Abstract
In the past years, catheter ablation has evolved into an effective treatment option for symptomatic, drug-resistant atrial fibrillation (AF) and it has recently been implemented as a primary treatment strategy for patients with paroxysmal AF. Although a significant number of studies have evaluated the potential benefits of catheter ablation compared with anti-arrhythmic drug (AAD)-therapy, to date, there are only a small number of randomised controlled trials in the literature, and several issues remain unsolved. The aim of this review is to analyze the current literature regarding this important issue and further discuss the question, whether catheter ablation may be more beneficial when compared to AAD therapy.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, affecting more than 6 million patients throughout Europe; as the population ages, the prevalence is estimated to more than double within the next 50 years.[1] Besides affecting quality of life (QoL), AF is associated with a significant increase in morbidity and mortality due to the developement of heart failure or disabling stroke.[2] Consequently, treatment of AF and AF related complications have become an escalating burden to the health care system. In the past, the only option for the treatment of AF was medical therapy targeting either rate or rhythm control, often associated with adverse drug effects leading to limitations in compliance or even resulting in fatal adverse events due to proarrhythmic effects.[3] Unfortunately, the recurrence rate of AF was still high, even when effective antiarrhythmic drugs such as amiodarone were used.[4]
Within the last decade, catheter ablation of AF has developed from a novel therapeutic option for a highly selected patient population, to the most commonly performed ablation procedure in many electrophysiological laboratories around the world. Haïssaguerre and coworkers have demonstrated that pulmonary veins are a trigger for AF in a substantial proportion of patients.[5] Consequently, pulmonary vein isolation has become the most widely accepted procedural endpoint for AF ablation.[4,6,7]
Natural History of Atrial Fibrillation
The natural course of AF is a progression from paroxysmal atrial AF with only short lasting AF episodes, to more prolonged episodes resulting in persistent and longstanding persistent AF after several years.[8] The majority of patients with paroxysmal AF will eventually develop persistent AF after several decades, with only less than 5% remaining in paroxysmal AF.8 Although progression to chronic AF in patients without structural heart disease may be lower,[9] in the CARAF registry (The Canadian Registry of AF) 25% of patients with paroxysmal AF progressed to permanent AF after 5 years.[10]
Anticoagulation Therapy
Antithrombotic therapy is known as the most important medication for treatment of AF with regard to mortality since the early nineties.[11] To date, only antithrombotic therapy has been clearly associated with a substantial decrease in mortality due to the reduced rate of disabling and non-disabling ischemic strokes.[12] The most effective of these antithrombotic therapies is anticoagulation; this is associated with a comparable bleeding risk to antiplatetelet therapy, but with a significant reduction in thromboembolic risk.[13] In patients with inappropriate INRs (International Normalized Ratio) or after anticoagulation was discontinued in both the AFFIRM and RACE trials, there was an increase in stroke rate, and this fact emphasised the importance of appropriate anticoagulation therapy.[3,14] Novel anticoagulants have further reduced the risk of stroke, as was shown for dabigatran (RELY,[15]), rivaroxaban (Rocket-AF,[16]) and apixaban (AVERROES,[17]). This has allowed patients who have so far been intolerant of, or are unsuitable for treatment with vitamin K antagonists to be treated with anticoagulation therapy. According to the current guidelines, anticoagulation therapy should be individualized for each patient after stratification of risk for ischemic stroke as estimated by the CHA2DS2-Vasc-score (cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 and sex category); currently for patients with a CHA2DS2-Vasc-score ≥2, anticoagulation therapy is the treatment of choice (1) and is also recommended in patients with a CHA2DS2-Vasc-score of 1.
Rhythm or Rate Control
Although the optimal treatment strategy for AF is still controversial and even lenient rate control seems to be mostly effective for a disctinct group of patients,[18] rhythm control remains the therapy of choice for the majority of symptomatic AF patients. So far, no study has clearly demonstrated that rhythm control is superior to rate control with regard to mortality.[3,14,19,20,21] However, a large prospective, observational survey of the management of AF in community-based patients has shown that rhythm-controlled patients progressed less rapidly to permanent AF.[22]
In addition, in clinical trials successful catheter ablation of AF is usually defined as freedom of arrhythmia recurrence lasting more than 30 seconds.. Detection of the true AF burden including asymptomatic episodes of AF-recurrence remains difficult and it has been shown in recent trials, that implanted monitoring devices offer a much higher diagnostic yield than 24h up to 7-day Holter monitoring.[23] In comparison, success with antiarrhythmic therapy is defined as either rate or rhythm control. Finally, it may be due to the limited efficacy or the deleterious side effects such as proarrhythmia and organ toxicity of AAD therapy, that maintaining sinus rhythm has not been shown to be superior to rate control in AF.[3] However, in a subanalysis of the AFFIRM trial, sinus rhythm was associated with a lower risk of death.[3]
Furthermore, in a recently conducted study by Nademanee et al., 674 high-risk AF patients were evaluated for the clinical endpoints of sinus rhythm, death, stroke or bleeding during a mean follow-up period of 836±605 days; this study demonstrated that patients in sinus rhythm had a better 5-year survival rate compared to patients with AF (92% vs 64%; p <0,01); therefore, sinus rhythm after AF ablation was associated with relatively low mortality and stroke risk, and was the most important independant predictor for survival.[24]
Antiarrhythmic Drug Treatment for Rhythm Control
