Abstract
The relatively low success rates seen with pulmonary vein ablation in non-paroxysmal atrial fibrillation (AF) patients as compared to those with the paroxysmal form of the arrhythmia have prompted electrophysiologists to search for newer ablative strategies. A decade has passed since the initial description of complex fractionated atrial electrogram (CFAE) ablation aimed at targeting the electrophysiological substrate in atrial fibrillation. Despite intensive research, superiority of CFAE-based ablation over other contemporary approaches could not be demonstrated. Nevertheless, the technique has an adjunctive role to pulmonary vein ablation in non-paroxysmal AF patients. Perhaps our incomplete understanding of the complex AF pathophysiology and inadequate characterization or determination of CFAE has limited our success so far. This review aims to highlight the current challenges and future role of CFAE ablation.
Introduction
The foundation of catheter ablation for atrial fibrillation (AF) is based on the seminal finding of ectopic foci originating from the pulmonary veins initiating the arrhythmia.[1] However, in patients with long-lasting persistent AF, the current HRS/EHRA/ECAS expert consensus statement recommends more extensive ablation such as linear ablation or targeting of complex fractionated atrial electrograms (CFAE) since pulmonary vein ablation alone yielded much lower success rate than in those with paroxysmal AF.[2] The premise for CFAE ablation is built on the basis that CFAE sites may represent potential substrate sites that contribute to the maintenance of AF.[3] In 2004, Nademanee et al. reported on a novel CFAE ablation strategy with AF termination with or without concurrent anti-arrhythmic drugs in 91 or 63% of patients with persistent AF.[4] However, this high rate of acute procedural success has not been reproducible in other laboratories and CFAE ablation has not been shown to be superior to other contemporary ablation techniques in a recent systematic review.[5] Here, we explore the challenges inherent with characterization of CFAE as well as the current and future role of CFAE ablation in AF.
What is CFAE?
Depolarization of cardiomyocytes can be recorded as extracellular electrogram by an electrode placed in the vicinity. In principle, electrograms recorded from healthy normal myocardium show a simple morphology, but they can appear complex whenever electrical activation is dyssynchronous. Durrer et al. were first to describe broadening and fragmentation of electrogram using unipolar and bipolar recordings in the ischemic ventricular myocardium due to dyssynchronous activation.[6-10] Gardner et al. further extended this finding with demonstration of inhomogeneous activations and anatomical evidence of individual myocardial fiber separation in regions with CFAE.[3,11-13] More recent studies have identified other potential causes of such dyssynchronous activations: non-local activations from overlying or adjacent structures, tissue discontinuities or anisotropy, wave pivoting or collision, conduction block and endo-epicardial electrical dissociation; although measurement artifacts and electrogram filtering are also known to contribute to electrogram fractionation.[4,14-23]
While the electrical basis of CFAE is easily understood, its pathophysiology remains difficult to establish with diverse mechanisms being proposed to date. First, CFAE has been linked to high frequency AF drivers with several studies describing the proximity of CFAE to sites with high dominant frequency.[9,20,24-26] Likewise, the occurrence of CFAE had been associated with prior increase in atrial activation rate.[27,28] However, simultaneous activation mapping with monophasic action potentials recording showed that only a minority of CFAE sites were related to rapid AF drivers.[29] More recently, panoramic contact mapping failed to demonstrate CFAE in the vicinity of stable AF rotors.[30] Similarly, only a weak correlation was seen between CFAE and highest Shannon entropy sites taken to represent pivot zones of AF drivers.[31] Second, several activation mapping studies have associated CFAE with the re-entrant mechanism of AF. Using a high-density multi-electrode catheter, Rostock et al. observed activation gradients in their mapping field covering the entire AF cycle length, indicative of local re-entry in regions with the broadest CFAE.[27] In the human posterior left atrium, Atienza et al. also related CFAE to re-entry around a line of functional block.[28] Furthermore, non-contact mapping studies have documented CFAE in the pivot zones and regions of wave break or collisions of meandering re-entrant waves, although CFAE determined with non-contact mapping may not equate to those recorded with direct contact mapping.[6,9,10] However, associations with such re-entrant activation patterns were encountered only in the minority of instances. Third, sites with CFAE are purported to reflect structural abnormalities that may maintain AF as demonstrated in: post-infarct myocardium with separation of myocardial fibers;[11] aging hearts with uncoupling of side-to-side connections between neighboring myocardial fibers;[17] normal porcine atria subjected to acetylcholine infusion and rapid pacing with increased fibrosis and reduced connexin 43 expression;[32] and simulated human heart failure atrial tissue model with heterogeneous fibroblast proliferation.[7] Koduri et al. provided further correlation of CFAE with heterogeneous distribution of fat and fibrosis in the posterior left atrium of canine with induced heart failure.[33] However, clinical studies have also reported that sites with CFAE during AF did not necessary demonstrate low voltage during sinus rhythm or pacing, suggestive of the absence of large structural abnormalities in most cases.[34,35] More recently, combined magnetic resonance imaging for atrial fibrosis and high-density CFAE mapping showed that most CFAE occurred at sites without delayed enhancement in patients with persistent AF.[36] In this study, sites with dense delayed enhancement demonstrated electrograms of lower voltage, less fractionation and longer cycle length than areas without delayed enhancement.[36] Fourth, CFAE have been associated with the autonomic nervous system in initiating and maintaining AF. Several groups have reported the occurrence of CFAE in the proximity of the ganglionated plexi and preceding the onset of AF[15,37] or with parasympathetic activation.[38] Moreover, abatement of CFAE has also been reported following ablation of the ganglionated plexi or with pharmacological autonomic blockade.[15,33,39-41]
Challenges with CFAE Determinations
Linking the phenomenon of asynchronous electrical activation underlying CFAE to pathophysiological causes has been highly challenging. Perhaps, the techniques used in the acquisition, interpretation and characterization of CFAE may in part contribute to the variable findings reported to date.[42] As seen in the published literature on CFAE, there are significant technical variations among investigators in how electrograms are acquired or processed for CFAE determinations. This can range from the type of catheter used (electrode size, inter-electrode spacing and electrode density), the filtering applied, electrogram configuration (bipolar or unipolar) and the CFAE algorithms employed (manual or semi-automated classifications).
