Abstract
Atrial fibrillation and heart failure are closely linked cardiac conditions that are both increasing in prevalence due to shared risk factors and common disease mechanisms. The presence of both disease entities portends an increase in morbidity and mortality. There are significant similarities in the treatment strategies of these conditions, and the adequate management of one disease may prevent the development of the other. To this date, a rhythm control strategy, even in the heart failure population, has not been proven to be superior to a rate control strategy. This may in large be due to study design coupled with deleterious effects of antiarrhythmic agents. There have been considerable advances over the past decade in catheter and device based management of atrial fibrillation and studies aimed to examine their long-term effect in patients with heart failure are underway.
Keywords: Atrial fibrillation, Heart failure, Epidemiology, Pathophysiology, Therapy
Introduction
Epidemiology
Atrial fibrillation (AF) and congestive heart failure (CHF) have emerged as new cardiovascular epidemics over the last decade, and often manifest as coexistent conditions.[1] The high prevalence and progressive nature of these two disease entities is a cause for significant morbidity and mortality. Currently, an estimated 6.6 million patients in the United States, or 2.8% of the population, are affected by heart failure, with >670,000 new diagnoses each year.[2] Heart failure is the primary reason for 12 to 15 million office visits and 6.5 million hospital days yearly.[3] According to the National Hospital Discharge Survey data the annual number of hospitalization for heart failure as a primary diagnosis has increased from 409,000 in 1979 to 1,166,000 in 2004.[4] The steadily increasing number of patients with heart failure is in part due to increased “salvage” of patients with extensive myocardial infarction who previously would not have survived.[2] As the most common hospital discharge diagnosis, heart failure presents a significant economic burden on our society with more Medicare dollars spent in the diagnosis and treatment of heart failure than for any other diagnosis.[5] In 2007, the American Heart Association estimated than $33 billion was spent on heart failure alone.[6]
Atrial fibrillation is also a common diagnosis, with an estimated prevalence of AF in the United States ranging from 2.7 to 6.1 million in the year 2010, with a projected increase in its prevalence.[2] Population based studies based on the growing proportion of elderly individuals in the United States and the current rate of increase in AF incidence, propose a projected number ranging from 5.6 to 15.9 million persons with AF in the United States by 2050.[7-8] AF is the most common arrhythmia in clinical practice, accounting for approximately one third of admissions resulting from cardiac rhythm disturbances. During the last 20 years, hospital admissions for AF have increased by 66% for a number of reasons, including the aging of the population, the rising prevalence of chronic heart disease, and more frequent diagnosis as a result of increased monitoring.[9] An estimated 26 billion Medicare dollars was spent in the management of AF in the year 2008.[2]
AF and Heart Failure
The association between AF and heart failure was appreciated almost a century ago,[10] and in 1937, Paul Dudley White noted, “Since auricular fibrillation so often complicates very serious heart disease its occurrence may precipitate heart failure or even death, unless successful therapy is quickly instituted.”[11] Modern heart failure series report a prevalence of AF ranging from 13 to 27%,[12-17] and the prevalence of AF increases in parallel with the degree of heart failure present.[18] Patients with mild heart failure and New York Heart Association (NYHA) functional class I have an AF prevalence of <5%,[19-20] while those with severe heart failure and NYHA functional class IV symptoms have a prevalence of AF up to 50%.[21] NYHA functional class II or III heart failure patients have an intermediate prevalence of AF.[22-23] Heart failure and AF share common risk factors such as age, hypertension, diabetes, and obesity, along with ischemic, non-ischemic, and valvular heart diease. These factors are associated with myocardial cellular and extracellular alterations, electrophysiologic and neurohormonal changes that combine to create an environment that promotes the development of both heart failure and AF.[24]
Pathophysiology of AF and Heart Failure
AF Begets HF
The pathophysiologic changes that occur in patients with AF and heart failure are complex and only partially understood, with each disease process creating an environment promoting the development of the other (Figure 1). AF may facilitate the development or progression of heart failure. The incidence of heart failure in individuals with AF in Framingham, Massachusetts[17] and Olmsted County, Minnesota[8] ranged from 3.3 to 4.4 per 100 person-years of follow up. Compared with patients in sinus rhythm, patients with severe HF and AF have a reduction in stroke volume, cardiac output, peak oxygen consumption, and peak workload.[18] Cardiac output is decreased in patients with AF due to various mechanisms. Increase in resting heart rate and an exaggerated heart rate response to exercise results in shortening of diastolic filling time, with a resultant decrease in cardiac output. The loss of atrioventricular synchrony plays a significant role, by impairing diastolic filling, decreasing stroke volume, and increasing mean diastolic atrial pressure, resulting in an estimated 20% reduction in cardiac output.[18] In addition,the irregularity of the ventricular response may adversely affect ventricular function and hemodynamic status, with decreased cardiac output, independent of heart rate.[19,25]
Figure 1. AF and heart failure: a vicious pathophysiological cycle. LA indicates left atrial; MR, mitral regurgitation; and TR, tricuspid regurgitation.