Several AADs are available for rhythm control with propafenone, flecainide, amiodarone and sotalol being the most frequently AADs used in European countries. As shown in the AFFIRM trial, antiarrhythmic drug treatment resulted in sinus rhythm in 82.4% of patients after one year and 62.6% at 5 years.[3] Treatment with flecainide usually leads to an increased likelihood of maintaining SR, which is at least doubled as compared to placebo. Propafenone has a similar efficacy as compared to flecainide but due to its beta-adrenoceptor blocking effect no additional beta-blocker treatment is necessary. However, beta-blockers are commonly added to propafenone therapy. This often causes significant bradycardia, leading to the discontinuation of propafenone treatment. The conversion rate from atrial fibrillation to SR seems to be similar with sotalol and amiodarone, whereas amiodarone is more effective in maintaining sinus rhythm; however, sotalol has shown a similar efficacy as compared to amiodarone for maintanance of sinus rhythm in patients with structural heart disease.[4] Proarrhythmic side effects are more commonly seen with sotalol than with amiodarone. The most feared potential side effect is the torsade-de-pointes tachycardia, which may occur in up to 5% during sotalol therapy but is rarely seen during amiodarone treatment. To reduce the incidence of this complication, sotalol therapy should be terminated or be continued with a reduced dosage when QT-prolongation >500ms is evident.[4] Recently, dronedarone, a multichannel blocker that inhibits sodium and potassium channels was introduced as a novel antiarrhythmic drug. It has a similar efficacy for maintaining sinus rhythm as compared to class I AADs or sotalol but a lower efficacy as compared to amiodarone;.[25] In the ATHENA trial, in which patients with paroxysmal or persistent AF and moderate risk for cardiovascular events were enrolled, dronedarone was associated with a significant reduction in cardiovascular outcome events (composite endpoint of unplanned cardiovascular hospitalizations and all-cause mortality).[26] In the PALLAS trial patients with permanent AF and cardiovascular risk factors were randomized to receive dronedarone or placebo. The trial was stopped prematurely by the Data Monitroing Committee due to an increase in cardiovascular events including cardiovascular mortality in the dronedarone arm.[27] Based predominantly on these studies, according to the current ESC guidelines dronedarone is recommend for treatment of paroxysmal or persistent AF in patients with or without structural heart disease and is not recommended in patients with permanent AF, particularly those with a significant cardiovascular disease burden.[1]
Catheter Ablation for Rhythm Control
Catheter ablation is a highly effective option for treatment of patients with symptomatic AF.[1] The most commonly performed ablation strategy is circumferential pulmonary vein isolation, (Figure 1a) usually performed with radiofrequency (RF).[28] Balloon technologies such as the cryoballoon.[29] or the laserballoon[30] have been developed in order to facilitate PVI particularly in patients with paroxysmal AF. Cryoablation is now established as an alternative to RF catheter ablation due to its single-shot characteristic, with currently approximately 40% of german centers using this technology for catheter ablation of AF.[31] In addition, contact force measurement (Figure 1b) and remote navigation systems have been developed to enhance catheter stability and to potentially improve safety and efficacy of ablation within the left atrium.[32] Besides pulmonary vein isolation, alternative concepts of substrate modification such as ablation of complex atrial fractionated electrograms (CAFE) or ablation of ganglionated plexi have been introduced in the past years with variable results in efficacy.[33,34] A promising novel concept is AF rotor ablation (Figure 2); so far only limited data is available and larger randomized studies are necessary to confirm the impact of this ablation approach.[35,36]
Figure 1A. Circumferential Pulmonary Vein Isolation.

Figure 1B. Circumferential Pulmonary vein isolation using a contact force sensing catheter with ablation lines around the septal and lateral pulmonary veins displayed in a 3D CARTO image in posterior (left image) and left lateral (right image) view. Contact force paramters are displayed in the dashboard, the force vector and the real time graph viewer.

Figure 2. FIRM mapping and ablation during Atrial Fibrillation.
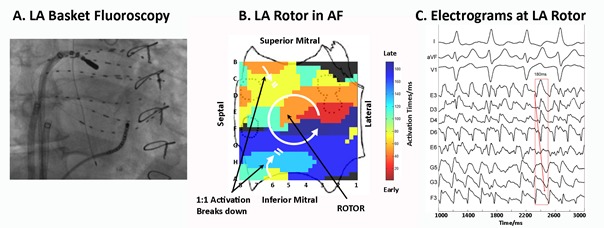
Figure 2 a: Basket and ablation catheters in the right atrium during atrial fibrillation Figure 2b:Counterclockwise rotor in the left atrium. Figure 2c: Electrograms around the rotor site, indicating counterclockwise rotation with variability at CL 180 ms. Catheter ablation for treatment of atrial fibrillation targeting atrial fibrillation rotors (with kind permission from Sanjiv Narayan)
Comparison of Catheter Ablation vs Antiarrhythmic Drug Treatment
Several issues have to be addressed when comparing catheter ablation and AAD therapy for treatment of AF. Firstly, effectiveness of catheter ablation or AAD therapy may vary amongst the different types of AF (i.e. paroxysmal, persistent or longstanding persistent). Secondly, at the present time several ablation strategies exist for the treatment of AF and therefore it may be difficult to compare different ablation strategies with AAD therapy.
Recurrence of Atrial Fibrillation
Only limited data exists comparing the efficacy of catheter ablation with AAD therapy in a randomized fashion; of the available studies, different AADs were used in the control groups, and furthermore, the follow-up (FU)-period of these trials were usually short (Table 1). The results of the first randomized trial were published 2005 by Wazni et al.[37] using a segmental ablation approach for pulmonary vein isolation (PVI) and included 70 patients. Although the study was small and the FU-time was short (12 months), it demonstrated that patients receiving antiarrhythmic drugs were more likely to have at least one recurrence of symptomatic AF, to be readmitted to hospital, and to present with a higher incidence of symptomatic AF recurrence as compared with patients who received PVI. Subsequent randomized trials have confirmed the superiority of catheter ablation for both patients with paroxysmal[38,39,40] and persistent [41,42] AF, or in mixed populations consisting of patients with paroxysmal and persistent AF[43,44] using a circumferential approach for PVI. Recently, the results of the MANTRA-PAF trial have shown that in patients with paroxysmal AF, the outcome did not differ significantly between the ablation group and the AAD group, at least with regards to the primary endpoint of the study, which was cumulative AF burden after 24 months. However, at the 24 months-follow-up the burden of AF was significantly lower in the ablation group as compared to patients treated with AADs; and patients in the ablation group were more likely to be free from any AF or from symptomatic AF.[45] In addition, the preliminary data of the RAAFT 2 trial, which randomized patients with paroxysmal AF to first-line catheter ablation vs AAD treatment, have shown superior results in prolonging time to first recurrence of symptomatic and asymptomatic atrial tachyarrhythmias in patients treated in the ablation group after a follow-up period up to 24 months.[46]
Table 1. Randomised controlled trials comparing AAD therapy with catheter ablation for treatment of atrial fibrillation.