Several reports have highlighted the effect of inter-electrode spacing on electrogram amplitude, morphology and fractionation.[43-45] In addition, amplification and filtering of electrograms are known to exacerbate artifacts caused by electrode motion that can resemble CFAE.[46] Bipolar electrode configuration has been the preferred recording technique in most electrophysiology laboratories with the advantage of capturing less far-field signal and compatibility with currently available semi-automated CFAE algorithms. However, it remains unknown whether the direction-dependent nature of bipolar electrograms has any significant impact on CFAE determinations although the impact of bipolar electrogram voltage on CFAE has previously been highlighted.[14,47] Furthermore, bipolar electrograms are not entirely immune from capturing non-local signals especially if these may be just millimeters away.[16] Even though unipolar electrograms are known to provide more precise determination of local activation, there remains a dearth of CFAE studies using unipolar electrode configuration.
The issue with current CFAE definitions has been highlighted recently. In Nademanee’s initial report, CFAEs were defined as those with ‘two or more deflections and/or perturbation of the baseline with continuous deflection of a prolonged activation complex’ or ‘those with very short cycle length (≤120ms)’ over a 10-second recording period.[4] Other investigators had defined CFAE with manual classifications that were slightly different to Nademanee’s while a more complex grading system has also been proposed recently.[27,29,48,49] Over the last decade, different semi-automated algorithms (Figure 1) have also been investigated and became incorporated into the commonly used 3-D electroanatomic mapping systems (Ensite NavX, St Jude Medical, St Paul, MN, USA or CARTO, Biosense Webster, Diamond Bar, CA, USA).[50-53] However, consistency of semi-automated algorithms are known to be dependent on user-adjustable thresholds of electrogram amplitude, fractionated interval criteria and recording duration.[47,54] Not only are there differences in manual CFAE classifications that are subjected to additional inter-observer variability, semi-automated CFAE algorithms are also known to vary in CFAE determinations.[26,42,54,55] As shown in Figure 2, four 2.5 seconds bipolar fibrillation electrograms of increasing complexity (A to D) were graded by four different CFAE algorithms semi-automatically using published or default criteria. Non-congruent results were seen in three of the four AF electrograms, even with the most fractionated example in D.
Figure 1. Semi-automated CFAE Algorithms .
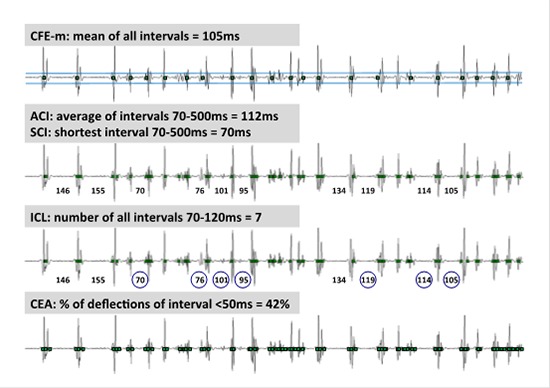
The 5 different semi-automated algorithms first tagged electrograms or deflections according to their specific amplitude and timing criteria adjusted to avoid noise or far-field detections. Based on the tagged deflections, each algorithm provides a quantitative measure of CFAE that is either interval or count based. In this example, the same 2.5s AF electrogram was subjected to the different algorithm. Complex fractionated electrogram mean (CFE-m) measures the mean of time intervals between all tagged deflections. Average complex interval (ACI) refers to the average interval of tagged deflections between 70 and 500ms. Shortest complex interval (SCI) denotes the shortest interval within the sample between 70 and 500ms. Interval confidence level (ICL) refers to the number of intervals between 70 and 120ms within a 2.5s sampling period. Continuous electrical activity (CEA) is defined by the presence of 2 or more successive tagged deflections with interval <50 ms and expressed as percentage of continuous activity.
Figure 2. Variability in CFAE Determinations with Semi-automated Algorithms.
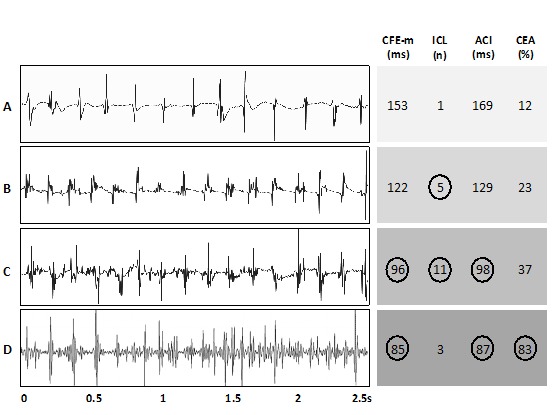
Four of the five algorithms shown in Figure 1 were applied to four different 2.5s AF electrograms of increasing fractionation (sample A to D). CFAE was defined by complex fractionated electrogram mean (CFE-m) <120ms, interval confidence level (ICL) ≥5, average complex interval (ACI) ≤100ms and continuous electrical activity (CEA) ≥75%. The result is circled when the individual algorithm deemed the electrogram to be CFAE. Full agreement is seen with sample A only. This figure is adapted from Lau et al [Reference 41, Figure 2].
Current mapping techniques in the electrophysiology laboratories utilize sequential acquisition of fibrillation electrograms aiming to cover most of the atrium. This technique provides multiple ‘snapshots’ of the arrhythmia ranging from 2.5 to 8 seconds in duration. The whole process can take between 10 to 15 minutes, depending on the density of mapping. Although studies have shown that a minimum of 5 seconds at each site is required to achieve good CFAE determinations, these comparisons were only made with the maximum allowable recordings of 8 seconds in the Ensite NavX system.[56,57] Similarly, 5 seconds recordings provided better consistency with CFAE detection as compared to 2.5 seconds using the CARTO mapping system.[54] Of course, the overriding limiting factor is that extensive sequential CFAE mapping will prolong the already lengthy AF ablation procedure. One may wonder whether a short recording segment at each site reflects the arrhythmia adequately. Although several investigators have reported that CFAE were stable and reproducible, Habel et al. was first to highlight the temporal variability in CFAE that constrains the validity of sequential mapping in human AF.[58] This is supported by a more recent systematic review where we reported that one out of four CFAE sites were no longer fractionated in a short time span of under two minutes.[42] Therefore, the inherent dynamic nature of CFAE presents further challenge for electrophysiologists in detecting and targeting them as substrate sites maintaining AF.