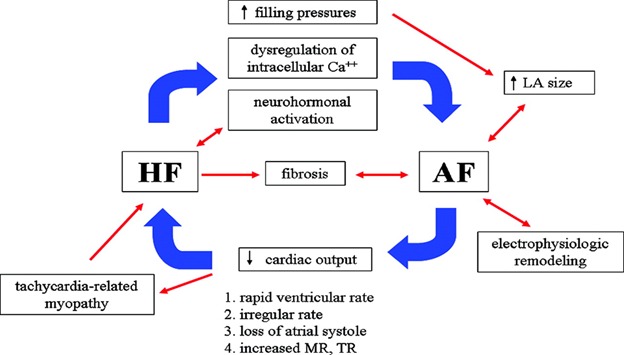
The relationship between AF and heart failure is most notable in the development of tachycardia-induced cardiomyopathy, in patients with poorly controlled ventricular rates during AF. AF is the most common cause of tachycardia-induced cardiomyopathy. The incidence of tachycardia-induced cardiomyopathy is unknown, as most reports have been small, retrospective series or case studies involving mostly patients with AF. Improvement in ejection fraction has been reported in patients who undergo radiofrequency ablation for AF or atrial flutter, and in this patient population the incidence of tachycardia-induced myopathy appears to be around 25-50%.[26-29] The first experimental model for this condition was presented by Whipple et al,[30] who demonstrated that chronic rapid atrial pacing led to low-output heart failure. The mechanisms responsible for tachycardia-induced cardiomyopathy have not been fully elucidated. However, experiments in animal models suggest that potential mechanisms for tachycardia-induced cardiomyopathy include myocardial ischemia, myocardial energy depletion, and abnormalities in calcium regulation.[31] Studies have confirmed that the elimination of these arrhythmias reverses the hemodynamic and clinical manifestations associated with this syndrome.[32]
HF Begets AF
Heart failure produces changes in the atrium which promote the development of AF. Various mechanisms including elevation of cardiac filling pressures, dysregulation of intracelluluar calcium, and autonomic and neuroendocrine dysfunction all play an important role (Figure 1). These changes result in decreased atrial refractory period, slowed atrial conduction, or increased heterogeneity of atrial repolarization, creating a substrate for the initiation and maintenance of AF.[18] Atrial stretch, as a consequence of increased atrial volume and pressure, activates stretch-activated ionic currents which result in increased dispersion of refractoriness and alterations in anisotropic and conduction properties.[33] Inhibition of these stretch-activated currents by gadolinium can reduce the susceptibility to AF in response to atrial pressure overload.[34] Dysregulation of intracellular calcium is an important shared mechanism in the pathophysiology of heart failure and AF. The key regulators of intracellular calcium metabolism, the ryanodine receptor and the sarcoplasmic reticulum Ca2+-ATPase, are downregulated in AF.[35-36] Furthermore, atrial ion channel remodeling has been demonstrated by an experimental HF model, with a notable increase in the Na+-Ca2+ exchanger current, which may cause delayed afterdepolarizations and triggered activity.[37] Heart failure has been associated with increased interstitial fibrosis.[38] Increased fibrosis in the atria leads to abnormal conduction and creates a substrate for AF in animal models.[38-40] Lastly, the neurohormonal alterations that occur in HF also promotes structural remodeling and atrial fibrosis.[38,41]
Prognostic Significance of AF in Heart Failure
The prognostic significance of AF in patients with heart failure remains controversial due to a lack of consensus that AF is an independent risk factor of adverse outcome (Table 1). Several recent trials have identified the presence of AF as an important predictor of mortality. In a retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trial which enrolled 6517 patients with LV ejection fraction (LVEF) < 35%, baseline AF was an independent predictor for all-cause mortality.[19] The increased mortality in AF patients compared to those in sinus rhythm was largely due to an increase in pump failure (16.7 vs. 9.4%). In the DIG trial which enrolled 7788 patients, 11% developed a supraventricular tachycardia (including, but not limited to AF) over a 3 year follow up period.[42] The development of supraventricular tachycardia independently increased the risk of total mortality (RR 2.45), stroke (RR 2.35), and hospitalization for worsening CHF (RR 3.00). In the Valsartan in Acute Myocardial Infarction (VALIANT) trial of 14 703 patients with acute myocardial infarction complicated by heart failure, AF was associated with a greater long-term morbidity and mortality.[43] AF is also associated with increased mortality in patients with heart failure and preserved ejection fraction. In a study evaluating 300 elderly patients with prior myocardial infarction and HF with preserved LVEF, AF was associated with a significantly higher 6 month mortality rate compared to sinus rhythm (11% vs. 2%).[44]
Table 1. Prognostic Significance of Atrial Fibrillation in Patients with Heart Failure.
SR indicates sinus rhythm; DIG, Digitalis Investigation Group; PRIME II, Prospective Randomized study of Ibopamine on Mortality and Efficacy. *After adjustment for important prognostic variables.
| Author/Substudy | Year | NYHA Class | Patients,n | AF,% | Follow-up,y | Patients in SR, n | Patients with AF, n | P | Predictor |
|---|---|---|---|---|---|---|---|---|---|
| Middlekauff et al[12] | 1991 | III-IV | 395 | 19 | 1.5 | 29 | 48 | 0.0013 | Yes |
| Carson et al[13] V-HeFT I V-HeFT II | 1993 | II-III II-III |
632 795 |
15 13 |
2.5 2.0 |
64 52 |
54 46 |
0.86 0.68 |
No |
| Dries et al[19]/SOLVD | 1998 | I-IV | 6517 | 6 | 2.8 | 23 | 34 | <0.001 | Yes |
| Mahoney et al[14] | 1999 | III-IV | 234 | 27 | 1.1 | 16 | 23 | 0.21 | No |
| Middlekauff et al[12] 1985-1989 1990-1993 | 1998 | III-IV III-IV |
359 391 |
20 24 |
2.0 2.0 |
45 25 |
61 34 |
0.002 0.09 |
Yes No |
| Mathew/DIG[42] | 2000 | I-IV | 7788 | 11 | 3.0 | 32 | 43 | 0.0001 | Yes |
| Crijns/PRIME II[47] | 2000 | III-IV | 409 | 84 | 3.4 | 47 | 60 | NS* | No |
| Køber/VALIANT[43] | 2006 | I-IV | 14703 | 15 | 3.0 | 20 | 37 | <0.0001 | Yes |
| Swedberg et al/COMET[51] | 2005 | II-IV | 3029 | 20 | 5.0 | 37 | 42 | NS | No* |
Interestingly, AF appears to be a stronger predictor of negative outcomes in the subset of patients with mild to moderate heart failure compared with patients with severe heart failure, in whom the contribution of AF to further impairment in survival is limited. Middlekauf et al[12] found that in patients with advanced heart failure with NYHA functional class III-IV, the presence of AF was predictive of decreased 1 year survival (44% vs. 83%) only in patients with a pulmonary capillary wedge pressure of less than 16mmHg on therapy, but not in patients with high pulmonary capillary wedge pressure. Corell et al[45] reported a similar finding in outpatients with AF and heart failure in whom AF is a stronger predictor of adverse outcome in patients with better cardiac function (LVEF>35%). In the Trandolapril Cardiac Evaluation (TRACE) study, long term mortality was increased in all subgroups of patients with AF except those with the most advanced disease (LVEF<25%).[46] These trials suggest that the independent effect of AF on mortality may be limited to patients with mild to moderate degrees of heart failure.
The results of these studies are in contrast to those in which AF does not appear to confer a mortality risk. In the Vasodilator Heart Failure Trial (V-HeFT) which enrolled 1427 patients with mild to moderate heart failure, the presence of AF was not associated with a worse outcome.[13] In a study of 409 patients with advanced heart failure, Crijns et al[47] found that the increased mortality in patients with AF (60% vs. 47%) was no longer significant after adjusting for age, LVEF, NYHA functional class, renal function, and blood pressure. Other relatively small studies have also concluded that AF is not an independent predictor for mortality in heart failure.[14,48-49] However, the negative results of these studies is likely related to the small number of patients and lack of power to detect a significant mortality difference.
