablation strategies is necessary to further reduce this complication rate. Strict maintenance of ACT >300 seconds, careful sheath management, minimizing char formation by discontinuing radiofrequency energy in the setting of abrupt impedance rises, and possibly performing RFA on anticoagulation may further decrease the risk of embolic events.[14]
Pulmonary Vein Stenosis
The incidence of pulmonary vein (PV) stenosis has significantly decreased in recent years due to improvements in ablation technique. With antral PV isolation, current studies cite an incidence of PV stenosis <0.01% to 0.04%[3,1,12] as compared to 5% when ostial PV isolation was routinely utilized.[20] Inflammation and smooth muscle constriction secondary to RFA are thought to be mediators of post procedure PV stenosis.[21] Location of ablation lesions in the PV ostia and increasing size of lesions are the primary risk factors in the development of PV stenosis.[14]
Late diagnosis of PV stenosis can be especially challenging as most primary care physicians are unaware of this complication. Patients may present with nonspecific symptoms such as dyspnea, chest pain, wheezing, and hemoptysis. Patients are often subjected to a multitude of unnecessary tests leading to misdiagnosis including pulmonary embolism, pneumonia, and lung carcinoma.[22] If a clinician suspects PV stenosis post ablation, multidetector computed tomography and magnetic resonance imaging are often used to establish a diagnosis. If patients are found to have severe stenosis with symptoms, most centers recommend angioplasty with or without stenting.
Total PV occlusion can be the result of a slow and insidious progression from a previous insignificant narrowing; therefore balloon angioplasty with or without stenting should be considered for any vein with a cumulative stenosis index (CSI) ≥ 75% regardless of symptomology (CSI = sum of percent stenosis of the unilateral veins divided by the total number of ipsilateral veins).[23] Because dilation of total PV occlusion is less successful, clinicians may consider angioplasty of severely stenosed PV regardless of symptomology in order to avoid progression to vein occlusion.[23]
Changes in ablation strategy have significantly reduced the incidence of PV stenosis in recent years. However, PV stenosis is a debilitating complication that must always be considered during the ablation procedure. Electrophysiologists must ensure that ablation lesions are delivered on the atrial side of the pulmonary veins avoiding the PV ostia. If severe flow limitations are noted in the setting of symptoms, balloon angioplasty with or without stenting should be performed to alleviate symptoms. Currently, there is no consensus regarding treatment of severe PV stenosis without symptoms; although stenting should be considered in order to avoid total occlusion. Many patients with single PV occlusion are asymptomatic and should undergo surveillance; however, patients with concomitant ipsilateral stenosis and a CSI ≥75% require intervention to restore flow in order to prevent associated lung disease.
Phrenic Nerve Injury
Phrenic nerve (PN) injury is a rare complication (<0.01%) of RFA due to its close proximity to common ablation sites.[3,11] The right PN courses laterally and slightly posteriorly to the superior vena cava and right atrium and descends anteriorly to the right superior PV, while the left PN tracks closely to the left atrial appendage.[24] The right PN has an especially close relationship to the right superior PV and is the most commonly injured phrenic nerve during RFA.[25]
High output pacing and monitoring of fluoroscopy during RFA are imperative to avoid permanent PN injury. However, PN injury secondary to RFA appears to have a benign prognosis and patients usually experience improvement in function over time (1-2 years).[26] Phrenic nerve injury is uncommon when ablation is performed outside the PV.
Atrio-Esophageal Fistula
Atrio-esophageal fistula (AEF) is an infrequent (<0.3%) however often fatal complication of RFA.[03,01,27] Due to the associated devastating consequences and mortality rate >80%, great care must be taken to avoid this catastrophic complication.[27]
The esophagus is a mobile structure with fixed points in the pharynx and gastroesophageal junction. As a result, movement within the thorax is dynamic, especially with peristalsis.[27] Several approaches have been suggested to prevent esophageal injury. One approach is real-time imaging of the esophagus with barium swallow or an esophageal probe and avoidance of RF applications in the immediate proximity of the esophagus.[29] Monitoring of intraesophageal temperature and termination of RF application after a critical rise in intraluminal temperature increase has also been suggested. However, with this approach intramural temperatures cannot be measured, and the sensor probe may not be close to the ablation site. Therefore, lack of a temperature rise may give a false sense of security and fistula has been reported after RFA using an intraesophageal temperature monitoring system.[30] Others have suggested limiting power and duration of RF applications in the posterior left atrium. However, it is not clear whether critical power settings ensure safety. Despite these safeguards, >25% of patients will exhibit structural changes in the mediastinum via endoscopy.[31] Patients with persistent AF, increased left atrial size, and ablation of the roof, coronary sinus, and mitral isthmus are more likely to develop esophageal ulceration.[32] The risk of atrioesophageal fistula may be higher during general anesthesia likely due to esophageal immobility.[33] Although acid reflux may develop after RFA,[34] the role of prophylactic proton pump inhibitors in prevention of fistula is unknown. A simple measure may be to administer a proton pump inhibitor prior to and for 2-4 weeks after ablation.