AF= atrial fibrillation; PAF= paroxysmal atrial fibrillation; PersAF= persistent atrial fibrillation; RA= right atrium; CPVA= circumferential pulmonary vein ablation; CTI= cavotricuspid isthmus, RF=radiofrequency; Cryo= Cryoablation
| Author | Year of publication | Patients (n) | AF type | Ablation technique (energy source) | Freedom of AF with AAD therapy ( at 1 year FU) | Freedom of AF after catheter ablation ( at 1 year FU) |
|---|---|---|---|---|---|---|
| Krittayaphong et al. | 2003 | 30 | PAF, PersAF | PVI, RA- lines (RF) | 40% | 79% |
| Wazni et al. | 2005 | 70 | PAF, PersAF | PVI (RF) | 37% | 87% |
| Oral et al. | 2006 | 245 | PersAF | CPVA (RF) | 58% | 74% |
| Pappone et al. | 2006 | 198 | PAF | CPVA (RF) | 22% | 86% |
| Jais et al. | 2008 | 112 | PAF | PVI, CTI (RF) | 23% | 89% |
| Forleo et al. | 2008 | 70 | PAF, PersAF | PVI, CTI (RF) | 43% | 80% |
| Packer et al. | 2010 | 245 | PAF | PVI (Cryo) | 7% | 70% |
| Mantra AF | 2012 | 294 | PAF | PVI (RF) | 68,8% | 85% |
To date, studies evaluating patients with reduced left ventricular function and AF, treated with either AAD therapy or additional catheter ablation are lacking. At the present time, there are currently two ongoing trials addressing this important question (AMICA and CASTLE-AF, ClinicalTrials.gov, see section future perstpectives for further details).
Complications and Mortality of Catheter Ablation and AAD therapy
Complications may arise from both therapeutic options, depending on the AADs used and on the modality and extent of catheter ablation.
Adverse Events of Antiarrhythmic Drug Therapy
Adverse effects of medical therapy vary depending on the type of AAD, ranging from gastrointestinal side effects affecting primarily patient compliance, up to proarrhythmic effects leading to life threatening events such as ventricular tachycardias or torsade de pointes tachycardia. These side effects are dependant on the type of AAD used and several class-effects of AADs have been described. In the CAST trial,[47] class I AADs such as flecainide or propafenone have been associated with an increased risk of deleterious events in patients after myocardial infarction and with significant coronary heart disease. Therefore, in the current ESC guidelines class I AADs are not recommended in patients with previous myocardial infarction, coronary artery disease, substantial LV hypertrophy and reduced ejection fraction.[1] However, when used in carefully selected patients, the incidence of ventricular arrhythmias in patients treated with flecainide is reported to be less than 3% 48 and adverse events seem not to be increased as compared to control groups.[49,50] In patients with structural heart disease, amiodarone and sotalol are recommended,[1] as treatment with these two drugs have not been associated with an increased mortality rate in these patients.[4] Careful monitoring of the QT-interval is pivotal when using sotalol and amiodarone,[51] however the incidence of drug-induced torsade de pointes tachycardias is low during treatment with amiodarone. Increased risk for proarrhythmia during sotalol-therapy is usually more often observed in patients with marked LV-hypertrophy, renal failure and hypokalemia.
Complications of Catheter Ablation
The incidence of periprocedural complications during catheter ablation varies depending on operator experience and the ablation technique used. The overall rate of major periprocedural complications is estimated to be 4.5% as evaluated by an international world-wide survey.[25] The most concerning complications are cardiac tamponade (the most frequent complication with an incidence of approximately 1.3%), transient ischemic attack or stroke, pulmonary vein stenosis, or the extremely rare but usually deleterious atrio-esophageal fistula.[25] The incidence of periprocedural death is reported to be as high as 0.15% mainly related to pericardial tamponade with fatal outcome. Minor complications include esophageal lesions, iatrogenic atrial septal defects or silent cerebral lesions,[52,53,54,55,56] most of which usually recover without sequelae and without altering cardiac or cerebral function. Vascular complications, although usually not deleterious, are frequent (up to 1.5%) and at least in part associated with a substantial morbidity caused by prolonged hospital stay or even vascular surgery.[25]
Mortality
Mortality data were only reported in a limited number of RCTs comparing AADs with catheter ablation of AF. In one study performed by Stabile et al. a mortality rate of 1.5% (1/68) in the catheter ablation group and 2.9% (2/69) in the group treated with AADs was described and did not differ significantly.[43] One patient in the AAD-group died due to cancer and another patient died due to sudden death. In comparison, a patient in the ablation arm suffered from stroke during the ablation procedure and died from cerebral hemorrhage nine months later. No deaths occurred in either the ablation or the medical treatment group in the RAAFT trial presented in 2012.[46] Currently, the ongoing (Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) CABANA Trial is recruiting patients to address this issue; thus, data evaluating mortality after catheter ablation as opposed to AAD treatment will be available in the near future (ClinicalTrials.gov).
Improvement of Symptoms and Quality of Life
AF is well known to be associated with a significant reduction in QoL.[57] So far, QoL has been evaluated in a significant number of clinical trials, including several RCTs.
Cost-Effectiveness
Due to the increasing number of PVIs performed world-wide, the cost-effectiveness of catheter ablation remains an important issue.
So far, catheter ablation has not been demonstrated to be more cost-effective than AAD treatment. In a meta-analysis analyzing three randomized trials performed by McKenna et al., a potential benefit was identified for patients suffering from paroxysmal AF, provided that the benefit gained in QoL in the catheter ablation group seen after 12 months is maintained beyond 5 years post ablation.[58]
Khaykin et al. estimated the costs of catheter ablation as compared to the cost of rate control or AAD treatment in this study. The costs were calculated over a five-year period, and the results showed that the costs of catheter ablation slightly exceeded those of medical therapy, ranging from $16,278 to $21,294.[59] The authors concluded that the costs of AF ablation and AAD therapy would most likely be comparable after a 3.2 to 8.4 year follow-up period.[59]
In a retrospective cost comparison of RF ablation versus drug therapy for patients with paroxysmal AF, the cost of RF ablation was calculated beginning in the year 2001 on the basis of resource use. After 5 years, the cost of RF ablation was below that of ongoing medical treatment and this continued to diverge thereafter. Therefore the authors concluded, that catheter ablation for treatment of AF may be more cost-effective compared to long-term drug therapy in patients with symptomatic paroxysmal AF.[60]
Another review also considered catheter ablation a cost-effective approach during long-term-follow-up when compared to medical treatment alone.[61]
The major limitation in interpreting these trials comparing catheter ablation with AADs is that the follow-up duration of these studies is usually short and data regarding long-term outcome after catheter ablation is still sparse. This makes it difficult to judge the definite cost-effectiveness of pulmonary vein isolation for the treatment of AF.