The timing and chronicity of AF has also been found to be an important prognostic factor. Many studies have found that new-onset AF carries a particularly grave prognosis in patients with heart failure. Almed and Perry[50] studied 944 elderly patients hospitalized for heart failure and found that compared with patients with no past or current AF, those with new onset AF had a 57% higher risk of death. Past or chronic AF was not associated with a significant higher risk of death. New onset AF, but not baseline AF, remained an independent predictor of all-cause mortality in an analysis of COMET.[51] The mortality risk is particularly elevated in the first few months after initial diagnosis. In a community-based cohort of patients newly diagnosed with AF, the mortality risk was substantially higher within the first 4 months, with a hazard ratio of 9.62 (95% CI, 8.93 to 10.32) compared with a hazard ratio of 1.66 (95% CI, 1.59 to 1.73) thereafter (Figure 3).[52] The transition from sinus rhythm to AF in patients with mild heart failure has been associated with clinical and hemodynamic deterioration, predisposition to systemic thromboembolism, and overall poorer prognosis.[53] Lastly, the temporal relationship between the diagnosis of AF and heart failure has a significant impact on mortality: patients who developed AF after the diagnosis of heart failure had an increased mortality risk (2.2 fold increase) compared to patients in whom AF was present before the diagnosis of heart failure.[54]
The role of the neuroadrenergic system in the pathophysiology and prognosis of heart failure is well established and markers of neuroadrenergic system activation have been correlated with disease progression and prognosis. The most widely used marker in clinical practice is the brain natriuretic peptide (BNP). Recent studies showed that plasma levels of BNP also correlate with the risk for AF recurrence following cardioversion and is a predictor for new AF during hospitalization in patients with acute ischemic stroke; reinforcing the pathophysiological association between the two diseases.[101 -[102 ]
In summary, there is a large body of evidence to suggest that AF confers worse prognosis in patients with heart failure. This is especially relevant to patients with less advanced heart failure to patients with recent onset of arrhythmia.
Therapeutic Considerations
Rate versus Rhythm Control
Heart failure patients who develop AF have an increased morbidity and mortality, which would suggest that the restoration and maintenance of sinus rhythm in these patients might improve their long-term outcomes. However, there is currently no data to support that pursuing a rhythm control strategy provides any benefit over rate control. The AF Follow-Up Investigation of Rhythm Management (AFFIRM)[55] and the Rate Control Versus Electrical Cardioversion for Persistent AF (RACE)[56] studies found no benefit for rhythm control strategy and actually showed a trend toward harm compared with rate control. Three other prospective randomized trials comparing rhythm to rate control including the How to Treat Chronic Atrial Fibrillation (HOT CAFE),[57] Strategies of Treatment of Atrial Fibrillation (STAF),[58] and Pharmacological Intervention in Atrial Fibrillation (PIAF)[59] trials all showed equivalent outcomes in both arms. It should be noted, however, that only 23% to 64% of patients assigned to rhythm control in these studies actually remained in sinus rhythm. Furthermore, the applicability of these trial data to patients with heart failure is questionable, given the small proportion of patients with heart failure. In the AFFIRM trial, for example, 76% of patients had a normal LVEF, and only 9% had an NYHA functional class of II or greater.[55]
Subgroup analyses of large trials focusing on patients with heart failure suggest favorable outcomes for the maintenance of sinus rhythm. In the RACE study, subgroup analysis of heart failure patients indicated an improved outcome with the maintenance of sinus rhythm after cardioversion.[60] Subgroup analyses of heart failure patients with AF who converted to sinus rhythm with amiodarone have demonstrated a survival benefit in the Congestive Heart Failure: Survival Trial of Antiarrhytmic Therapy (CHF-STAT)[61] and a significant improvement in cardiac function and quality of life in the CAFE-II trial, when compared with a rate control strategy.[62] In the Danish Investigators of Arrhythmia and Mortality on Dofetilide (DIAMOND) study, improved survival was seen in heart failure patients maintained in sinus rhythm with dofetilide.[63] The first prospective trial designed to examine AF therapy strategies in patients with heart failure was the AF Congestive Heart Failure (AF-CHF) trial. In this prospective and randomized study, rhythm control was not superior to a rate control strategy.[64] A total of 1,376 patients with AF and systolic heart failure were randomized to rhythm control (typically with amiodarone) versus rate control. After a mean follow-up of 3 years, the investigators found that rhythm control did not improve mortality, hospitalization due to heart failure exacerbation or stroke when compared to rate control. This study confirmed the applicability of the AFFIRM[55] and RACE[56] trials also to patients with heart failure. However, caution is warranted in interpretation and acceptance of these data. First, patients assigned to rate control strategy were able to achieve adequate rate control at rest and at low-level exercise, which may not reflect “real-life” patients. Second, the benefit of sinus rhythm could have been counterbalanced by the harm of antiarrhythmic medications in a similar fashion to the AFFIRM study. Third, although the prevalence of sinus rhythm in the group assigned to rhythm control was as high as 80%, the actual percentage of patients free of AF following randomization may have been diluted due to significant cross over between the two groups, reflecting a more traditional success rate of amiodarone in the range of 60%. In addition, there is a wide variability in mechanisms of heart failure and underlying structural and hemodynamic abnormalities. Some patients, especially those with diastolic dysfunction, are highly symptomatic in AF and derive significant benefit in the restoration of sinus rhythm while other patients do not significantly benefit from AV synchrony.
Rate Control
Although optimal ventricular rate control in AF is a matter for debate, the guidelines advocates for ventricular rate of 60-80 beats per minutes at rest and 90-110 beats per minutes during moderate exertion. Therefore, adequate rate control should be determined with assessment of chronotropic response with exertion or with a 24-hour Holter monitor. Beta-blockers are the first line agent for rate control in patients with AF and chronic heart failure. In addition to controlling ventricular response, beta-blockers (in particular, bisoprolol, metoprolol succinate, and carvedilol) have shown to decrease mortality in heart failure.[66-68] Nondihydropyridine calcium channel blockers (including verapamil and diltiazem) are also effective rate-controlling agents, but may not be tolerated in patients with a low LVEF due to their negative inotropic effect. Digoxin is a second line agent for rate control and can be used in conjunction with other rate modulating drugs, and has been shown to improve symptoms and decrease hospitalizations in patients with heart failure.[69]
Rhythm Control
Antiarrhythmic drug options are limited in patients with heart failure. The use of class IC agents was associated with increased mortality in the Cardiac Arrhythmia Suppression Trial (CAST)[70] in patients with ventricular ectopy after myocardial infarction, and their use is not recommended in patients with structural heart disease. Antiarrhythmic drug choices in heart failure patients are limited to amiodarone, dofetilide and sotalol. Amiodarone, a class III agent, has been shown to be safe and effective, but is associated with an increase risk for symptomatic bradycardia in patients with advanced heart failure.[71-72] Another class III agent, dofetilide, was found to be safe and effective in heart failure patients in the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) study.[62] Sotalol should be used with caution given its increased risk for torsades de pointes, especially in the setting of electrolyte abnormalities, LVEF ≤ 40%, acute onset or decompensated heart failure or renal failure.[73-74]
AV nodal Ablation and Pacemaker Implantation
Pharmacologic therapy is often ineffective or associated with significant side effects. In patients with symptomatic AF radiofrequency atrioventricular (AV) nodal ablation with subsequent pacemaker placement may be an attractive therapeutic option. In addition to providing symptomatic relief, ablate and pace strategy has been shown to improve cardiac performance.[75] Over a follow up period of 2 years, patients who underwent an AV nodal ablation with pacemaker placement had an improvement in NYHA functional class and decreased hospitalizations. In addition, the LVEF improved from a mean of 42±16% to 50±14%, with the greatest improvement seen in patients with baseline depressed LVEF with an increase from a mean of 35±9% to 46±8%.