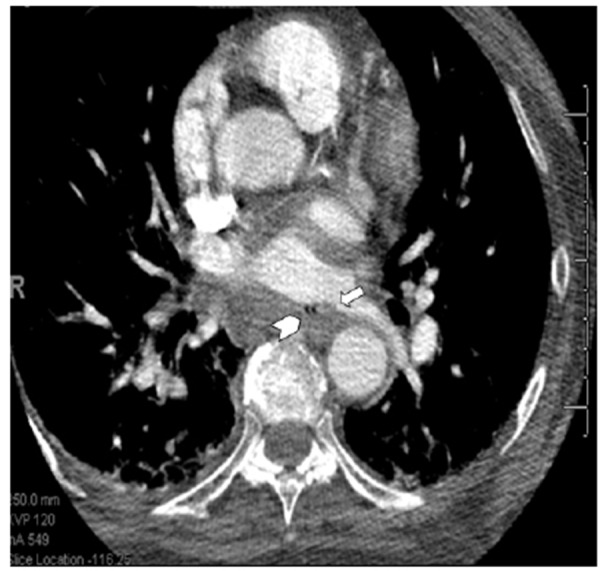
Nonspecific symptoms may result in delayed diagnosis of AEF. Patients may present with acute pericarditis, pneumonitis, sepsis, seizures, strokes, gastrointestinal bleeding, and circulatory collapse due to the intense inflammatory response resulting from atrial and esophageal tissue necrosis.[14] Any patient presenting with a swallowing difficulty, fever, neurological deficit, or sepsis after ter RFA must immediately undergo evaluation for AEF. Imaging of the mediastinum with CT or MRI with or without barium swallow with specific attention to the presence of air in the mediastinum, and integrity of the atrioesophageal wall is very helpful for establishing the diagnosis ([Figure 1]). Esophageal instrumentation with TEE or endoscopy is often considered contraindicated due to the risk of introduction of air and potential for embolization. Treatment often involves surgical repair; however, cases of stenting of the esophagus and keeping the patient NPO with appropriate antibiotic coverage have also been reported.[35] Surgical consultation must be obtained immediately.
Figure 1. Magnetic resonance imaging of a 66 year old woman presenting with atrio-esophageal fistula four weeks after atrial fibrillation ablation.
Death
Death is an infrequent complication of RFA for AF occurring in 1 of 1,000 patients.[7] Operators must be aware of the causes of death Table 1 and clinical decision making must be performed in order to decrease this risk whenever possible. The most common causes of mortality are tamponade with cardiac arrest (22%), atrioesophageal fistula (16%), stroke (9%), and massive pneumonia (6%).
Table 1. Potential Causes of Mortality Associated with Radiofrequency Atrial Fibrillation Ablation.