The Anticoagulation Issue
Stroke is still the most devastating complication of AF, leading to a significantly increased morbidity and mortality.[1] Therefore, anticoagulation is a very important component in the overall therapeutic strategy of AF treatment. The question remains, whether anticoagulation should be maintained according to CHA2DS2-Vasc-score, or if one can safely discontinue anticoagulation therapy after successful catheter ablation, if there is no documented recurrence of AF. According to the current guidelines, catheter ablation is determined to be successful when the patient is free of symptoms and free of documented arrhythmic episodes after a 12-month follow-up period.[62] After several years however, a substantial number of patients may develop recurrence of AF; therefore the risk of stroke remains even after a so-called successful ablation. Furthermore, follow-up of AF patients is still limited with the current monitoring tools. It is common practice toperform follow-up using holter monitoring only for a period up to 72 hours, and thus a high rate of AF recurrence may remain undetected.[63,64] This is important, as recurrence of arrhythmia has been shown to be associated with a higher incidence of thrombembolic events after PVI independent of CHADS2-score.[65] However, several non-randomised studies indicate that it might be safe to stop anticoagulation after successful catheter ablation of AF. Themistoclakis et al. showed in a multi-center study that after a mean of greater than two years, no differences in the incidence of stroke were found in patients with continued anticoagulation therapy as compared to those treated only with aspirin after PVI.[66] Even when the CHADS2-score was ≥2 the risk of stroke was not significantly increased, although the number of patients with a higher CHADS2-score was substantially low in this trial. The authors of another study performed by Saad et al. investigating the thrombembolic risk after PVI in patients with a CHADS2-score ≤3 concluded that discontinuation of anticoagulation is safe in this patient group when patients are maintained on antiplatelet therapy. Another trial supports these data for patients with very low risk (CHADS2 0-1); stroke rate was not increased in patients discharged with only antiplatelet therapy as compared to warfarin at one-year follow-up after PVI.[68] Although the current guidelines still recommend continuation of anticoagulation according to CHA2DS2-vasc-score even after successful PVI (1), a Canadian study recäently showed that 11% of physicians would discontinue anticoagulation therapy in their patients after 1 year when no arrhythmia recurrence is documented.[69]
However, as long as data from randomized controlled multicenter trials are lacking, anticoagulation should be continued after PVI according to the CHA2DS2-Vasc-score. The OAT-Pilot study recently initiated by Natale et al evaluating the safety of Oral Anticoagulation Therapy withdrawal after successful pulmonary vein isolation in patients with AF and associated high hisk factors for embolic events will contribute valuable data to further discuss this issue in the near future.
Future Perspectives
Novel tools such as contact force guided ablation and balloon technologies, as well as novel ablation strategies including ablation of rotors may improve efficacy and safety of catheter ablation.
In the future, several issues have to be adressed when comparing catheter ablation with antiarrhythmic drug therapy, including improvement of hemodynamics in patients with heart failure, reducing the risk of ischemic stroke, or overall mortality.
Previous non-randomized studies have shown potential benefit of sinus rhythm over AF in patients with congestive heart failure.[70] To prove this hypothesis, there are currently two prospective randomized multicenter studies recruiting patients with severely reduced left ventricular ejection fraction (LV-EF ≤35%). In the AMICA (AtrialFibrillation Management in Congestive Heart Failure With Ablation) trial the primary endpoint will be to evaluate the influence of best medical treatment as compared to pulmonary vein isolation on LV-EF in patients with AF with a reduced LV-EF of <35% requiring ICD (implantable cardioverter defibrillator) or CRT-D (cardiac resynchronisation and defibrillator therapy) implantation (ClinicalTrials.gov). The AMICA trial started in 2008 (ClinicalTrials.gov) and is now recruiting patients worldwide in a randomized multicenter fashion. Similarly, the Castle-AF trial (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF), which was started in the same year, is evaluating a similar patient population with different clinical endpoints (i.e. all-cause mortality or worsening heart failure requiring unplanned hospitalization)(ClinicalTrials.org).
Furthermore, the CABANA Trial (Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) is currently testing the hypothesis that left atrial catheter ablation for treatment of AF will be superior to current state-of-the-art therapy with either rate control or rhythm control drugs for reducing total mortality (ClinicalTrials.gov). The EAST (early treatment of atrial fibrillation for stroke prevention trial) study is estimated to be finalized in 2017 and addresses, as the primary endpoint, a composite of cardiovascular death, stroke and hospitalization due to worsening heart failure or due to acute coronary syndrome. Two co-primary outcome parameters are defined and those are firstly, the time to first occurrence of a composite of cardiovascular death, stroke/transient ischemic attack and hospitalization due to worsening of heart failure or due to acute coronary syndrome and secondly, nights spent in hospital per year (ClinicalTrials.gov).
Conclusions
In patients with paroxysmal AF, catheter ablation has been established as an effective alternative treatment to medical AAD-therapy, and is now considered as first line therapy in selected patients in the current European guidelines. Novel ablation strategies may further improve the efficacy of catheter ablation, whereas novel AADs have been shown to be of limited value.There is still limited data in regards to the impact of catheter ablation on the risk of stroke and mortality , however several large randomized trials which are currently being conducted may provide answers to these important questions in the future. Novel anticoagulants will further help to reduce the risk of stroke in patients with AF; as long as larger randomized trials are lacking, anticoagulation should be continued lifelong according to the CHA2DS2-Vasc-score independent of the documented rhythm after catheter ablation of AF.
Disclosures
Andreas Rillig received travel grants from Hansen Medical and St. Jude Medical and lecture fees from St. Jude Medical. Tina Lin received fellowship grants from St. Jude Medical. Roland Tilz received research grants from Hansen Medical and St. Jude Medical, travel grants from St. Jude medical, Biosense Webster, lecture fees from St. Jude Medical, Biosense Webster, Hansen Medical. Prof. Kuck has received research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, and Cryocath; and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis.