Sites with CFAE: Where to Ablate?
Due to our limited pathophysiological understanding and variable technical determinations of CFAE, it is plausible that not all CFAE sites are mechanistically important, while important CFAE sites may have been missed using sequential mapping technique. In general, CFAEs are more prevalent in patients with persistent than paroxysmal AF with higher fractionation, higher percentage area and greater proportion of mapped points with fractionated electrograms.[24,35,59] On the contrary, it has also been reported that greater degree of atrial remodeling, as gauged by larger left atrial dimension and lower atrial voltage, was associated with longer cycle length and smaller percentage area of CFAE.[60] This phenomenon can perhaps be explained by higher left atrial wall stress in those with greater left atrial volume, to account for lower atrial voltage and reduced CFAE.[61]
Nevertheless, numerous pre-clinical and human studies have indicated an association between CFAE and the underlying substrate complexity as a result of progressive atrial remodeling and senescence. [23,62,63] Ciaccio et al. further demonstrated significant differences in electrogram morphology in patients with paroxysmal and long-standing persistent AF independent of activation rate, with lower variability, greater repeatability and more uniform distribution seen at disparate left atrial sites in the latter.[64,65] CFAE are widely distributed in the human atria with preponderance to the left over right atrium and at specific atrial sites where there are abrupt changes in muscle fiber orientation. Early work by Centurion et al in the right atrium of patients with sick sinus syndrome showed a wider distribution of CFAE in those with AF than those without.[66] More recent high-density mapping studies have demonstrated longer CFAE maximum duration, higher fractionation and higher prevalence of CFAE in the left compared to the right atria of AF patients.[24,27,51,52] The sites where CFAE are encountered more frequently differ according to reports. These include the inter-atrial septum, pulmonary vein ostia, posterior left atrium, left atrial roof, left atria appendage, mitral annulus region and coronary sinus.[35,51-53,60,67-70] Specifically, CFAE have been noted to concentrate around the pulmonary veins in paroxysmal AF patients but more evenly distributed over all left atrial regions in those with persistent AF.[52]
The widespread nature of CFAE calls for means of differentiating sites with critical CFAE that maintain AF from non-critical or ‘by-stander’ sites. Several advantages can be gained by avoiding ablation of non-critical CFAE sites including: reduced procedural time, radiation exposure, risk of complications or collateral damage and longer-term sequelae of iatrogenic atrial tachycardia or ‘stiff left atrial syndrome’.[71] Pre-clinical work examining pharmacologic cardioversion of persistent AF in the goats showed that cibenzoline (Class I anti-arrhythmic drug) resulted in AF slowing and organization while reducing electrogram fractionation.[72] Whether anti-arrhythmic drugs can help to distinguish critical CFAE sites and minimize unnecessary ablation of ‘by-stander’ sites were of interest subsequently. Kumagai and Toyama reported a reduction in CFAE sites following administration of nifekalant and pilsicainide in 60 AF patients undergoing catheter ablation. They were able to terminate AF in about 1 in 4 patients after targeting of the persistent CFAE sites following anti-arrhythmic drug administration.[73] The same investigators also reported higher AF termination rate, shorter procedural time and fewer radiofrequency ablation lesions in patients where adjuvant CFAE ablation was undertaken following CFAE localization with nifekalant than those without, to achieve similar success rates at 12 months.[74] Likewise, in a smaller study, intraprocedural administration of ibutilide has been used to organize AF activity and minimize the adjuvant CFAE ablation lesion set without a clear reduction in longer-term success rates.[75] A larger prospective double-blinded multi-center study is now underway to further investigate whether ibutilide use can indeed improve current CFAE ablation strategy.[76] Other investigators have also shown that selective ablation of CFAE with certain morphologies led to greater prolongation in AF cycle length than others, indicating a likely usefulness of their CFAE classification system in minimizing ablation of non-critical sites.[49,77]
Current and Future Role of CFAE ablation
The decade since the initial report by Nademanee has seen a significant number of studies examining the utility of CFAE ablation in AF patients. Pulmonary vein ablation remains the cornerstone for patients with paroxysmal AF and additional CFAE ablation in this patient cohort has not shown significant additional benefits.[78,79] Studies reporting CFAE ablation as a lone strategy in patients with persistent AF have not been able to demonstrate superior success over other established techniques such as pulmonary vein antrum isolation, box isolation or the stepwise ablation approach.[5] The largest of these studies by Oral et al. showed a single procedural drug-free success rate of 33% in 100 chronic AF patients at mean follow-up duration of 14 months.[80] However, recent meta-analyses have shown a beneficial role for adjunctive CFAE ablation following pulmonary vein ablation in patients with non-paroxysmal AF.[78,79] This is despite variability in the CFAE ablation endpoints used, which can range from elimination of fractionated potentials, transformation to discrete electrograms, organization of AF cycle length and termination into sinus rhythm or atrial tachycardia. Although termination of AF appears to confer superior longer-term success rates, this has not been consistently demonstrated, with alternative measures such as dominant frequency assessment having been proposed.[81-84]
At present, CFAE ablation remains as an adjunctive approach following pulmonary vein ablation in patients with non-paroxysmal AF. Its utility and success appear to be hampered by several factors as mentioned in this review: 1) CFAE can be due to a multitude of causes that can result in dyssynchronous activations with diverse pathophysiological mechanisms; 2) Current techniques in CFAE acquisition, interpretation and characterization remains suboptimal and challenging; 3) Difficulty in distinguishing critical from ‘by-stander’ CFAE sites that promote AF maintenance and risk of excessive or unnecessary ablation that may result in higher complications; 4) Lack of well-defined electrophysiological endpoints for CFAE ablation.
Conclusions:
This review highlights the many challenges with current CFAE-based ablation. Further work is necessary to improve the limitations with regard to our understanding and utility of CFAE targeting in catheter ablation for AF.
