Abstract
Background
In some epidemiological studies, blood lipids are determined at non-fasting state, which may impact cardiovascular risk estimation. The aim of this study was to evaluate postprandial LDL-C changes in men with newly diagnosed metabolic syndrome (MetSy).
Methods
36 male patients were examined: 12 men with and 24 men without MetSy. The fat tolerance test was performed before and after a three-month hypolipidemic treatment. Serum lipids were measured using routine methods, lipid peroxides (LPO) colorimetrically, apolipoproteins A-I, B, and hsCRP immunoturbidimetrically.
Results
The postprandial increase in triglycerides was associated with a decrease in LDL-C and a small decrease in apo B. In men with MetSy, the mean change in LDL-C (−19.5 ± 2.3 mg/dl) was greater than in healthy men (−5.7 ± 3.8 mg/dl). All lipid changes (ΔTG, ΔLDL-C and ΔLPO) were linearly dependent on the postprandial non-LDL-cholesterol. After three months of hypolipidemic treatment, in all men with MetSy, the apoB/apoA-I ratio remained the same as before the therapy.
Conclusion
In men diagnosed with MetSy, postprandial decreases in LDL-cholesterol may cause underestimation of cardiovascular risk. After three months of hypolipidemic treatment, there was only a partial reduction in this risk, as the apoB/apoA-I ratio remained the same.
Keywords: Postprandial LDL-cholesterol, metabolic syndrome, apoB/apoA ratio
1 Introduction
Metabolic syndrome (MetSy) is defined as a cluster of cardiovascular risk factors, including: central obesity, insulin resistance, prediabetes or diabetes, atherogenic dyslipidemia and hypertension. However, the special treatment of patients with MetSy is a subject of controversy. Gerald M. Reaven, recognized as the greatest authority in the field of research on MetSy, a few years ago stated that metabolic syndrome “is dead” [1]. The World Health Organisation has recently concluded that this syndrome has “limited practical utility as a diagnostic or management tool” [2]. On the other hand, it has been well documented that metabolic syndrome is associated with arterial stiffness [3], maladaptive vascular remodeling [4] and early development of atherosclerosis, especially in carotid arteries [5]. Furthemore, meta-analysis of prospective epidemiological studies confirmed that metabolic syndrome is an important risk factor for cardiovascular diseases’ incidence and mortality [6–9].
Atherogenic dyslipidemia is an abnormal blood lipid pattern which includes: decreased high density cholesterol (HDL-C), hypertriglyceridemia (HTG) and a presence of small low density lipoproteins (LDL). The anti-atherogenic, multidirectional action of HDL is well documented [10–13]. Also, the atherogenic effect of hypertriglyceridemia, connected with their pro-inflammatory [14], pro-thrombotic [15] and pro-oxidative [16] action is well recognized. In subjects with HTG, all lipoproteins, including HDL, may be oxidatively modified, which leads to procoagulation state [17].
At present, the elevation of non-fasting triglycerides (TG) is recognized as an independent residual risk factor for cardiovascular diseases, although there is no consensus about appropriate target level [15,18]. A level of non-fasting TG ≥5 mmol/L vs <1 mmol/L determines a significantly increased risk of myocardial infarction, ischemic stroke and early death in the general population [19]. There is an agreement that after a meal, typically, there is an increase in chylomicrons, VLDL and lipid peroxides (LPO) levels [18–22].
As modern men most of the day remain in a state of postprandial lipemia, the lipid pattern should be determined not only at fasting, but also in postprandial state. Furthermore, some questions arise, regarding the significance of postprandial measurements, especially LDL cholesterol, in cardiovascular risk estimation. Data on how a meal impacts LDL-C is scarce [20]. This problem is especially relevant for patients diagnosed with MetSy, who generally display normal or slightly increased total- and LDL-cholesterol levels, as is presented in this study.
The aim of this study was to evaluate postprandial LDL cholesterol changes in men diagnosed with metabolic syndrome in comparison to men without MetSy, and to assess meal-induced changes in LDL cholesterol after a three-month hypolipemic treatment. Particular attention was paid not only to serum LDL-cholesterol fraction, but also to HDL2- and HDL3 subfractions, as well as to apoB- and apoA-lipoprotein concentrations, all measured at fasting state, and three hours after a standardized fat-rich breakfast.