References
- 1.Camm A John, Kirchhof Paulus, Lip Gregory Y H, Schotten Ulrich, Savelieva Irene, Ernst Sabine, Van Gelder Isabelle C, Al-Attar Nawwar, Hindricks Gerhard, Prendergast Bernard, Heidbuchel Hein, Alfieri Ottavio, Angelini Annalisa, Atar Dan, Colonna Paolo, De Caterina Raffaele, De Sutter Johan, Goette Andreas, Gorenek Bulent, Heldal Magnus, Hohloser Stefan H, Kolh Philippe, Le Heuzey Jean-Yves, Ponikowski Piotr, Rutten Frans H. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010 Oct;12 (10):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof Paulus, Auricchio Angelo, Bax Jeroen, Crijns Harry, Camm John, Diener Hans-Christoph, Goette Andreas, Hindricks Gerd, Hohnloser Stefan, Kappenberger Lukas, Kuck Karl-Heinz, Lip Gregory Y H, Olsson Bertil, Meinertz Thomas, Priori Silvia, Ravens Ursula, Steinbeck Gerhard, Svernhage Elisabeth, Tijssen Jan, Vincent Alphons, Breithardt Günter. Outcome parameters for trials in atrial fibrillation: executive summary. Eur. Heart J. 2007 Nov;28 (22):2803–17. doi: 10.1093/eurheartj/ehm358. [DOI] [PubMed] [Google Scholar]
- 3.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 5;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 4.Singh Bramah N, Singh Steven N, Reda Domenic J, Tang X Charlene, Lopez Becky, Harris Crystal L, Fletcher Ross D, Sharma Satish C, Atwood J Edwin, Jacobson Alan K, Lewis H Daniel, Raisch Dennis W, Ezekowitz Michael D. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 2005 May 5;352 (18):1861–72. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 5.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang Feifan, Tilz Roland, Chun Julian, Schmidt Boris, Wissner Erik, Zerm Thomas, Neven Kars, Köktürk Bulent, Konstantinidou Melanie, Metzner Andreas, Fuernkranz Alexander, Kuck Karl-Heinz. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010 Dec 7;122 (23):2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 7.Tilz Roland Richard, Rillig Andreas, Thum Anna-Maria, Arya Anita, Wohlmuth Peter, Metzner Andreas, Mathew Shibu, Yoshiga Yasuhiro, Wissner Erik, Kuck Karl-Heinz, Ouyang Feifan. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012 Nov 6;60 (19):1921–9. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 8.Jahangir Arshad, Lee Victor, Friedman Paul A, Trusty Jane M, Hodge David O, Kopecky Stephen L, Packer Douglas L, Hammill Stephen C, Shen Win-Kuang, Gersh Bernard J. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007 Jun 19;115 (24):3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 9.Kato Takeshi, Yamashita Takeshi, Sagara Kouichi, Iinuma Hiroyuki, Fu Long-Tai. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14-year follow-up study. Circ. J. 2004 Jun;68 (6):568–72. doi: 10.1253/circj.68.568. [DOI] [PubMed] [Google Scholar]
- 10.Kerr Charles R, Humphries Karin H, Talajic Mario, Klein George J, Connolly Stuart J, Green Martin, Boone John, Sheldon Robert, Dorian Paul, Newman David. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am. Heart J. 2005 Mar;149 (3):489–96. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Hart R G, Benavente O, McBride R, Pearce L A. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 1999 Oct 5;131 (7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hylek Elaine M, Go Alan S, Chang Yuchiao, Jensvold Nancy G, Henault Lori E, Selby Joe V, Singer Daniel E. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N. Engl. J. Med. 2003 Sep 11;349 (11):1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 13.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006 Jun 10;367 (9526):1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 14.Van Gelder Isabelle C, Hagens Vincent E, Bosker Hans A, Kingma J Herre, Kamp Otto, Kingma Tsjerk, Said Salah A, Darmanata Julius I, Timmermans Alphons J M, Tijssen Jan G P, Crijns Harry J G M. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 Dec 5;347 (23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 15.Connolly Stuart J, Ezekowitz Michael D, Yusuf Salim, Eikelboom John, Oldgren Jonas, Parekh Amit, Pogue Janice, Reilly Paul A, Themeles Ellison, Varrone Jeanne, Wang Susan, Alings Marco, Xavier Denis, Zhu Jun, Diaz Rafael, Lewis Basil S, Darius Harald, Diener Hans-Christoph, Joyner Campbell D, Wallentin Lars. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009 Sep 17;361 (12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 16.Patel Manesh R, Mahaffey Kenneth W, Garg Jyotsna, Pan Guohua, Singer Daniel E, Hacke Werner, Breithardt Günter, Halperin Jonathan L, Hankey Graeme J, Piccini Jonathan P, Becker Richard C, Nessel Christopher C, Paolini John F, Berkowitz Scott D, Fox Keith A A, Califf Robert M. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011 Sep 8;365 (10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 17.Connolly Stuart J, Eikelboom John, Joyner Campbell, Diener Hans-Christoph, Hart Robert, Golitsyn Sergey, Flaker Greg, Avezum Alvaro, Hohnloser Stefan H, Diaz Rafael, Talajic Mario, Zhu Jun, Pais Prem, Budaj Andrzej, Parkhomenko Alexander, Jansky Petr, Commerford Patrick, Tan Ru San, Sim Kui-Hian, Lewis Basil S, Van Mieghem Walter, Lip Gregory Y H, Kim Jae Hyung, Lanas-Zanetti Fernando, Gonzalez-Hermosillo Antonio, Dans Antonio L, Munawar Muhammad, O'Donnell Martin, Lawrence John, Lewis Gayle, Afzal Rizwan, Yusuf Salim. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011 Mar 3;364 (9):806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder Isabelle C, Groenveld Hessel F, Crijns Harry J G M, Tuininga Ype S, Tijssen Jan G P, Alings A Marco, Hillege Hans L, Bergsma-Kadijk Johanna A, Cornel Jan H, Kamp Otto, Tukkie Raymond, Bosker Hans A, Van Veldhuisen Dirk J, Van den Berg Maarten P. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010 Apr 15;362 (15):1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson Jörg, Miketic Sinisa, Windeler Jürgen, Cuneo Alessandro, Haun Sebastian, Micus Stefan, Walter Sabine, Tebbe Ulrich. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J. Am. Coll. Cardiol. 2003 May 21;41 (10):1690–6. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 20.Hohnloser S H, Kuck K H, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000 Nov 25;356 (9244):1789–94. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 21.Opolski Grzegorz, Torbicki Adam, Kosior Dariusz A, Szulc Marcin, Wozakowska-Kaplon Beata, Kolodziej Piotr, Achremczyk Piotr. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004 Aug;126 (2):476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 22.Camm A John, Breithardt Günter, Crijns Harry, Dorian Paul, Kowey Peter, Le Heuzey Jean-Yves, Merioua Ihsen, Pedrazzini Laurence, Prystowsky Eric N, Schwartz Peter J, Torp-Pedersen Christian, Weintraub William. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). J. Am. Coll. Cardiol. 2011 Jul 26;58 (5):493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Ritter Martin A, Kochhäuser Simon, Duning Thomas, Reinke Florian, Pott Christian, Dechering Dirk G, Eckardt Lars, Ringelstein E Bernd. Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke. 2013 May;44 (5):1449–52. doi: 10.1161/STROKEAHA.111.676189. [DOI] [PubMed] [Google Scholar]
- 24.Nademanee Koonlawee, Schwab Mark C, Kosar Erol M, Karwecki Margaret, Moran Michael D, Visessook Nithi, Michael Anthony Don, Ngarmukos Tachapong. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):843–9. doi: 10.1016/j.jacc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Le Heuzey Jean-Yves, De Ferrari Gaetano M, Radzik David, Santini Massimo, Zhu Junren, Davy Jean-Marc. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J. Cardiovasc. Electrophysiol. 2010 Jun 1;21 (6):597–605. doi: 10.1111/j.1540-8167.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- 26.Hohnloser Stefan H, Crijns Harry J G M, van Eickels Martin, Gaudin Christophe, Page Richard L, Torp-Pedersen Christian, Connolly Stuart J. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 2009 Feb 12;360 (7):668–78. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 27.Connolly Stuart J, Camm A John, Halperin Jonathan L, Joyner Campbell, Alings Marco, Amerena John, Atar Dan, Avezum Álvaro, Blomström Per, Borggrefe Martin, Budaj Andrzej, Chen Shih-Ann, Ching Chi Keong, Commerford Patrick, Dans Antonio, Davy Jean-Marc, Delacrétaz Etienne, Di Pasquale Giuseppe, Diaz Rafael, Dorian Paul, Flaker Greg, Golitsyn Sergey, Gonzalez-Hermosillo Antonio, Granger Christopher B, Heidbüchel Hein, Kautzner Josef, Kim June Soo, Lanas Fernando, Lewis Basil S, Merino Jose L, Morillo Carlos, Murin Jan, Narasimhan Calambur, Paolasso Ernesto, Parkhomenko Alexander, Peters Nicholas S, Sim Kui-Hian, Stiles Martin K, Tanomsup Supachai, Toivonen Lauri, Tomcsányi János, Torp-Pedersen Christian, Tse Hung-Fat, Vardas Panos, Vinereanu Dragos, Xavier Denis, Zhu Jun, Zhu Jun-Ren, Baret-Cormel Lydie, Weinling Estelle, Staiger Christoph, Yusuf Salim, Chrolavicius Susan, Afzal Rizwan, Hohnloser Stefan H. Dronedarone in high-risk permanent atrial fibrillation. N. Engl. J. Med. 2011 Dec 15;365 (24):2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 28.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 29.Chun Kyoung-Ryul Julian, Schmidt Boris, Metzner Andreas, Tilz Roland, Zerm Thomas, Köster Ilka, Fürnkranz Alexander, Koektuerk Buelent, Konstantinidou Melanie, Antz Matthias, Ouyang Feifan, Kuck Karl Heinz. The 'single big cryoballoon' technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 2009 Mar;30 (6):699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzner Andreas, Wissner Erik, Schmidt Boris, Chun Julian, Hindricks Gerhard, Piorkowski Christopher, Ouyang Feifan, Kuck Karl-Heinz. Acute and long-term clinical outcome after endoscopic pulmonary vein isolation: results from the first prospective, multicenter study. J. Cardiovasc. Electrophysiol. 2013 Jan;24 (1):7–13. doi: 10.1111/j.1540-8167.2012.02427.x. [DOI] [PubMed] [Google Scholar]
- 31.Neuberger H.-R., Tilz R. R., Bonnemeier H., Deneke T., Estner H. L., Kriatselis C., Kuniss M., Luik A., Sommer P., Steven D., von Bary C., Voss F., Eckardt L.. A survey of German centers performing invasive electrophysiology in 2010: Structure, procedures, and training positions; Europace, accepted for publication. 2013 doi: 10.1093/europace/eut149. [DOI] [PubMed] [Google Scholar]
- 32.Rillig Andreas, Schmidt Boris, Steven Daniel, Meyerfeldt Udo, DI Biase Luigi, Wissner Erik, Becker Rüdiger, Thomas Dierk, Wohlmuth Peter, Gallinghouse G Joseph, Scholz Eberhardt, Jung Werner, Willems Stefan, Natale Andrea, Ouyang Feifan, Kuck Karl Heinz, Tilz Roland. Study design of the man and machine trial: a prospective international controlled noninferiority trial comparing manual with robotic catheter ablation for treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013 Jan;24 (1):40–6. doi: 10.1111/j.1540-8167.2012.02418.x. [DOI] [PubMed] [Google Scholar]