Acknowledgements
Dr. Lau is supported by a Postdoctoral Fellowship from the National Health and Medical Research Council of Australia and CRB Blackburn Overseas Travelling Fellowship from the Royal Australasian College of Physicians. This work was supported by grant funding from The Center for Translational Molecular Medicine (CTMM - COHFAR), The Netherlands and The European Network for Translational Research in Atrial Fibrillation (EUTRAF - 261057), European Union.
Disclosures
None.
References
- 1.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Schotten Ulrich, Verheule Sander, Kirchhof Paulus, Goette Andreas. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol. Rev. 2011 Jan;91 (1):265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 4.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 02;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 5.Brooks Anthony G, Stiles Martin K, Laborderie Julien, Lau Dennis H, Kuklik Pawel, Shipp Nicholas J, Hsu Li-Fern, Sanders Prashanthan. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010 Jun;7 (6):835–46. doi: 10.1016/j.hrthm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Yamabe Hiroshige, Morihisa Kenji, Tanaka Yasuaki, Uemura Takashi, Enomoto Koji, Kawano Hiroaki, Ogawa Hisao. Mechanisms of the maintenance of atrial fibrillation: role of the complex fractionated atrial electrogram assessed by noncontact mapping. Heart Rhythm. 2009 Aug;6 (8):1120–8. doi: 10.1016/j.hrthm.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Ashihara Takashi, Haraguchi Ryo, Nakazawa Kazuo, Namba Tsunetoyo, Ikeda Takanori, Nakazawa Yuko, Ozawa Tomoya, Ito Makoto, Horie Minoru, Trayanova Natalia A. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram-based catheter ablation. Circ. Res. 2012 Jan 20;110 (2):275–84. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DURRER D, FORMIJNE P, van DAM R, van LIER A, BULLER J, MEYLER F L. The electrocardiogram in normal and some abnormal conditions; in revived human fetal heart and in acute and chronic coronary occlusion. Am. Heart J. 1961 Mar;61 ():303–16. doi: 10.1016/0002-8703(61)90599-3. [DOI] [PubMed] [Google Scholar]
- 9.Lo Li-Wei, Higa Satoshi, Lin Yenn-Jiang, Chang Shih-Lin, Tuan Ta-Chuan, Hu Yu-Feng, Tsai Wen-Chin, Tsao Hsuan-Ming, Tai Ching-Tai, Ishigaki Sugako, Oyakawa Asuka, Maeda Minetaka, Suenari Kazuyoshi, Chen Shih-Ann. The novel electrophysiology of complex fractionated atrial electrograms: insight from noncontact unipolar electrograms. J. Cardiovasc. Electrophysiol. 2010 Jun 01;21 (6):640–8. doi: 10.1111/j.1540-8167.2009.01679.x. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld Edward P, Lavi Nimrod, Bazan Victor, Gojraty Sattar, Kim Steven J, Michele John. Mechanism of complex fractionated electrograms recorded during atrial fibrillation in a canine model. Pacing Clin Electrophysiol. 2011 Jul;34 (7):844–57. doi: 10.1111/j.1540-8159.2011.03071.x. [DOI] [PubMed] [Google Scholar]
- 11.Gardner P I, Ursell P C, Fenoglio J J, Wit A L. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985 Sep;72 (3):596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 12.Koduri Hemantha, Ng Jason, Cokic Ivan, Aistrup Gary L, Gordon David, Wasserstrom J Andrew, Kadish Alan H, Lee Richard, Passman Rod, Knight Bradley P, Goldberger Jeffrey J, Arora Rishi. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012 Aug 01;5 (4):640–9. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konings K T, Smeets J L, Penn O C, Wellens H J, Allessie M A. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997 Mar 04;95 (5):1231–41. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 14.de Bakker Jacques M T, Wittkampf Fred H M. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):204–13. doi: 10.1161/CIRCEP.109.904763. [DOI] [PubMed] [Google Scholar]
- 15.Lu Zhibing, Scherlag Benjamin J, Lin Jiaxiong, Niu Guodong, Ghias Muhammad, Jackman Warren M, Lazzara Ralph, Jiang Hong, Po Sunny S. Autonomic mechanism for complex fractionated atrial electrograms: evidence by fast fourier transform analysis. J. Cardiovasc. Electrophysiol. 2008 Aug;19 (8):835–42. doi: 10.1111/j.1540-8167.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 16.Shah Dipen, Burri Haran, Sunthorn Henri, Gentil-Baron Pascale. Identifying far-field superior vena cava potentials within the right superior pulmonary vein. Heart Rhythm. 2006 Aug;3 (8):898–902. doi: 10.1016/j.hrthm.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Spach M S, Dolber P C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ. Res. 1986 Mar;58 (3):356–71. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Berenfeld Omer, Zaitsev Alexey V, Mironov Sergey F, Pertsov Arkady M, Jalife José. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ. Res. 2002 Jun 14;90 (11):1173–80. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 19.Verheule Sander, Tuyls Els, van Hunnik Arne, Kuiper Marion, Schotten Ulrich, Allessie Maurits. Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Dec;3 (6):590–9. doi: 10.1161/CIRCEP.109.931634. [DOI] [PubMed] [Google Scholar]