2 Patients and methods
2.1 Patients
36 male volunteers were included in the study: 12 men newly diagnosed with MetSy and 24 men without metabolic syndrome. The sample was collected among hospital employees and their friends, interested in blood lipid measurements. In each of the examined subject, medical history and physical examination were performed, arterial blood pressure, height and weight were measured and body mass index (BMI) was calculated. The sample size was small. Nevertheless, it exceeded the value appointed automatically in power analysis by Statistica software (N = 32, the power of the analysis: 0,9044).
A diagnosis of the metabolic syndrome was established on the basis of a “harmonized” version of definition from the Adult Treatment Panel III and the International Diabetes Federation [2]. Exclusion criteria were: already diagnosed MetSy or diabetes and taking lipid-lowering or anti-diabetic drugs prior to the study. Among subjects, nobody had been diagnosed with diabetes, while all men newly diagnosed with metabolic syndrome had also newly diagnosed glucose intolerance (the fasting glucose concentration was 115 ± 10 mg/dl, and postprandial glucose did not reach value of 140 mg/dl (122 ± 9 mg/dl).
Arterial hypertension has been identified in the all groups. All hypertensive men were treated with an angiotensin-converting-enzyme inhibitor or a sartan, in some cases in combination with a diuretic or a beta blocker. Until the beginning of our study, no other drugs, including lipid-lowering drugs, anti-diabetic drugs or aspirin had been given.
The study took place in Department of Internal Diseases and Hypertension, Wroclaw Medical University in February–November 2011. The period of our investigation lasted three months. The written informed consent was obtained from all men taking part in the study. The study was approved by Local Ethical Committee in Wroclaw and followed the Declaration of Helsinki.
2.2 Fat tolerance test (FTT)
Regular diet prior to the study was defined by all subjects as rich in fat. After an 8-hour fast, all patients were given a single standardized fat-rich breakfast meal: 100g of fat, 25g of carbohydrates and 100g of protein (1500 kcal). Only drinking water was allowed postprandially. Venous blood samples were drawn before and 3h after meal consumption. After the analysis of fasting lipid serum values, men diagnosed with MetSy or mixed hyperlipemia started individualized therapy. The applied therapies were non-pharmacological and pharmacological; consisting of therapeutic diet and therapeutic lifestyle in all patients, fibrates in men with MetSy, statin or statin plus fibrate in men with mixed hyperlipemia. The kind and dose of fibrate or statin was dependent on the degree of lipid disturbances. The therapies were prescribed by a clinical physician and the same doctor followed all patients. After a three-month therapy, FTT was repeated. Not all participants were tested after 3 months, as healthy normolipidemic patients did not attend the second postprandial test. In the groups of men with MetSy or mixed hyperlipidemia, no one dropped out of the study.
2.3 Biochemical measurements
Blood was centrifuged at 1000 g for 20 minutes at 4°C. Each serum sample was divided into three tubes and stored at −20°C. Serum lipids were measured using standard methods. Total cholesterol (TC), triglycerides (TG), and HDL cholesterol (HDL-C) were measured using enzymatic assay SPINREACT (SantEsteve De Bas, Girona, Spain). LDL-cholesterol (LDL-C) was estimated among patients with a TG concentration lower than 400 mg/dl by the Friedewald equation. To assess HDL2 and HDL3 cholesterol QUANTOLIP® HDL (Technoclone GmbH, Vienna, Austria) precipitation test was used. Remnant cholesterol (R-C) was calculated as postprandial total cholesterol minus low-density lipoprotein cholesterol minus high-density lipoprotein cholesterol [19].
Serum lipid peroxides (LPO) level was measured colorimetrically, using Satoh method. Glucose was also measured by a colorimetric method, using DADE Behringer test. Serum apolipoprotein A-I (apoA-I) and apolipoprotein B (apoB) were determined by the immunoturbidimetric method DADE Behringer Marburg GmbH (Marburg, Germany). The serum high-sensitivity C-reactive protein (hsCRP) was determined using the CardioPhasehs CRP Dade Behringer preparation with the molecular immunonephelometric method, accordingly to N Rheumatology Standard SL (BCR-CRM 470).
2.4 Statistical analysis
Statistical analysis was performed with the use of ‘Statistica PL 10.0’ computer program (Stat Soft, Poland). The distribution of the variables was checked with W-Shapiro-Wilk test. All variables and their changes showed an approximately normal distribution, and were reported as mean ± standard error (SE). Statistical analysis included t tests and analysis of variance. Statistically significant differences between the independent means were estimated with LSD post-hoc test. Values of p<0.05 were assumed as statistically significant. Correlations between parameters were assessed by Spearman’s correlation coefficient.