- 33.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 2;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 34.Hou Yinglong, Scherlag Benjamin J, Lin Jiaxiong, Zhang Ying, Lu Zhibing, Truong Kim, Patterson Eugene, Lazzara Ralph, Jackman Warren M, Po Sunny S. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J. Am. Coll. Cardiol. 2007 Jul 3;50 (1):61–8. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 35.Narayan Sanjiv M, Patel Jigar, Mulpuru Siva, Krummen David E. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study. Heart Rhythm. 2012 Sep;9 (9):1436–9. doi: 10.1016/j.hrthm.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wazni Oussama M, Marrouche Nassir F, Martin David O, Verma Atul, Bhargava Mandeep, Saliba Walid, Bash Dianna, Schweikert Robert, Brachmann Johannes, Gunther Jens, Gutleben Klaus, Pisano Ennio, Potenza Dominico, Fanelli Raffaele, Raviele Antonio, Themistoclakis Sakis, Rossillo Antonio, Bonso Aldo, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005 Jun 1;293 (21):2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 38.Pappone Carlo, Augello Giuseppe, Sala Simone, Gugliotta Filippo, Vicedomini Gabriele, Gulletta Simone, Paglino Gabriele, Mazzone Patrizio, Sora Nicoleta, Greiss Isabelle, Santagostino Andreina, LiVolsi Laura, Pappone Nicola, Radinovic Andrea, Manguso Francesco, Santinelli Vincenzo. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J. Am. Coll. Cardiol. 2006 Dec 5;48 (11):2340–7. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Jaïs Pierre, Cauchemez Bruno, Macle Laurent, Daoud Emile, Khairy Paul, Subbiah Rajesh, Hocini Mélèze, Extramiana Fabrice, Sacher Fréderic, Bordachar Pierre, Klein George, Weerasooriya Rukshen, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008 Dec 9;118 (24):2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 40.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 41.Oral Hakan, Pappone Carlo, Chugh Aman, Good Eric, Bogun Frank, Pelosi Frank, Bates Eric R, Lehmann Michael H, Vicedomini Gabriele, Augello Giuseppe, Agricola Eustachio, Sala Simone, Santinelli Vincenzo, Morady Fred. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 2006 Mar 2;354 (9):934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 42.Krittayaphong Rungroj, Raungrattanaamporn Ongkarn, Bhuripanyo Kiertijai, Sriratanasathavorn Charn, Pooranawattanakul Sukanya, Punlee Kesaree, Kangkagate Charuwan. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai. 2003 May;86 Suppl 1:S8–16. [PubMed] [Google Scholar]
- 43.Stabile Giuseppe, Bertaglia Emanuele, Senatore Gaetano, de Simone Antonio, Zerbo Francesca, Carreras Giovanni, Turco Pietro, Pascotto Pietro, Fazzari Massimo. Feasibility of pulmonary vein ostia radiofrequency ablation in patients with atrial fibrillation: a multicenter study (CACAF pilot study). Pacing Clin Electrophysiol. 2003 Jan;26 (1 Pt 2):284–7. doi: 10.1046/j.1460-9592.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 44.Forleo Giovanni B, Mantica Massimo, De Luca Lucia, Leo Roberto, Santini Luca, Panigada Stefania, De Sanctis Valerio, Pappalardo Augusto, Laurenzi Francesco, Avella Andrea, Casella Michela, Dello Russo Antonio, Romeo Francesco, Pelargonio Gemma, Tondo Claudio. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J. Cardiovasc. Electrophysiol. 2009 Jan;20 (1):22–8. doi: 10.1111/j.1540-8167.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 45.Cosedis Nielsen Jens, Johannessen Arne, Raatikainen Pekka, Hindricks Gerhard, Walfridsson Håkan, Kongstad Ole, Pehrson Steen, Englund Anders, Hartikainen Juha, Mortensen Leif Spange, Hansen Peter Steen. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med. 2012 Oct 25;367 (17):1587–95. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 46.Morillo, MD, FHRS Carlos, Verma, MD, FRCP Atul, Kuck, MD, FHRS Karl H, Champagne, MD, FRCP Jean, Nair, MBBS Girish, Sterns, MD, FRCP Lawrence, Beresh, MSc Heather, Connolly, MD, FRCP Stuart J, Natale, MD, FHRS Andrea. Radiofrequency Ablation vs Antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: (RAAFT 2): a randomized trial. Heart Rhythm. 0;0:0–0. [Google Scholar]
- 47.Echt D S, Liebson P R, Mitchell L B, Peters R W, Obias-Manno D, Barker A H, Arensberg D, Baker A, Friedman L, Greene H L. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991 Mar 21;324 (12):781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 48.McNamara Robert L, Tamariz Leonardo J, Segal Jodi B, Bass Eric B. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann. Intern. Med. 2003 Dec 16;139 (12):1018–33. doi: 10.7326/0003-4819-139-12-200312160-00012. [DOI] [PubMed] [Google Scholar]
- 49.Wehling Martin. Meta-analysis of flecainide safety in patients with supraventricular arrhythmias. Arzneimittelforschung. 2002;52 (7):507–14. doi: 10.1055/s-0031-1299923. [DOI] [PubMed] [Google Scholar]
- 50.Pritchett E L, Wilkinson W E. Mortality in patients treated with flecainide and encainide for supraventricular arrhythmias. Am. J. Cardiol. 1991 May 1;67 (11):976–80. doi: 10.1016/0002-9149(91)90170-p. [DOI] [PubMed] [Google Scholar]
- 51.Kääb Stefan, Hinterseer Martin, Näbauer Michael, Steinbeck Gerhard. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome--a case-control pilot study using i.v. sotalol. Eur. Heart J. 2003 Apr;24 (7):649–57. doi: 10.1016/s0195-668x(02)00806-0. [DOI] [PubMed] [Google Scholar]
- 52.Tilz Roland R, Chun K R Julian, Metzner Andreas, Burchard Andre, Wissner Erik, Koektuerk Buelent, Konstantinidou Melanie, Nuyens Dieter, De Potter Tom, Neven Kars, Fürnkranz Alexander, Ouyang Feifan, Schmidt Boris. Unexpected high incidence of esophageal injury following pulmonary vein isolation using robotic navigation. J. Cardiovasc. Electrophysiol. 2010 Aug 1;21 (8):853–8. doi: 10.1111/j.1540-8167.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- 53.Rillig Andreas, Meyerfeldt Udo, Birkemeyer Ralf, Wiest Stefan, Sauer Bernd M, Staritz Martin, Jung Werner. Oesophageal temperature monitoring and incidence of oesophageal lesions after pulmonary vein isolation using a remote robotic navigation system. Europace. 2010 May;12 (5):655–61. doi: 10.1093/europace/euq061. [DOI] [PubMed] [Google Scholar]