- 20.Kalifa Jérôme, Tanaka Kazuhiko, Zaitsev Alexey V, Warren Mark, Vaidyanathan Ravi, Auerbach David, Pandit Sandeep, Vikstrom Karen L, Ploutz-Snyder Robert, Talkachou Arkadzi, Atienza Felipe, Guiraudon Gérard, Jalife José, Berenfeld Omer. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006 Feb 07;113 (5):626–33. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 21.Konings K T, Smeets J L, Penn O C, Wellens H J, Allessie M A. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997 Mar 04;95 (5):1231–41. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 22.Allessie Maurits A, de Groot Natasja M S, Houben Richard P M, Schotten Ulrich, Boersma Eric, Smeets Joep L, Crijns Harry J. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010 Dec;3 (6):606–15. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 23.Eckstein Jens, Maesen Bart, Linz Dominik, Zeemering Stef, van Hunnik Arne, Verheule Sander, Allessie Maurits, Schotten Ulrich. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc. Res. 2011 Mar 01;89 (4):816–24. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 24.Stiles Martin K, Brooks Anthony G, Kuklik Pawel, John Bobby, Dimitri Hany, Lau Dennis H, Wilson Lauren, Dhar Shashi, Roberts-Thomson Ross L, Mackenzie Lorraine, Young Glenn D, Sanders Prashanthan. High-density mapping of atrial fibrillation in humans: relationship between high-frequency activation and electrogram fractionation. J. Cardiovasc. Electrophysiol. 2008 Dec;19 (12):1245–53. doi: 10.1111/j.1540-8167.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 25.Suenari Kazuyoshi, Lin Yenn-Jiang, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Tuan Ta-Chuan, Huang Shih-Yu, Tai Ching-Tai, Nakano Yukiko, Kihara Yasuki, Tsao Hsuan-Ming, Wu Tsu-Juey, Chen Shih-Ann. Relationship between arrhythmogenic pulmonary veins and the surrounding atrial substrate in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011 Apr;22 (4):405–10. doi: 10.1111/j.1540-8167.2010.01932.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee Geoffrey, Roberts-Thomson Kurt, Madry Andrew, Spence Steven, Teh Andrew, Heck Patrick M, Kumar Saurabh, Kistler Peter M, Morton Joseph B, Sanders Prashanthan, Kalman Jonathan M. Relationship among complex signals, short cycle length activity, and dominant frequency in patients with long-lasting persistent AF: a high-density epicardial mapping study in humans. Heart Rhythm. 2011 Nov;8 (11):1714–9. doi: 10.1016/j.hrthm.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Rostock Thomas, Rotter Martin, Sanders Prashanthan, Takahashi Yoshihide, Jaïs Pierre, Hocini Mélèze, Hsu Li-Fern, Sacher Fréderic, Clémenty Jacques, Haïssaguerre Michel. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006 Jan;3 (1):27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Atienza Felipe, Calvo David, Almendral Jesús, Zlochiver Sharon, Grzeda Krzysztof R, Martínez-Alzamora Nieves, González-Torrecilla Esteban, Arenal Angel, Fernández-Avilés Francisco, Berenfeld Omer. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J. Am. Coll. Cardiol. 2011 Mar 01;57 (9):1081–92. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan Sanjiv M, Wright Matthew, Derval Nicolas, Jadidi Amir, Forclaz Andrei, Nault Isabelle, Miyazaki Shinsuke, Sacher Frédéric, Bordachar Pierre, Clémenty Jacques, Jaïs Pierre, Haïssaguerre Michel, Hocini Mélèze. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011 Feb;8 (2):244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayan Sanjiv M, Shivkumar Kalyanam, Krummen David E, Miller John M, Rappel Wouter-Jan. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol. 2013 Feb;6 (1):58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganesan Anand N, Kuklik Pawel, Lau Dennis H, Brooks Anthony G, Baumert Mathias, Lim Wei Wen, Thanigaimani Shivshankar, Nayyar Sachin, Mahajan Rajiv, Kalman Jonathan M, Roberts-Thomson Kurt C, Sanders Prashanthan. Bipolar electrogram shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013 Feb;6 (1):48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 32.Liu Xu, Shi Hai-feng, Tan Hong-wei, Wang Xin-hua, Zhou Li, Gu Jia-ning. Decreased connexin 43 and increased fibrosis in atrial regions susceptible to complex fractionated atrial electrograms. Cardiology. 2009;114 (1):22–9. doi: 10.1159/000210398. [DOI] [PubMed] [Google Scholar]
- 33.Koduri Hemantha, Ng Jason, Cokic Ivan, Aistrup Gary L, Gordon David, Wasserstrom J Andrew, Kadish Alan H, Lee Richard, Passman Rod, Knight Bradley P, Goldberger Jeffrey J, Arora Rishi. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012 Aug 01;5 (4):640–9. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teh Andrew W, Kistler Peter M, Lee Geoffrey, Medi Caroline, Heck Patrick M, Spence Steven J, Sparks Paul B, Morton Joseph B, Sanders Prashanthan, Kalman Jonathan M. The relationship between complex fractionated electrograms and atrial low-voltage zones during atrial fibrillation and paced rhythm. Europace. 2011 Dec;13 (12):1709–16. doi: 10.1093/europace/eur197. [DOI] [PubMed] [Google Scholar]
- 35.Jadidi Amir S, Duncan Edward, Miyazaki Shinsuke, Lellouche Nicolas, Shah Ashok J, Forclaz Andrei, Nault Isabelle, Wright Matthew, Rivard Lena, Liu Xingpeng, Scherr Daniel, Wilton Stephen B, Sacher Frédéric, Derval Nicolas, Knecht Sebastien, Kim Steven J, Hocini Mélèze, Narayan Sanjiv, Haïssaguerre Michel, Jaïs Pierre. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):32–42. doi: 10.1161/CIRCEP.111.964197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadidi Amir S, Cochet Hubert, Shah Ashok J, Kim Steven J, Duncan Edward, Miyazaki Shinsuke, Sermesant Maxime, Lehrmann Heiko, Lederlin Matthieu, Linton Nick, Forclaz Andrei, Nault Isabelle, Rivard Lena, Wright Matthew, Liu Xingpeng, Scherr Daniel, Wilton Stephen B, Roten Laurent, Pascale Patrizio, Derval Nicolas, Sacher Frédéric, Knecht Sebastien, Keyl Cornelius, Hocini Mélèze, Montaudon Michel, Laurent Francois, Haïssaguerre Michel, Jaïs Pierre. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J. Am. Coll. Cardiol. 2013 Aug 27;62 (9):802–12. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 37.Choi Eue-Keun, Shen Mark J, Han Seongwook, Kim Daehyeok, Hwang Samuel, Sayfo Sameh, Piccirillo Gianfranco, Frick Kyle, Fishbein Michael C, Hwang Chun, Lin Shien-Fong, Chen Peng-Sheng. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010 Jun 22;121 (24):2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lellouche Nicolas, Buch Eric, Celigoj Andrew, Siegerman Carin, Cesario David, De Diego Carlos, Mahajan Aman, Boyle Noel G, Wiener Isaac, Garfinkel Alan, Shivkumar Kalyanam. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J. Am. Coll. Cardiol. 2007 Oct 02;50 (14):1324–31. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Lin Jiaxiong, Scherlag Benjamin J, Zhou Jing, Lu Zhibing, Patterson Eugene, Jackman Warren M, Lazzara Ralph, Po Sunny S. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE). J. Cardiovasc. Electrophysiol. 2007 Nov;18 (11):1197–205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 40.Katritsis Demosthenes, Sougiannis Demetrios, Batsikas Kimon, Giazitzoglou Eleftherios, Mersinias John, Katritsis George, Po Sunny S. Autonomic modulation of complex fractionated atrial electrograms in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2011 Sep;31 (3):217–23. doi: 10.1007/s10840-011-9558-0. [DOI] [PubMed] [Google Scholar]
- 41.Knecht Sébastien, Wright Matthew, Matsuo Seiichiro, Nault Isabelle, Lellouche Nicolas, Sacher Frédéric, Kim Steven J, Morgan Dennis, Afonso Valtino, Shinzuke Miyazaki, Hocini Mélèze, Clémenty Jacques, Narayan Sanjiv M, Ritter Phillipe, Jaïs Pierre, Haïssaguerre Michel. Impact of pharmacological autonomic blockade on complex fractionated atrial electrograms. J. Cardiovasc. Electrophysiol. 2010 Jul;21 (7):766–72. doi: 10.1111/j.1540-8167.2009.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau Dennis H, Maesen Bart, Zeemering Stef, Verheule Sander, Crijns Harry J, Schotten Ulrich. Stability of complex fractionated atrial electrograms: a systematic review. J. Cardiovasc. Electrophysiol. 2012 Sep;23 (9):980–7. doi: 10.1111/j.1540-8167.2012.02335.x. [DOI] [PubMed] [Google Scholar]
- 43.Baerman J M, Ropella K M, Sahakian A V, Kirsh J A, Swiryn S. Effect of bipole configuration on atrial electrograms during atrial fibrillation. Pacing Clin Electrophysiol. 1990 Jan;13 (1):78–87. doi: 10.1111/j.1540-8159.1990.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 44.Correa de Sa Daniel D, Thompson Nathaniel, Stinnett-Donnelly Justin, Znojkiewicz Pierre, Habel Nicole, Müller Joachim G, Bates Jason H T, Buzas Jeffrey S, Spector Peter S. Electrogram fractionation: the relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ Arrhythm Electrophysiol. 2011 Dec;4 (6):909–16. doi: 10.1161/CIRCEP.111.965145. [DOI] [PubMed] [Google Scholar]
- 45.Nagashima Koichi, Okumura Yasuo, Watanabe Ichiro, Nakai Toshiko, Ohkubo Kimie, Kofune Tatsuya, Kofune Masayoshi, Mano Hiroaki, Sonoda Kazumasa, Hirayama Atsushi. Effects of inter-electrode spacing on complex fractionated atrial electrograms and dominant frequency detection. J Interv Card Electrophysiol. 2012 Jun;34 (1):51–7. doi: 10.1007/s10840-011-9654-1. [DOI] [PubMed] [Google Scholar]
- 46.Ideker R E, Lofland G K, Bardy G H, Smith W M, Worley S J, Wallace A G, Cox J L, Gallagher J J. Late fractionated potentials and continuous electrical activity caused by electrode motion. Pacing Clin Electrophysiol. 1983 Sep;6 (5 Pt 1):908–14. doi: 10.1111/j.1540-8159.1983.tb04412.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsai Wen-Chin, Lin Yenn-Jiang, Tsao Hsuan-Ming, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Chang Chien-Jung, Tang Wei-Hua, Tuan Ta-Chun, Udyavar Ameya R, Wang Hi-Hung, Chen Shih-Ann. The optimal automatic algorithm for the mapping of complex fractionated atrial electrograms in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010 Jan;21 (1):21–6. doi: 10.1111/j.1540-8167.2009.01567.x. [DOI] [PubMed] [Google Scholar]
- 48.Roberts-Thomson Kurt C, John Bobby, Worthley Stephen G, Brooks Anthony G, Stiles Martin K, Lau Dennis H, Kuklik Pawel, Shipp Nicholas J, Kalman Jonathan M, Sanders Prashanthan. Left atrial remodeling in patients with atrial septal defects. Heart Rhythm. 2009 Jul;6 (7):1000–6. doi: 10.1016/j.hrthm.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 49.Hunter Ross J, Diab Ihab, Thomas Glyn, Duncan Edward, Abrams Dominic, Dhinoja Mehul, Sporton Simon, Earley Mark J, Schilling Richard J. Validation of a classification system to grade fractionation in atrial fibrillation and correlation with automated detection systems. Europace. 2009 Dec;11 (12):1587–96. doi: 10.1093/europace/eup351. [DOI] [PubMed] [Google Scholar]
- 50.Verma Atul, Novak Paul, Macle Laurent, Whaley Bonnie, Beardsall Marianne, Wulffhart Zaev, Khaykin Yaariv. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008 Feb;5 (2):198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Calò Leonardo, De Ruvo Ermenegildo, Sciarra Luigi, Gricia Roberto, Navone Giovanna, De Luca Lucia, Nuccio Francesca, Sette Antonella, Pristipino Cristian, Dulio Alessandro, Gaita Fiorenzo, Lioy Ernesto. Diagnostic accuracy of a new software for complex fractionated electrograms identification in patients with persistent and permanent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Oct;19 (10):1024–30. doi: 10.1111/j.1540-8167.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu Jinjin, Estner Heidi, Luik Armin, Ucer Ekrem, Reents Tilko, Pflaumer Andreas, Zrenner Bernhard, Hessling Gabriele, Deisenhofer Isabel. Automatic 3D mapping of complex fractionated atrial electrograms (CFAE) in patients with paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Sep;19 (9):897–903. doi: 10.1111/j.1540-8167.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 53.Scherr Daniel, Dalal Darshan, Cheema Aamir, Cheng Alan, Henrikson Charles A, Spragg David, Marine Joseph E, Berger Ronald D, Calkins Hugh, Dong Jun. Automated detection and characterization of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. Heart Rhythm. 2007 Aug;4 (8):1013–20. doi: 10.1016/j.hrthm.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Tsai Wen-Chin, Wang Ji-Hung, Lin Yenn-Jiang, Tsao Hsuan-Ming, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Chang Chien-Jung, Tang Wei-Hua, Huang Shih-Yu, Suenari Kazuyoshi, Tuan Ta-Chun, Chen Shih-Ann. Consistency of the automatic algorithm in detecting complex fractionated electrograms using an electroanatomical navigation system. Pacing Clin Electrophysiol. 2012 Aug;35 (8):980–9. doi: 10.1111/j.1540-8159.2012.03444.x. [DOI] [PubMed] [Google Scholar]