3 Results
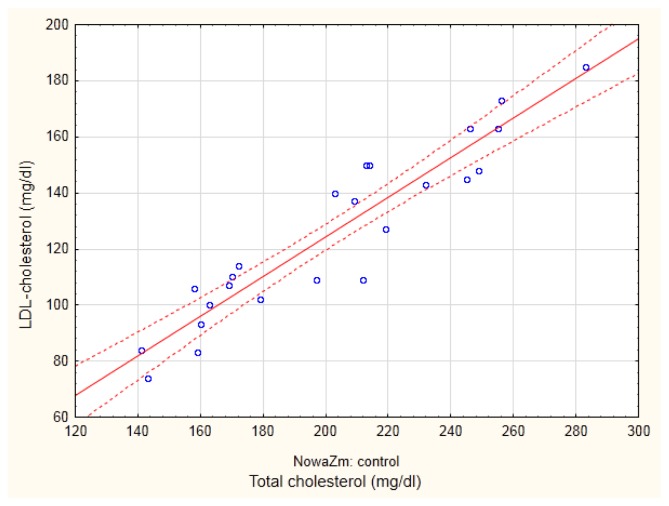
In all groups of men (with MetSy and without MetSy), physiological dependences between apoA and HDL-cholesterol (r = 0.6734; p<0.05 and r = 0.4439, p<0.05) and between apoB, and LDL-cholesterol level (r = 0.8356; p<0.001 and r = 0.5201, p<0.01, in men with and without MetSy, respectively) were shown. On the basis of Figure 1, showing the correlation between total- and LDL-cholesterol in the group of men without MetSy, two subgroups of men may be identified: with total cholesterol level lower or greater than 200 mg/dl. Therefore, the control group was divided into control I and control II, depending on the total cholesterol concentration. Whereas the control I subgroup included healthy men with normal lipid pattern, men from the control II group were newly diagnosed with mixed hyperlipemia.
Figure 1.
The dependence between total and low density lipoprotein (LDL) - cholesterol in the group of men without metabolic syndrome
There were many differences between the three groups at the beginning of the study, Table 1. In comparison to normolipemic men, men that we diagnosed with MetSy, according to our findings, displayed i.a. higher BMI, serum TG, total cholesterol, glucose and hsCRP, and lower HDL cholesterol concentration.
Table 1.
Basal characteristics of group of men diagnosed with MetS, healthy normolipemic men (control I) and group of men diagnosed with mixed hyperlipidemia (control II) in a fasting state
| Group | |||||
|---|---|---|---|---|---|
| MetS (12 men) | Control I (14 men) total cholesterol < 200 mg/dl | Control II (10 men) total cholesterol > 200 mg/dl | |||
| Age | years | 51.91 ± 2.34* | 40.75 ± 2.73 | 48.30 ± 4.13 | |
|
| |||||
| BMI | kg/m2 | 33.83 ± 1.52*** | 26.78 ± 0.94 | 25.30 ± 0.65 | |
|
| |||||
| Smokers | number/all | 9/12 | 4/14 | 4/10 | |
| % | 75% | 29% | 40% | ||
|
| |||||
| Hard drinkers | number/all | 0/12 | 0/14 | 0/10 | |
| % | 0% | 0% | 0% | ||
|
| |||||
| Hypertension | number/all | 9/12* | 5/24 | 5/10 | |
| % | 75% | 21% | 50% | ||