- 54.Rillig Andreas, Meyerfeldt Udo, Tilz Roland Richard, Talazko Jochen, Arya Anita, Zvereva Vlada, Birkemeyer Ralf, Miljak Tomislav, Hajredini Bajram, Wohlmuth Peter, Fink Ulrich, Jung Werner. Incidence and long-term follow-up of silent cerebral lesions after pulmonary vein isolation using a remote robotic navigation system as compared with manual ablation. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):15–21. doi: 10.1161/CIRCEP.111.967497. [DOI] [PubMed] [Google Scholar]
- 55.Rillig Andreas, Meyerfeldt Udo, Kunze Markus, Birkemeyer Ralf, Miljak Tomislav, Jäckle Sebastian, Hajredini Bajram, Treusch Fabian, Jung Werner. Persistent iatrogenic atrial septal defect after a single-puncture, double-transseptal approach for pulmonary vein isolation using a remote robotic navigation system: results from a prospective study. Europace. 2010 Mar;12 (3):331–6. doi: 10.1093/europace/eup428. [DOI] [PubMed] [Google Scholar]
- 56.Rillig Andreas, Meyerfeldt Udo, Birkemeyer Ralf, Treusch Fabian, Kunze Markus, Jung Werner. Persistent iatrogenic atrial septal defect after pulmonary vein isolation : incidence and clinical implications. J Interv Card Electrophysiol. 2008 Sep;22 (3):177–81. doi: 10.1007/s10840-008-9257-7. [DOI] [PubMed] [Google Scholar]
- 57.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers G M, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J. Am. Coll. Cardiol. 2000 Oct;36 (4):1303–9. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 58.McKenna C, Palmer S, Rodgers M, Chambers D, Hawkins N, Golder S, Van Hout S, Pepper C, Todd D, Woolacott N. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart. 2009 Apr;95 (7):542–9. doi: 10.1136/hrt.2008.147165. [DOI] [PubMed] [Google Scholar]
- 59.Khaykin Yaariv, Wang Xiaoyin, Natale Andrea, Wazni Oussama M, Skanes Allan C, Humphries Karin H, Kerr Charles R, Verma Atul, Morillo Carlos A. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J. Cardiovasc. Electrophysiol. 2009 Jan;20 (1):7–12. doi: 10.1111/j.1540-8167.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 60.Weerasooriya Rukshen, Jaïs Pierre, Le Heuzey Jean-Yves, Scaveé Christophe, Choi Kee-Joon, Macle Laurent, Raybaud Florence, Hocini Mélèze, Shah Dipen C, Lavergne Thomas, Clémenty Jacques, Haïssaguerre Michel. Cost analysis of catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2003 Jan;26 (1 Pt 2):292–4. doi: 10.1046/j.1460-9592.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 61.Andrikopoulos George, Tzeis Stylianos, Maniadakis Nikos, Mavrakis Hercules E, Vardas Panos E. Cost-effectiveness of atrial fibrillation catheter ablation. Europace. 2009 Feb;11 (2):147–51. doi: 10.1093/europace/eun342. [DOI] [PubMed] [Google Scholar]
- 62.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 63.Steven Daniel, Rostock Thomas, Lutomsky Boris, Klemm Hanno, Servatius Helge, Drewitz Imke, Friedrichs Kai, Ventura Rodolfo, Meinertz Thomas, Willems Stephan. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur. Heart J. 2008 Apr;29 (8):1037–42. doi: 10.1093/eurheartj/ehn024. [DOI] [PubMed] [Google Scholar]
- 64.Martinek Martin, Aichinger Josef, Nesser Hans-Joachim, Ziegler Paul D, Purerfellner Helmut. New insights into long-term follow-up of atrial fibrillation ablation: full disclosure by an implantable pacemaker device. J. Cardiovasc. Electrophysiol. 2007 Aug;18 (8):818–23. doi: 10.1111/j.1540-8167.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 65.Tao Hailong, Ma Changsheng, Dong Jianzeng, Liu Xingpeng, Long Deyong, Yu Ronghui. Late thromboembolic events after circumferential pulmonary vein ablation of atrial fibrillation. J Interv Card Electrophysiol. 2010 Jan;27 (1):33–9. doi: 10.1007/s10840-009-9455-y. [DOI] [PubMed] [Google Scholar]
- 66.Themistoclakis Sakis, Corrado Andrea, Marchlinski Francis E, Jais Pierre, Zado Erica, Rossillo Antonio, Di Biase Luigi, Schweikert Robert A, Saliba Walid I, Horton Rodney, Mohanty Prasant, Patel Dimpi, Burkhardt David J, Wazni Oussama M, Bonso Aldo, Callans David J, Haissaguerre Michel, Raviele Antonio, Natale Andrea. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J. Am. Coll. Cardiol. 2010 Feb 23;55 (8):735–43. doi: 10.1016/j.jacc.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 67.Saad Eduardo B, d'Avila André, Costa Ieda P, Aryana Arash, Slater Charles, Costa Rodrigo E, Inácio Luiz A, Maldonado Paulo, Neto Dario M, Camiletti Angelina, Camanho Luiz E, Polanczyk Carisi A. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):615–21. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- 68.Bunch T Jared, Crandall Brian G, Weiss J Peter, May Heidi T, Bair Tami L, Osborn Jeffrey S, Anderson Jeffrey L, Lappe Donald L, Muhlestein J Brent, Nelson Jennifer, Allison Scott, Foley Thomas, Anderson Lars, Day John D. Warfarin is not needed in low-risk patients following atrial fibrillation ablation procedures. J. Cardiovasc. Electrophysiol. 2009 Sep;20 (9):988–93. doi: 10.1111/j.1540-8167.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 69.Mardigyan Vartan, Verma Atul, Birnie David, Guerra Peter, Redfearn Damian, Becker Giuliano, Champagne Jean, Sapp John, Gula Lorne, Parkash Ratika, Macle Laurent, Crystal Eugene, O'Hara Gilles, Khaykin Yaariv, Sturmer Marcio, Veenhuyzen George D, Greiss Isabelle, Sarrazin Jean-Francois, Mangat Iqwal, Novak Paul, Skanes Allan, Roux Jean-Francois, Chauhan Vijay, Hadjis Tom, Morillo Carlos A, Essebag Vidal. Anticoagulation management pre- and post atrial fibrillation ablation: a survey of canadian centres. Can J Cardiol. 2013 Feb;29 (2):219–23. doi: 10.1016/j.cjca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Dries D L, Exner D V, Gersh B J, Domanski M J, Waclawiw M A, Stevenson L W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998 Sep;32 (3):695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]