| Cardiac tamponade |
|---|
| Atrioesophageal fistula |
| Peripheral embolism |
| Stroke |
| Massive pneumonia |
| Myocardial Infarction |
| Intractable torsades de pointes |
| Septicemia |
| Sudden respiratory arrest |
| Extrapericardial pulmonary vein perforation |
| Occlusion of bilateral pulmonary veins |
| Hemothorax |
| Anaphylaxis |
| Asphyxia from tracheal compression secondary to subclavian hematoma |
| Intracranial bleeding |
| Acute respiratory distress syndrome |
| Esophageal perforation from intraoperative transesophageal echocardiography |
References
- 1.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 2.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Packer Douglas, Skanes Allan. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005 Mar 08;111 (9):1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 3.Baman Timir S, Jongnarangsin Krit, Chugh Aman, Suwanagool Arisara, Guiot Aurelie, Madenci Arin, Walsh Spencer, Ilg Karl J, Gupta Sanjaya K, Latchamsetty Rakesh, Bagwe Suveer, Myles James D, Crawford Thomas, Good Eric, Bogun Frank, Pelosi Frank, Morady Fred, Oral Hakan. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011 Jun;22 (6):626–31. doi: 10.1111/j.1540-8167.2010.01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spragg David D, Dalal Darshan, Cheema Aamir, Scherr Daniel, Chilukuri Karuna, Cheng Alan, Henrikson Charles A, Marine Joseph E, Berger Ronald D, Dong Jun, Calkins Hugh. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):627–31. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 5.Bunch T Jared, Asirvatham Samuel J, Friedman Paul A, Monahan Kristi H, Munger Thomas M, Rea Robert F, Sinak Lawrence J, Packer Douglas L. Outcomes after cardiac perforation during radiofrequency ablation of the atrium. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1172–9. doi: 10.1111/j.1540-8167.2005.50135.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao Ling-Yun, Tang Ri-Bo, Dong Jian-Zeng, Liu Xing-Peng, Long De-Yong, Yu Rong-Hui, Jiang Chen-Xi, Chen Gang, Sang Cai-Hua, Zhang Xin-Yong, Ning Man, Ma Chang-Sheng. Autotransfusion in the management of cardiac tamponade occurring during catheter ablation of atrial fibrillation. Chin. Med. J. 2010 Apr 05;123 (7):961–3. [PubMed] [Google Scholar]
- 7.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2009 May 12;53 (19):1798–803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase Luigi, Burkhardt J David, Mohanty Prasant, Sanchez Javier, Horton Rodney, Gallinghouse G Joseph, Lakkireddy Dhanunjay, Verma Atul, Khaykin Yaariv, Hongo Richard, Hao Steven, Beheiry Salwa, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Santangeli Pasquale, Wang Paul, Al-Ahmad Amin, Patel Dimpi, Themistoclakis Sakis, Bonso Aldo, Rossillo Antonio, Corrado Andrea, Raviele Antonio, Cummings Jennifer E, Schweikert Robert A, Lewis William R, Natale Andrea. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation. 2010 Jun 15;121 (23):2550–6. doi: 10.1161/CIRCULATIONAHA.109.921320. [DOI] [PubMed] [Google Scholar]
- 9.Mortada M Eyman, Chandrasekaran K, Nangia Vikram, Dhala Anwer, Blanck Zalmen, Cooley Ryan, Bhatia Atul, Gilbert Carol, Akhtar Masood, Sra Jasbir. Periprocedural anticoagulation for atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2008 Apr;19 (4):362–6. doi: 10.1111/j.1540-8167.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 10.Latchamsetty Rakesh, Gautam Sandeep, Bhakta Deepak, Chugh Aman, John Roy M, Epstein Laurence M, Miller John M, Michaud Gregory F, Oral Hakan, Morady Fred, Jongnarangsin Krit. Management and outcomes of cardiac tamponade during atrial fibrillation ablation in the presence of therapeutic anticoagulation with warfarin. Heart Rhythm. 2011 Jun;8 (6):805–8. doi: 10.1016/j.hrthm.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Bertaglia Emanuele, Zoppo Franco, Tondo Claudio, Colella Andrea, Mantovan Roberto, Senatore Gaetano, Bottoni Nicola, Carreras Giovanni, Corò Leonardo, Turco Pietro, Mantica Massimo, Stabile Giuseppe. Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multicenter prospective registry on procedural safety. Heart Rhythm. 