- 55.Ng Jason, Borodyanskiy Aleksey I, Chang Eric T, Villuendas Roger, Dibs Samer, Kadish Alan H, Goldberger Jeffrey J. Measuring the complexity of atrial fibrillation electrograms. J. Cardiovasc. Electrophysiol. 2010 Jun 01;21 (6):649–55. doi: 10.1111/j.1540-8167.2009.01695.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin Yenn-Jiang, Tai Ching-Tai, Kao Tsair, Chang Shih-Lin, Wongcharoen Wanwarang, Lo Li-Wei, Tuan Ta-Chuan, Udyavar Ameya R, Chen Yi-Jen, Higa Satoshi, Ueng Kuo-Chang, Chen Shih-Ann. Consistency of complex fractionated atrial electrograms during atrial fibrillation. Heart Rhythm. 2008 Mar;5 (3):406–12. doi: 10.1016/j.hrthm.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Stiles Martin K, Brooks Anthony G, John Bobby, Wilson Lauren, Kuklik Pawel, Dimitri Hany, Lau Dennis H, Roberts-Thomson Ross L, Mackenzie Lorraine, Willoughby Scott, Young Glenn D, Sanders Prashanthan. The effect of electrogram duration on quantification of complex fractionated atrial electrograms and dominant frequency. J. Cardiovasc. Electrophysiol. 2008 Mar;19 (3):252–8. doi: 10.1111/j.1540-8167.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- 58.Habel Nicole, Znojkiewicz Pierre, Thompson Nathaniel, Müller Joachim G, Mason Bryan, Calame James, Calame Susan, Sharma Shruti, Mirchandani Gagan, Janks Deborah, Bates Jason, Noori Arshia, Karnbach Andreas, Lustgarten Daniel L, Sobel Burton E, Spector Peter. The temporal variability of dominant frequency and complex fractionated atrial electrograms constrains the validity of sequential mapping in human atrial fibrillation. Heart Rhythm. 2010 May;7 (5):586–93. doi: 10.1016/j.hrthm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Solheim Eivind, Off Morten Kristian, Hoff Per Ivar, Schuster Peter, Ohm Ole-Jørgen, Chen Jian. Characteristics and distribution of complex fractionated atrial electrograms in patients with paroxysmal and persistent atrial fibrillation. J Interv Card Electrophysiol. 2010 Aug;28 (2):87–93. doi: 10.1007/s10840-010-9479-3. [DOI] [PubMed] [Google Scholar]
- 60.Park Jae Hyung, Park Sang Weon, Kim Jong Youn, Kim Sook Kyoung, Jeoung Boyoung, Lee Moon-Hyung, Hwang Chun, Kim Young-Hoon, Kim Sung Soon, Pak Hui-Nam. Characteristics of complex fractionated atrial electrogram in the electroanatomically remodeled left atrium of patients with atrial fibrillation. Circ. J. 2010 Aug;74 (8):1557–63. doi: 10.1253/circj.cj-10-0048. [DOI] [PubMed] [Google Scholar]
- 61.Hunter Ross J, Liu Yankai, Lu Yiling, Wang Wen, Schilling Richard J. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2012 Apr;5 (2):351–60. doi: 10.1161/CIRCEP.111.965541. [DOI] [PubMed] [Google Scholar]
- 62.Roberts-Thomson Kurt C, Kistler Peter M, Sanders Prashanthan, Morton Joseph B, Haqqani Haris M, Stevenson Irene, Vohra Jitendra K, Sparks Paul B, Kalman Jonathan M. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage, and conduction velocity. Heart Rhythm. 2009 May;6 (5):587–91. doi: 10.1016/j.hrthm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Lau Dennis H, Mackenzie Lorraine, Kelly Darren J, Psaltis Peter J, Brooks Anthony G, Worthington Michael, Rajendram Arumuga, Kelly Douglas R, Zhang Yuan, Kuklik Pawel, Nelson Adam J, Wong Christopher X, Worthley Stephen G, Rao Mohan, Faull Randall J, Edwards James, Saint David A, Sanders Prashanthan. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010 Sep;7 (9):1282–90. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Ciaccio Edward J, Biviano Angelo B, Whang William, Gambhir Alok, Garan Hasan. Different characteristics of complex fractionated atrial electrograms in acute paroxysmal versus long-standing persistent atrial fibrillation. Heart Rhythm. 2010 Sep;7 (9):1207–15. doi: 10.1016/j.hrthm.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciaccio Edward J, Biviano Angelo B, Whang William, Vest John A, Gambhir Alok, Einstein Andrew J, Garan Hasan. Differences in repeating patterns of complex fractionated left atrial electrograms in longstanding persistent atrial fibrillation as compared with paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Aug;4 (4):470–7. doi: 10.1161/CIRCEP.110.960153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centurion O A, Fukatani M, Konoe A, Tanigawa M, Shimizu A, Isomoto S, Kaibara M, Hashiba K. Different distribution of abnormal endocardial electrograms within the right atrium in patients with sick sinus syndrome. Br Heart J. 1992 Dec;68 (6):596–600. doi: 10.1136/hrt.68.12.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Clémenty J. Regional disparities of endocardial atrial activation in paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1996 Nov;19 (11 Pt 2):1998–2003. doi: 10.1111/j.1540-8159.1996.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 68.Katritsis Demosthenes, Sougiannis Demetrios, Giazitzoglou Eleftherios, Kourlaba Georgia, Ellenbogen Kenneth A. Regional endocardial left atrial voltage and electrogram fractionation in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Dec;19 (12):1254–8. doi: 10.1111/j.1540-8167.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 69.Park Jae H, Pak Hui-Nam, Kim Sook K, Jang Jin K, Choi Jong I, Lim Hong E, Hwang Chun, Kim Young-Hoon. Electrophysiologic characteristics of complex fractionated atrial electrograms in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 Mar;20 (3):266–72. doi: 10.1111/j.1540-8167.2008.01321.x. [DOI] [PubMed] [Google Scholar]