The long-term outcomes of the “ablate and pace” strategy is less clear. In a study comparing AV node ablation with AF ablation in[71] elderly patients with pharmacologically refractory AF, AV nodal ablation with pacing with an increased incidence of new heart failure (53% vs. 24%), lower LVEF (44±8% versus 51±10%), and a higher NYHA functional class (1.7±0.9 versus 1.4±0.7).[76] A growing body of evidence underscores the harmful effects of long-term right ventricular pacing. This was evidenced by the DAVID[77] trial which found that in patients with a LVEF ≤40% with an indication for ICD implantation but no indication for antibradycardia pacing, there was trend towards increased mortality and HF hospitalization in patients with chronic RV pacing. Mechanical ventricular dyssynchrony is an established contributor to heart failure and the LV dyssynchrony imposed by right ventricular apical pacing can lead to LV remodeling with dilatation and decreases in LVEF.[78]
Cardiac resynchronization therapy (CRT) may be a preferable pacing method in these patients; however, there is insufficient data at this point to support its routine use. Small randomized studies comparing CRT versus RV pacing in patients undergoing AV nodal ablation for refractory AF have yielded conflicting results with some studies showing a benefit of CRT[79-81] with significant improvements in 6 minute walk test,[79] reduction in exacerbation of HF and hospitalization,[80] and prevention of the reverse remodeling of the left atrium and left ventricle,[81] while another study failed to show any additional benefit of CRT beyond that conferred by rate regularization.[82] A meta-analysis of five randomized clinical trials of patients with AF undergoing AV nodal ablation found no significant reduction in mortality with CRT.[83]
The Block-HF study that was recently published showed that biventricular pacing was superior to conventional RV pacing in patients with AV block and heart failure. Although this study was not designed to examine the effect of pacing solely in patients with refractory AF, about 50% of all participants had AF.[100]
The theoretical benefit of CRT in conjunction with AV nodal ablation needs to be further evaluated in large-scale, multicenter, randomized controlled trials which are more adequately powered to detect major clinical outcomes, including mortality.
AF Ablation
Our ever expanding understanding of the mechanisms of atrial fibrillation and rapidly advancing technologies have made catheter-based ablation of atrial fibrillation an increasingly effective and safe modality of treating patients with atrial fibrillation. Despite studies suggesting an equivalent outcome for pharmacologic rhythm or rate control, many patients derive much symptomatic benefit from the maintenance of sinus rhythm.[55-56,64] The benefit of rhythm control may be counterbalanced by the lack of effective antiarrhythmic drugs, coupled with their significant adverse effects. Catheter-based ablation for AF offers the unique opportunity to retain the benefits of rhythm control without the detrimental effects of antiarrhythmic drugs.[84-87] In a prospective study of 58 patients with systolic heart failure, AF ablation resulted in significant improvement in LV function, exercise capacity, symptoms, and quality of life with the majority of patients (78%) remained in sinus rhythm after a mean follow-up of 1 year.[84] In the more recent the Pulmonary-Vein Isolation for AF in Patients With Heart Failure (PABA-CHF) pulmonary vein isolation was superior to AV nodal ablation combined with biventricular pacing in patients with heart failure.[88] A more recent study randomized patients with advanced heart failure and severe left ventricular dysfunction to a rhythm control with pulmonary vein isolation and rate control strategy.[99] Rhythm control strategy with AF ablation yielded less favorable results without improvement in left ventricular function or 6 minute walk. In addition, only 50% of patients in the ablation group maintained sinus rhythm at 6 months of follow up. Furthermore, this patient population showed increased procedural complication rate of 15%. Patients assigned to ablation in this study were older (mean age 62), had more severe systolic dysfunction (LVEF 16%), and had long-standing and persistent AF (mean 44 months), all predictors of decreased ablation success.[89-90] This study underscores the importance of patient selection for rhythm control strategies, including ablative approach
AF Prevention
Once AF develops in patients with heart failure, this is usually accompanied by progressive and irreversible structural changes leading to disease progression.[91] Hence, an ideal strategy in the management of heart failure patients should involve treatments aimed at prevention of AF. In addition to optimal heart failure therapy aimed to restrict and potentially reverse structural abnormalities, several other non-antiarrhythmic therapies have been shown to be effective in reducing the incidence and recurrence rates of AF in both the general population and those with heart failure. Clinical studies have shown that the inhibition of the renin-angiotensin-aldosterone system can decrease the incidence and recurrence of AF in select patients groups with heart failure.[92-94] In the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) study angiotensin receptor-1 blockers were shown to decrease the incidence of AF in a broad spectrum of >7500 patients with heart failure.[95] A recent study analyzing the EMPHASIS-HF database, found that aldosterone antagonism with eplerenone in NYHA functional class II patients with systolic heart failure reduced the new onset of AF.[96]
Beta-blocker therapy is also associated with a decreased risk for AF. A meta-analysis of 7 randomized, placebo-controlled trials which included 11 952 patients with heart failure already on angiotensin-converting enzyme inhibitors found that beta-blockers reduced the incidence of new AF from 39 to 28 per 1000 patient-years, with a relative risk reduction of 27%.[97] Lastly, statin therapy has been shown to reduce the incidence and recurrence of AF in heart failure patients. A recent meta-analysis of 6 randomized trials with statins including 3 557 patients showed that their use was associated with a significant decreased risk of AF compared with controls subjects (oddsratio, 0.39; 95% CI 0.18-0.85, p = 0.02), with a more marked benefit in the secondary prevention of AF (odds ratio 0.33) than for new onset or postoperative AF (odds ratio, 0.60).[98]
Conclusions:
AF and heart failure are common cardiac conditions which often coexist, due to common risk factors and a complex interplay of the pathophysiology of these two disease entities. Their joint association correlates with adverse outcomes. AF and heart failure share common disease mechanisms and treatment strategies. Optimal medical management of heart failure may protect against the occurrence of AF and therapies targeting AF may prevent the development of congestive heart failure. The debate between a rate control and rhythm control strategy is now fueled with new studies comparing rate control with catheter ablation, potentially increasing the efficacy of rate control while minimizing drug-related side effects. Our choice of antiarrhythmic agents remains limited in this sick population due to their deleterious effects. Further data is needed to guide our decision making in the appropriate use of catheter ablation in this patient population. In the meantime, it remains critical for us as caregivers to take into account the unique complexities of our patients in determining their optimal treatment strategy.