2007 Oct;4 (10):1265–71. doi: 10.1016/j.hrthm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Patel Dimpi, Mohanty Prasant, Di Biase Luigi, Sanchez Javier E, Shaheen Mazen H, Burkhardt J David, Bassouni Mohammed, Cummings Jennifer, Wang Yan, Lewis William R, Diaz Alberto, Horton Rodney P, Beheiry Salwa, Hongo Richard, Gallinghouse G Joseph, Zagrodzky Jason D, Bailey Shane M, Al-Ahmad Amin, Wang Paul, Schweikert Robert A, Natale Andrea. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7 (2):167–72. doi: 10.1016/j.hrthm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Viles-Gonzalez Juan F, Mehta Davendra. Thromboembolic risk and anticoagulation strategies in patients undergoing catheter ablation for atrial fibrillation. Curr Cardiol Rep. 2011 Feb;13 (1):38–42. doi: 10.1007/s11886-010-0153-2. [DOI] [PubMed] [Google Scholar]
- 14.Dixit Sanjay, Marchlinski Francis E. How to recognize, manage, and prevent complications during atrial fibrillation ablation. Heart Rhythm. 2007 Jan;4 (1):108–15. doi: 10.1016/j.hrthm.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffery L, Antman Elliott M, Halperin Jonathan L, Hunt Sharon Ann, Nishimura Rick, Ornato Joseph P, Page Richard L, Riegel Barbara, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm A John, Dean Veronica, Deckers Jaap W, Despres Catherine, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais Joao, Osterspey Ady, Tamargo Juan Luis, Zamorano José Luis. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114 (7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 16.Lickfett Lars, Hackenbroch Matthias, Lewalter Thorsten, Selbach Stephanie, Schwab Jörg O, Yang Alexander, Balta Osman, Schrickel Jan, Bitzen Alexander, Lüderitz Berndt, Sommer Torsten. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation. J. Cardiovasc. Electrophysiol. 2006 Jan;17 (1):1–7. doi: 10.1111/j.1540-8167.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz Niko, Kuniss Malte, Nedelmann Max, Kaps Manfred, Bachmann Georg, Neumann Thomas, Pitschner Heinz-Friedrich, Gerriets Tibo. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm. 2010 Dec;7 (12):1761–7. doi: 10.1016/j.hrthm.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Gaita Fiorenzo, Caponi Domenico, Pianelli Martina, Scaglione Marco, Toso Elisabetta, Cesarani Federico, Boffano Carlo, Gandini Giovanni, Valentini Maria Consuelo, De Ponti Roberto, Halimi Franck, Leclercq Jean François. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. 2010 Oct 26;122 (17):1667–73. doi: 10.1161/CIRCULATIONAHA.110.937953. [DOI] [PubMed] [Google Scholar]
- 19.Ren Jian-Fang, Marchlinski Francis E, Callans David J. Left atrial thrombus associated with ablation for atrial fibrillation: identification with intracardiac echocardiography. J. Am. Coll. Cardiol. 2004 May 19;43 (10):1861–7. doi: 10.1016/j.jacc.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Saad Eduardo B, Marrouche Nassir F, Saad Cynthia P, Ha Edward, Bash Dianna, White Richard D, Rhodes John, Prieto Lourdes, Martin David O, Saliba Walid I, Schweikert Robert A, Natale Andrea. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann. Intern. Med. 2003 Apr 15;138 (8):634–8. doi: 10.7326/0003-4819-138-8-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Combes Nicolas, Boveda Serge, Lapeyre Matthieu, Marijon Eloi. Acute pulmonary vein stenosis after radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2009 May;20 (5):569–70. doi: 10.1111/j.1540-8167.2008.01368.x. [DOI] [PubMed] [Google Scholar]
- 22.Barrett Conor D, Di Biase Luigi, Natale Andrea. How to identify and treat patient with pulmonary vein stenosis post atrial fibrillation ablation. Curr. Opin. Cardiol. 2009 Jan;24 (1):42–9. doi: 10.1097/hco.0b013e32831bef70. [DOI] [PubMed] [Google Scholar]
- 23.Di Biase Luigi, Fahmy Tamer S, Wazni Oussama M, Bai Rong, Patel Dimpi, Lakkireddy Dhanunjaya, Cummings Jennifer E, Schweikert Robert A, Burkhardt J David, Elayi Claude S, Kanj Mohamed, Popova Lucie, Prasad Subramanya, Martin David O, Prieto Lourdes, Saliba Walid, Tchou Patrick, Arruda Mauricio, Natale Andrea. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: clinical implications after long-term follow-up. J. Am. Coll. Cardiol. 2006 Dec 19;48 (12):2493–9. doi: 10.1016/j.jacc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Fell S C. Surgical anatomy of the diaphragm and the phrenic nerve. Chest Surg. Clin. N. Am. 1998 May;8 (2):281–94. [PubMed] [Google Scholar]