- 70.Roux Jean-François, Gojraty Sattar, Bala Rupa, Liu Christopher F, Hutchinson Mathew D, Dixit Sanjay, Callans David J, Marchlinski Francis, Gerstenfeld Edward P. Complex fractionated electrogram distribution and temporal stability in patients undergoing atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2008 Aug;19 (8):815–20. doi: 10.1111/j.1540-8167.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 71.Gibson Douglas N, Di Biase Luigi, Mohanty Prasant, Patel Jigar D, Bai Rong, Sanchez Javier, Burkhardt J David, Heywood J Thomas, Johnson Allen D, Rubenson David S, Horton Rodney, Gallinghouse G Joseph, Beheiry Salwa, Curtis Guy P, Cohen David N, Lee Mark Y, Smith Michael R, Gopinath Devi, Lewis William R, Natale Andrea. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011 Sep;8 (9):1364–71. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Shan Zhaoliang, Van Der Voort Pepijn H, Blaauw Yuri, Duytschaever Mattias, Allessie Maurits A. Fractionation of electrograms and linking of activation during pharmacologic cardioversion of persistent atrial fibrillation in the goat. J. Cardiovasc. Electrophysiol. 2004 May;15 (5):572–80. doi: 10.1046/j.1540-8167.2004.03402.x. [DOI] [PubMed] [Google Scholar]
- 73.Kumagai K, Toyama H. Impact of Antiarrhythmic Drugs on Complex Fractionated Atrial Electrograms. Journal of Arrhythmia. 2011;27:314–323. [Google Scholar]
- 74.Kumagai Koichiro, Toyama Hideko. Usefulness of ablation of complex fractionated atrial electrograms using nifekalant in persistent atrial fibrillation. J Cardiol. 2013 Jan;61 (1):44–8. doi: 10.1016/j.jjcc.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 75.Singh Sheldon M, D'Avila Andre, Kim Steven J, Houghtaling Christopher, Dukkipati Srinivas R, Reddy Vivek Y. Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms: preliminary assessment of a modified step-wise approach to ablation of persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010 Jun 01;21 (6):608–16. doi: 10.1111/j.1540-8167.2009.01671.x. [DOI] [PubMed] [Google Scholar]
- 76.Singh Sheldon M, D'Avila Andre, Kim Young-Hoon, Aryana Arash, Mangrum J Michael, Michaud Gregory F, Dukkipati Srinivas R, Callans David J, Barrett Conor D, Beras-Jovine Maximo R, Reddy Vivek Y. The Modified Ablation Guided by Ibutilide Use in Chronic Atrial Fibrillation (MAGIC-AF) Study: clinical background and study design. J. Cardiovasc. Electrophysiol. 2012 Apr;23 (4):352–8. doi: 10.1111/j.1540-8167.2011.02198.x. [DOI] [PubMed] [Google Scholar]
- 77.Hunter Ross J, Diab Ihab, Tayebjee Muzahir, Richmond Laura, Sporton Simon, Earley Mark J, Schilling Richard J. Characterization of fractionated atrial electrograms critical for maintenance of atrial fibrillation: a randomized, controlled trial of ablation strategies (the CFAE AF trial). Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):622–9. doi: 10.1161/CIRCEP.111.962928. [DOI] [PubMed] [Google Scholar]
- 78.Li Wei-ju, Bai Yong-yi, Zhang Hong-yin, Tang Ri-bo, Miao Cheng-long, Sang Cai-hua, Yin Xian-dong, Dong Jian-zeng, Ma Chang-sheng. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Circ Arrhythm Electrophysiol. 2011 Apr;4 (2):143–8. doi: 10.1161/CIRCEP.110.958405. [DOI] [PubMed] [Google Scholar]
- 79.Hayward Robert M, Upadhyay Gaurav A, Mela Theofanie, Ellinor Patrick T, Barrett Conor D, Heist E Kevin, Verma Atul, Choudhry Niteesh K, Singh Jagmeet P. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm. 2011 Jul;8 (7):994–1000. doi: 10.1016/j.hrthm.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oral Hakan, Chugh Aman, Good Eric, Wimmer Alan, Dey Sujoya, Gadeela Nitesh, Sankaran Sundar, Crawford Thomas, Sarrazin Jean F, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Frederick Melissa, Fortino Jackie, Benloucif-Moore Suzanne, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007 May 22;115 (20):2606–12. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 81.O'Neill Mark D, Wright Matthew, Knecht Sébastien, Jaïs Pierre, Hocini Mélèze, Takahashi Yoshihide, Jönsson Anders, Sacher Frédéric, Matsuo Seiichiro, Lim Kang Teng, Arantes Leonardo, Derval Nicolas, Lellouche Nicholas, Nault Isabelle, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur. Heart J. 2009 May;30 (9):1105–12. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]
- 82.Elayi Claude S, Verma Atul, Di Biase Luigi, Ching Chi Keong, Patel Dimpi, Barrett Conor, Martin David, Rong Bai, Fahmy Tamer S, Khaykin Yaariv, Hongo Richard, Hao Steven, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Arruda Mauricio, Schweikert Robert A, Natale Andrea. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008 Dec;5 (12):1658–64. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Lau DH, Brooks AG, Sanders P. AF Termination: the Holy Grail of persistent AF ablation? . Journal of atrial fibrillation . 2010;1:702–704. doi: 10.4022/jafib.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshida Kentaro, Chugh Aman, Good Eric, Crawford Thomas, Myles James, Veerareddy Srikar, Billakanty Sreedhar, Wong Wai S, Ebinger Matthew, Pelosi Frank, Jongnarangsin Krit, Bogun Frank, Morady Fred, Oral Hakan. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010 Mar;7 (3):295–302. doi: 10.1016/j.hrthm.2009.11.024. [DOI] [PubMed] [Google Scholar]