Disclosures
None.
References
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 1997 Nov 06;337 (19):1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Roger Véronique L, Go Alan S, Lloyd-Jones Donald M, Benjamin Emelia J, Berry Jarett D, Borden William B, Bravata Dawn M, Dai Shifan, Ford Earl S, Fox Caroline S, Fullerton Heather J, Gillespie Cathleen, Hailpern Susan M, Heit John A, Howard Virginia J, Kissela Brett M, Kittner Steven J, Lackland Daniel T, Lichtman Judith H, Lisabeth Lynda D, Makuc Diane M, Marcus Gregory M, Marelli Ariane, Matchar David B, Moy Claudia S, Mozaffarian Dariush, Mussolino Michael E, Nichol Graham, Paynter Nina P, Soliman Elsayed Z, Sorlie Paul D, Sotoodehnia Nona, Turan Tanya N, Virani Salim S, Wong Nathan D, Woo Daniel, Turner Melanie B. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 03;125 (1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connell J B, Bristow M R. Economic impact of heart failure in the United States: time for a different approach. J. Heart Lung Transplant. 1994 Jul 1;13 (4):S107–12. [PubMed] [Google Scholar]
- 4.Fang Jing, Mensah George A, Croft Janet B, Keenan Nora L. Heart failure-related hospitalization in the U.S., 1979 to 2004. J. Am. Coll. Cardiol. 2008 Aug 05;52 (6):428–34. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Massie B M, Shah N B. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am. Heart J. 1997 Jun;133 (6):703–12. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 6.Rosamond Wayne, Flegal Katherine, Friday Gary, Furie Karen, Go Alan, Greenlund Kurt, Haase Nancy, Ho Michael, Howard Virginia, Kissela Brett, Kissela Bret, Kittner Steven, Lloyd-Jones Donald, McDermott Mary, Meigs James, Moy Claudia, Nichol Graham, O'Donnell Christopher J, Roger Veronique, Rumsfeld John, Sorlie Paul, Steinberger Julia, Thom Thomas, Wasserthiel-Smoller Sylvia, Hong Yuling. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 06;115 (5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 7.Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 09;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 8.Miyasaka Yoko, Barnes Marion E, Gersh Bernard J, Cha Stephen S, Bailey Kent R, Abhayaratna Walter P, Seward James B, Tsang Teresa S M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114 (2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 9.Friberg Jens, Buch Pernille, Scharling Henrik, Gadsbphioll Niels, Jensen Gorm B. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003 Nov;14 (6):666–72. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie J. disease of the Heart 3rd ed. London, UK: Oxford Medical Publications; 1914;0:0–0. [Google Scholar]
- 11.White PD. Heart Diseas0e. New York, NY: The McMillan Co; 1937;0:0–0. [Google Scholar]
- 12.Middlekauff H R, Stevenson W G, Stevenson L W. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991 Jul;84 (1):40–8. doi: 10.1161/01.cir.84.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Carson P E, Johnson G R, Dunkman W B, Fletcher R D, Farrell L, Cohn J N. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993 Jun;87 (6 Suppl):VI102–10. [PubMed] [Google Scholar]
- 14.Mahoney P, Kimmel S, DeNofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am. J. Cardiol. 1999 Jun 01;83 (11):1544–7. doi: 10.1016/s0002-9149(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 15.Senni M, Tribouilloy C M, Rodeheffer R J, Jacobsen S J, Evans J M, Bailey K R, Redfield M M. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998 Nov 24;98 (21):2282–9. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 16.Deedwania P C, Singh B N, Ellenbogen K, Fisher S, Fletcher R, Singh S N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998 Dec 08;98 (23):2574–9. doi: 10.1161/01.cir.98.23.2574. [DOI] [PubMed] [Google Scholar]
- 17.Wang Thomas J, Larson Martin G, Levy Daniel, Vasan Ramachandran S, Leip Eric P, Wolf Philip A, D'Agostino Ralph B, Murabito Joanne M, Kannel William B, Benjamin Emelia J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003 Jun 17;107 (23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 18.Maisel William H, Stevenson Lynne Warner. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003 Mar 20;91 (6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 19.Dries D L, Exner D V, Gersh B J, Domanski M J, Waclawiw M A, Stevenson L W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998 Sep;32 (3):695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 20.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N. Engl. J. Med. 1992 Sep 03;327 (10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 21.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N. Engl. J. Med. 1987 Jun 04;316 (23):1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 22.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N. Engl. J. Med. 1991 Aug 01;325 (5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 23.Carson P E, Johnson G R, Dunkman W B, Fletcher R D, Farrell L, Cohn J N. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993 Jun;87 (6 Suppl):VI102–10. [PubMed] [Google Scholar]
- 24.Kareti Kiran R, Chiong Jun R, Hsu Steve S, Miller Alan B. Congestive heart failure and atrial fibrillation: rhythm versus rate control. J. Card. Fail. 2005 Apr;11 (3):164–72. doi: 10.1016/j.cardfail.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Naito M, David D, Michelson EL , Schaffenberg M , Dreifus LS . The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J. 1983;106:284–291. doi: 10.1016/0002-8703(83)90194-1. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger J A, Steinberg J S. Resolution of cardiomyopathy after ablation of atrial flutter. J. Am. Coll. Cardiol. 1998 Jul;32 (1):205–10. doi: 10.1016/s0735-1097(98)00183-1. [DOI] [PubMed] [Google Scholar]
- 27.Redfield M M, Kay G N, Jenkins L S, Mianulli M, Jensen D N, Ellenbogen K A. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin. Proc. 2000 Aug;75 (8):790–5. doi: 10.4065/75.8.790. [DOI] [PubMed] [Google Scholar]
- 28.Edner M, Caidahl K, Bergfeldt L, Darpö B, Edvardsson N, Rosenqvist M. Prospective study of left ventricular function after radiofrequency ablation of atrioventricular junction in patients with atrial fibrillation. Br Heart J. 1995 Sep;74 (3):261–7. doi: 10.1136/hrt.74.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentlesk Philip J, Sauer William H, Gerstenfeld Edward P, Lin David, Dixit Sanjay, Zado Erica, Callans David, Marchlinski Francis E. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 30.Whipple GH, Sheffield LT, Woodman EG, Theophilis C, Friedman S. Reversible congestive heart failure due to chronic rapid stimulation of the normal heart. Proc N Engl Cardiovasc soc. 1962;20:39–40. [Google Scholar]