- 25.Sánchez-Quintana Damian, Cabrera José Angel, Climent Vicente, Farré Jerónimo, Weiglein Andreas, Ho Siew Yen. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J. Cardiovasc. Electrophysiol. 2005 Mar;16 (3):309–13. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
- 26.Bai Rong, Patel Dimpi, Di Biase Luigi, Fahmy Tamer S, Kozeluhova Marketa, Prasad Subramanya, Schweikert Robert, Cummings Jennifer, Saliba Walid, Andrews-Williams Michelle, Themistoclakis Sakis, Bonso Aldo, Rossillo Antonio, Raviele Antonio, Schmitt Claus, Karch Martin, Uriarte Jorge A Salerno, Tchou Patrick, Arruda Mauricio, Natale Andrea. Phrenic nerve injury after catheter ablation: should we worry about this complication? J. Cardiovasc. Electrophysiol. 2006 Sep;17 (9):944–8. doi: 10.1111/j.1540-8167.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 27.Ghia Kasturi K, Chugh Aman, Good Eric, Pelosi Frank, Jongnarangsin Krit, Bogun Frank, Morady Fred, Oral Hakan. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009 Jan;24 (1):33–6. doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 28.Good Eric, Oral Hakan, Lemola Kristina, Han Jihn, Tamirisa Kamala, Igic Petar, Elmouchi Darryl, Tschopp David, Reich Scott, Chugh Aman, Bogun Frank, Pelosi Frank, Morady Fred. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2005 Dec 06;46 (11):2107–10. doi: 10.1016/j.jacc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 29.Martinek M, Bencsik G, Aichinger J, Hassanein S, Schoefl R, Kuchinka P, Nesser H J, Purerfellner H. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J. Cardiovasc. Electrophysiol. 2009 Jul;20 (7):726–33. doi: 10.1111/j.1540-8167.2008.01426.x. [DOI] [PubMed] [Google Scholar]
- 30.Gilcrease Glynn W, Stein Joseph B. A delayed case of fatal atrioesophageal fistula following radiofrequency ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010 Jun 01;21 (6):708–11. doi: 10.1111/j.1540-8167.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 31.Zellerhoff Stephan, Ullerich Hansjörg, Lenze Frank, Meister Tobias, Wasmer Kristina, Mönnig Gerold, Köbe Julia, Milberg Peter, Bittner Alex, Domschke Wolfram, Breithardt Günter, Eckardt Lars. Damage to the esophagus after atrial fibrillation ablation: Just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):155–9. doi: 10.1161/CIRCEP.109.915918. [DOI] [PubMed] [Google Scholar]
- 32.Martinek Martin, Meyer Christian, Hassanein Said, Aichinger Josef, Bencsik Gabor, Schoefl Rainer, Boehm Gernot, Nesser Hans-Joachim, Purerfellner Helmut. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm. 2010 Sep;7 (9):1224–30. doi: 10.1016/j.hrthm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Di Biase Luigi, Saenz Luis Carlos, Burkhardt David J, Vacca Miguel, Elayi Claude S, Barrett Conor D, Horton Rodney, Bai Rong, Siu Alan, Fahmy Tamer S, Patel Dimpi, Armaganijan Luciana, Wu Chia Tung, Kai Sonne, Ching Ching Keong, Phillips Karen, Schweikert Robert A, Cummings Jennifer E, Arruda Mauricio, Saliba Walid I, Dodig Milan, Natale Andrea. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009 Apr;2 (2):108–12. doi: 10.1161/CIRCEP.108.815266. [DOI] [PubMed] [Google Scholar]
- 34.Martinek Martin, Hassanein Said, Bencsik Gabor, Aichinger Josef, Schoefl Rainer, Bachl Andrea, Gerstl Sebastian, Nesser Hans-Joachim, Purerfellner Helmut. Acute development of gastroesophageal reflux after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2009 Oct;6 (10):1457–62. doi: 10.1016/j.hrthm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Bunch T Jared, Nelson Jennifer, Foley Tom, Allison Scott, Crandall Brian G, Osborn Jeffrey S, Weiss J Peter, Anderson Jeffrey L, Nielsen Peter, Anderson Lars, Lappe Donald L, Day John D. Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J. Cardiovasc. Electrophysiol. 2006 Apr;17 (4):435–9. doi: 10.1111/j.1540-8167.2006.00464.x. [DOI] [PubMed] [Google Scholar]