- 31.Shinbane J S, Wood M A, Jensen D N, Ellenbogen K A, Fitzpatrick A P, Scheinman M M. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J. Am. Coll. Cardiol. 1997 Mar 15;29 (4):709–15. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 32.Van Gelder I C, Crijns H J, Blanksma P K, Landsman M L, Posma J L, Van Den Berg M P, Meijler F L, Lie K I. Time course of hemodynamic changes and improvement of exercise tolerance after cardioversion of chronic atrial fibrillation unassociated with cardiac valve disease. Am. J. Cardiol. 1993 Sep 01;72 (7):560–6. doi: 10.1016/0002-9149(93)90352-d. [DOI] [PubMed] [Google Scholar]
- 33.Solti F, Vecsey T, Kékesi V, Juhász-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc. Res. 1989 Oct;23 (10):882–6. doi: 10.1093/cvr/23.10.882. [DOI] [PubMed] [Google Scholar]
- 34.Bode F, Katchman A, Woosley R L, Franz M R. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000 May 09;101 (18):2200–5. doi: 10.1161/01.cir.101.18.2200. [DOI] [PubMed] [Google Scholar]
- 35.Beuckelmann D J, Näbauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992 Mar;85 (3):1046–55. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 36.Ohkusa T, Ueyama T, Yamada J, Yano M, Fujumura Y, Esato K, Matsuzaki M. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J. Am. Coll. Cardiol. 1999 Jul;34 (1):255–63. doi: 10.1016/s0735-1097(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000 Jun 06;101 (22):2631–8. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Fareh S, Leung T K, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999 Jul 06;100 (1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Guerra Jose M, Everett Thomas H, Lee Ken W, Wilson Emily, Olgin Jeffrey E. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006 Jul 11;114 (2):110–8. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 40.Lee Ken W, Everett Thomas H, Rahmutula Dulkon, Guerra Jose M, Wilson Emily, Ding Chunhua, Olgin Jeffrey E. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006 Oct 17;114 (16):1703–12. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cha Yong-Mei, Dzeja Petras P, Shen Win K, Jahangir Arshad, Hart Chari Y T, Terzic Andre, Redfield Margaret M. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am. J. Physiol. Heart Circ. Physiol. 2003 Apr;284 (4):H1313–20. doi: 10.1152/ajpheart.00337.2002. [DOI] [PubMed] [Google Scholar]
- 42.Mathew J, Hunsberger S, Fleg J, Mc Sherry F, Williford W, Yusuf S. Incidence, predictive factors, and prognostic significance of supraventricular tachyarrhythmias in congestive heart failure. Chest. 2000 Oct;118 (4):914–22. doi: 10.1378/chest.118.4.914. [DOI] [PubMed] [Google Scholar]
- 43.Køber Lars, Swedberg Karl, McMurray John J V, Pfeffer Marc A, Velazquez Eric J, Diaz Rafael, Maggioni Aldo P, Mareev Viatcheslav, Opolski Grzegorz, Van de Werf Frans, Zannad Faiez, Ertl Georg, Solomon Scott D, Zelenkofske Steven, Rouleau Jean-Lucien, Leimberger Jeffrey D, Califf Robert M. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur. J. Heart Fail. 2006 Oct;8 (6):591–8. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Aronow W S, Ahn C, Kronzon I. Prognosis of congestive heart failure after prior myocardial infarction in older persons with atrial fibrillation versus sinus rhythm. Am. J. Cardiol. 2001 Jan 15;87 (2):224–5, A8-9. doi: 10.1016/s0002-9149(00)01324-2. [DOI] [PubMed] [Google Scholar]
- 45.Corell Pernille, Gustafsson Finn, Schou Morten, Markenvard John, Nielsen Tonny, Hildebrandt Per. Prevalence and prognostic significance of atrial fibrillation in outpatients with heart failure due to left ventricular systolic dysfunction. Eur. J. Heart Fail. 2007 Mar;9 (3):258–65. doi: 10.1016/j.ejheart.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen Ole Dyg, Bagger Henning, Køber Lars, Torp-Pedersen Christian. Impact of congestive heart failure and left ventricular systolic function on the prognostic significance of atrial fibrillation and atrial flutter following acute myocardial infarction. Int. J. Cardiol. 2005 Apr 08;100 (1):65–71. doi: 10.1016/j.ijcard.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Crijns H J, Tjeerdsma G, de Kam P J, Boomsma F, van Gelder I C, van den Berg M P, van Veldhuisen D J. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur. Heart J. 2000 Aug;21 (15):1238–45. doi: 10.1053/euhj.1999.2107. [DOI] [PubMed] [Google Scholar]
- 48.Likoff M J, Chandler S L, Kay H R. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am. J. Cardiol. 1987 Mar 01;59 (6):634–8. doi: 10.1016/0002-9149(87)91183-0. [DOI] [PubMed] [Google Scholar]
- 49.Keogh A M, Baron D W, Hickie J B. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am. J. Cardiol. 1990 Apr 01;65 (13):903–8. doi: 10.1016/0002-9149(90)91434-8. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed Ali, Perry Gilbert J. Incident atrial fibrillation and mortality in older adults with heart failure. Eur. J. Heart Fail. 2005 Dec;7 (7):1118–21. doi: 10.1016/j.ejheart.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Swedberg Karl, Olsson Lars G, Charlesworth Andrew, Cleland John, Hanrath Peter, Komajda Michel, Metra Marco, Torp-Pedersen Christian, Poole-Wilson Philip. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur. Heart J. 2005 Jul;26 (13):1303–8. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 52.Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 53.Pozzoli M, Cioffi G, Traversi E, Pinna G D, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J. Am. Coll. Cardiol. 1998 Jul;32 (1):197–204. doi: 10.1016/s0735-1097(98)00221-6. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlain Alanna M, Redfield Margaret M, Alonso Alvaro, Weston Susan A, Roger Véronique L. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011 Nov;4 (6):740–6. doi: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 56.Van Gelder Isabelle C, Hagens Vincent E, Bosker Hans A, Kingma J Herre, Kamp Otto, Kingma Tsjerk, Said Salah A, Darmanata Julius I, Timmermans Alphons J M, Tijssen Jan G P, Crijns Harry J G M. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 57.Opolski Grzegorz, Torbicki Adam, Kosior Dariusz A, Szulc Marcin, Wozakowska-Kaplon Beata, Kolodziej Piotr, Achremczyk Piotr. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004 Aug;126 (2):476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 58.Carlsson Jörg, Miketic Sinisa, Windeler Jürgen, Cuneo Alessandro, Haun Sebastian, Micus Stefan, Walter Sabine, Tebbe Ulrich. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J. Am. Coll. Cardiol. 2003 May 21;41 (10):1690–6. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 59.Hohnloser S H, Kuck K H, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000 Nov 25;356 (9244):1789–94. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 60.Hagen V, Van Veldhuisen D, Kamp O, Rienstra M, Bosker HA, Veeger NJ, Tijssen JG, Crigns HJ, Van Gelder IC. Rate Control versus Electrical Cardioversion for Persistent Atrail Fibrillation Study Group. Effect of rate and rhythm control on left ventricle function and cardiac dimensions in patients with persistent atrial fibrillation: results from the RAte Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) study. Heart Rhythm. 2005;2:19–24. doi: 10.1016/j.hrthm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Deedwania P C, Singh B N, Ellenbogen K, Fisher S, Fletcher R, Singh S N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998 Dec 08;98 (23):2574–9. doi: 10.1161/01.cir.98.23.2574. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen O D, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001 Jul 17;104 (3):292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 63.Shelton R J, Clark A L, Goode K, Rigby A S, Houghton T, Kaye G C, Cleland J G F. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart. 2009 Jun;95 (11):924–30. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 64.Roy Denis, Talajic Mario, Nattel Stanley, Wyse D George, Dorian Paul, Lee Kerry L, Bourassa Martial G, Arnold J Malcolm O, Buxton Alfred E, Camm A John, Connolly Stuart J, Dubuc Marc, Ducharme Anique, Guerra Peter G, Hohnloser Stefan H, Lambert Jean, Le Heuzey Jean-Yves, O'Hara Gilles, Pedersen Ole Dyg, Rouleau Jean-Lucien, Singh Bramah N, Stevenson Lynne Warner, Stevenson William G, Thibault Bernard, Waldo Albert L. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008 Jun 19;358 (25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 65.Talajic Mario, Khairy Paul, Levesque Sylvie, Connolly Stuart J, Dorian Paul, Dubuc Marc, Guerra Peter G, Hohnloser Stefan H, Lee Kerry L, Macle Laurent, Nattel Stanley, Pedersen Ole D, Stevenson Lynne Warner, Thibault Bernard, Waldo Albert L, Wyse D George, Roy Denis. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J. Am. Coll. Cardiol. 2010 Apr 27;55 (17):1796–802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 66.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999 Jan 02;353 (9146):9–13. [PubMed] [Google Scholar]
- 67.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999 Jun 12;353 (9169):2001–7. [PubMed] [Google Scholar]
- 68.Joglar J A, Acusta A P, Shusterman N H, Ramaswamy K, Kowal R C, Barbera S J, Hamdan M H, Page R L. Effect of carvedilol on survival and hemodynamics in patients with atrial fibrillation and left ventricular dysfunction: retrospective analysis of the US Carvedilol Heart Failure Trials Program. Am. Heart J. 2001 Sep;142 (3):498–501. doi: 10.1067/mhj.2001.117318. [DOI] [PubMed] [Google Scholar]
- 69.Rich M W, McSherry F, Williford W O, Yusuf S. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J. Am. Coll. Cardiol. 2001 Sep;38 (3):806–13. doi: 10.1016/s0735-1097(01)01442-5. [DOI] [PubMed] [Google Scholar]
- 70.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW, the CAST Investigators. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: the Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 71.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg M J, Green M, Kus T, Lambert J, Dubuc M, Gagné P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000 Mar 30;342 (13):913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 72.Weinfeld M S, Drazner M H, Stevenson W G, Stevenson L W. Early outcome of initiating amiodarone for atrial fibrillation in advanced heart failure. J. Heart Lung Transplant. 2000 Jul;19 (7):638–43. doi: 10.1016/s1053-2498(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 73.Soyka L F, Wirtz C, Spangenberg R B. Clinical safety profile of sotalol in patients with arrhythmias. Am. J. Cardiol. 1990 Jan 02;65 (2):74A–81A. doi: 10.1016/0002-9149(90)90207-h. [DOI] [PubMed] [Google Scholar]
- 74.Lehmann M H, Hardy S, Archibald D, quart B, MacNeil D J. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation. 1996 Nov 15;94 (10):2535–41. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 75.Manolis A G, Katsivas A G, Lazaris E E, Vassilopoulos C V, Louvros N E. Ventricular performance and quality of life in patients who underwent radiofrequency AV junction ablation and permanent pacemaker implantation due to medically refractory atrial tachyarrhythmias. J Interv Card Electrophysiol. 1998 Mar;2 (1):71–6. doi: 10.1023/a:1009721008761. [DOI] [PubMed] [Google Scholar]
- 76.Hsieh Ming-Hsiung, Tai Ching-Tai, Lee Shih-Huang, Tsao Huan-Ming, Lin Yung-Kuo, Huang Jin-Long, Chan Paul, Chen Yi-Jen, Kuo Jen-Yuan, Tuan Ta-Chuan, Hsu Tsui-Lieh, Kong Chi-Woon, Chang Shih-Lin, Chen Shih-Ann. Catheter ablation of atrial fibrillation versus atrioventricular junction ablation plus pacing therapy for elderly patients with medically refractory paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2005 May;16 (5):457–61. doi: 10.1111/j.1540-8167.2005.40632.x. [DOI] [PubMed] [Google Scholar]
- 77.Wilkoff Bruce L, Cook James R, Epstein Andrew E, Greene H Leon, Hallstrom Alfred P, Hsia Henry, Kutalek Steven P, Sharma Arjun. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002 Dec 25;288 (24):3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 78.O'Keefe James H, Abuissa Hussam, Jones Philip G, Thompson Randall C, Bateman Timothy M, McGhie A Iain, Ramza Brian M, Steinhaus David M. Effect of chronic right ventricular apical pacing on left ventricular function. Am. J. Cardiol. 2005 Mar 15;95 (6):771–3. doi: 10.1016/j.amjcard.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 79.Doshi Rahul N, Daoud Emile G, Fellows Christopher, Turk Kyong, Duran Aurelio, Hamdan Mohamed H, Pires Luis A. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1160–5. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- 80.Orlov Michael V, Gardin Julius M, Slawsky Mara, Bess Renee L, Cohen Gerald, Bailey William, Plumb Vance, Flathmann Horst, de Metz Katerina. Biventricular pacing improves cardiac function and prevents further left atrial remodeling in patients with symptomatic atrial fibrillation after atrioventricular node ablation. Am. Heart J. 2010 Feb;159 (2):264–70. doi: 10.1016/j.ahj.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Brignole Michele, Botto Gianluca, Mont Lluis, Iacopino Saverio, De Marchi Giuseppe, Oddone Daniele, Luzi Mario, Tolosana Jose M, Navazio Alessandro, Menozzi Carlo. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur. Heart J. 2011 Oct;32 (19):2420–9. doi: 10.1093/eurheartj/ehr162. [DOI] [PubMed] [Google Scholar]
- 82.Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, Sutton R, Vardas P, Bongiorni M G, Bergfeldt L, Menozzi C, Musso G. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur. Heart J. 2005 Apr;26 (7):712–22. doi: 10.1093/eurheartj/ehi069. [DOI] [PubMed] [Google Scholar]
- 83.Stavrakis Stavros, Garabelli Paul, Reynolds Dwight W. Cardiac resynchronization therapy after atrioventricular junction ablation for symptomatic atrial fibrillation: a meta-analysis. Europace. 2012 Oct;14 (10):1490–7. doi: 10.1093/europace/eus193. [DOI] [PubMed] [Google Scholar]
- 84.Hsu Li-Fern, Jaïs Pierre, Sanders Prashanthan, Garrigue Stéphane, Hocini Mélèze, Sacher Fréderic, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Scavée Christophe, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004 Dec 02;351 (23):2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 85.Gentlesk Philip J, Sauer William H, Gerstenfeld Edward P, Lin David, Dixit Sanjay, Zado Erica, Callans David, Marchlinski Francis E. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 86.Tondo Claudio, Mantica Massimo, Russo Giovanni, Avella Andrea, De Luca Lucia, Pappalardo Augusto, Fagundes Rafael Lopes, Picchio Edo, Laurenzi Francesco, Piazza Vito, Bisceglia Irma. Pulmonary vein vestibule ablation for the control of atrial fibrillation in patients with impaired left ventricular function. Pacing Clin Electrophysiol. 2006 Sep;29 (9):962–70. doi: 10.1111/j.1540-8159.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- 87.Chen Michael S, Marrouche Nassir F, Khaykin Yaariv, Gillinov A Marc, Wazni Oussama, Martin David O, Rossillo Antonio, Verma Atul, Cummings Jennifer, Erciyes Demet, Saad Eduardo, Bhargava Mandeep, Bash Dianna, Schweikert Robert, Burkhardt David, Williams-Andrews Michelle, Perez-Lugones Alejandro, Abdul-Karim Ahmad, Saliba Walid, Natale Andrea. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004 Mar 17;43 (6):1004–9. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 88.Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 89.Oakes Robert S, Badger Troy J, Kholmovski Eugene G, Akoum Nazem, Burgon Nathan S, Fish Eric N, Blauer Joshua J E, Rao Swati N, DiBella Edward V R, Segerson Nathan M, Daccarett Marcos, Windfelder Jessiciah, McGann Christopher J, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009 Apr 07;119 (13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò M P, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001 Nov 20;104 (21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 91.Burstein Brett, Nattel Stanley. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 92.Pedersen O D, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999 Jul 27;100 (4):376–80. doi: 10.1161/01.cir.100.4.376. [DOI] [PubMed] [Google Scholar]
- 93.Vermes Emmanuelle, Tardif Jean-Claude, Bourassa Martial G, Racine Normand, Levesque Sylvie, White Michel, Guerra Peter G, Ducharme Anique. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003 Jun 17;107 (23):2926–31. doi: 10.1161/01.CIR.0000072793.81076.D4. [DOI] [PubMed] [Google Scholar]
- 94.Maggioni Aldo P, Latini Roberto, Carson Peter E, Singh Steven N, Barlera Simona, Glazer Robert, Masson Serge, Cerè Elisabetta, Tognoni Gianni, Cohn Jay N. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am. Heart J. 2005 Mar;149 (3):548–57. doi: 10.1016/j.ahj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 95.Ducharme Anique, Swedberg Karl, Pfeffer Marc A, Cohen-Solal Alain, Granger Christopher B, Maggioni Aldo P, Michelson Eric L, McMurray John J V, Olsson Lars, Rouleau Jean L, Young James B, Olofsson Bertil, Puu Margareta, Yusuf Salim. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am. Heart J. 2006 Jul;152 (1):86–92. [PubMed] [Google Scholar]
- 96.Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B. EMPHASIS-HF Study Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 97.Nasr Imad Abi, Bouzamondo Anissa, Hulot Jean-Sébastien, Dubourg Olivier, Le Heuzey Jean-Yves, Lechat Philippe. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur. Heart J. 2007 Feb;28 (4):457–62. doi: 10.1093/eurheartj/ehl484. [DOI] [PubMed] [Google Scholar]
- 98.Fauchier Laurent, Pierre Bertrand, de Labriolle Axel, Grimard Caroline, Zannad Noura, Babuty Dominique. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):828–35. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 99.MacDonald Michael R, Connelly Derek T, Hawkins Nathaniel M, Steedman Tracey, Payne John, Shaw Morag, Denvir Martin, Bhagra Sai, Small Sandy, Martin William, McMurray John J V, Petrie Mark C. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011 May;97 (9):740–7. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 100.Curtis Anne B, Worley Seth J, Adamson Philip B, Chung Eugene S, Niazi Imran, Sherfesee Lou, Shinn Timothy, Sutton Martin St John. Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013 Apr 25;368 (17):1585–93. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 101.Shibazaki Kensaku, Kimura Kazumi, Fujii Shuichi, Sakai Kenichiro, Iguchi Yasuyuki. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am. J. Cardiol. 2012 May 01;109 (9):1303–7. doi: 10.1016/j.amjcard.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 102.Tang Y, Yang H, Qiu J. Relationship between brain natriuretic peptide and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J. Int. Med. Res. 2011;39 (5):1618–24. doi: 10.1177/147323001103900504. [DOI] [PubMed] [Google Scholar]