|
| |||||
| T-C | mg/dl | mmol/l | 219.91 ± 8.67*** | 174.35 ± 6.38 | 240.60 ± 7.24*** |
| 5.68 ± 0.22 | 5.19 ± 0.21 | 6.22 ± 0.18 | |||
|
| |||||
| TG | mg/dl | mmol/l | 275.41 ± 21.93*** | 100.00 ± 6.85 | 188.70 ± 24.23** |
| 3.11 ± 0.25 | 1.55 ± 0.16 | 2.12 ± 0.27 | |||
|
| |||||
| HDL-C | mg/dl | mmol/l | 39.38 ± 1.84* | 46.64 ± 1.9 | 53.50 ± 3.28* |
| 1.01 ± 0.05 | 1.29 ± 0.05 | 1.37 ± 0.09 | |||
|
| |||||
| Glucose | mg/dl | mmol/l | 114.83 ± 10.23* | 92.14 ± 3.29 | 89.70 ± 1.82 |
| 6.39 ± 0.57 | 5.11 ± 0.18 | 5.0 ± 0.10 | |||
|
| |||||
| LPO | nmol/ml | 3.75 ± 0.46 | 2.76 ± 0.37 | 2.10 ± 0.34 | |
|
| |||||
| hsCRP | mg/l | 2.36 ± 0.51* | 1.05 ± 0.27 | 1.71 ± 0.54 | |
|
| |||||
| Apo A-I | g/l | 1.43 ± 0.10 | 1.49 ± 0.04 | 1.56 ± 0.08 | |
| Apo B | g/l | 1.11 ± 0.12 | 1.06 ± 0.04 | 1.28 ± 0.06 | |
Results are presented as mean ± standard error (SE). T-C: total cholesterol; TG: triglycerides; HDL-C: HDL cholesterol; LPO: lipid peroxides; hsCRP: high sensitivity C-reactive protein; apo A-I: apolipoprotein A-I; apo B: apolipoprotein B
differences statistically significant in comparison to group of normolipemic men (control I): *p<0.05; **p<0.01; ***p<0.001
The postprandial changes in triglycerides, LDL cholesterol and apolipoprotein B
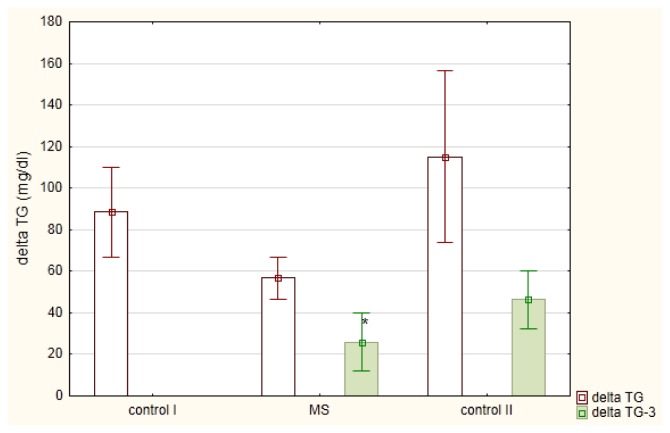
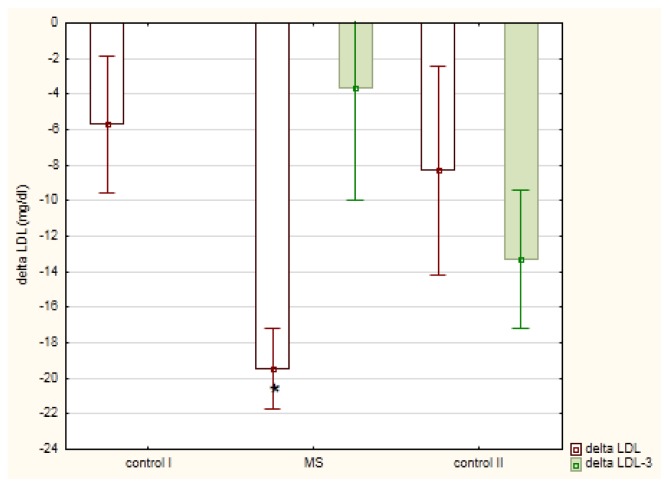
The meal-induced postprandial changes in serum TG (ΔTG) were not dependent on fasting TG levels (r = −0.0805) but were linearly dependent on postprandial non-LDL cholesterol (r = 0.5208; p<0.01) and remnant cholesterol (R-C; r = 0.500, p< 0.01). These TG changes (mean ΔTG ± SE) in men diagnosed with MetSy (56.7 ± 10.1 mg/dl) were slightly lower in comparison to healthy men (88.4 ± 21.6 mg/dl) or men diagnosed with mixed hyperlipemia (115.1 ± 41.1 mg/dl), Figure 2. In MetSy group, the postprandial increase in TG was associated with a significant decrease in LDL cholesterol (p < 0.01), Table 2. This decrease (mean ΔLDL-C ± SE) in MetSy group (−19.5 ± 2.3 mg/dl) was greater (p = 0.0125), in comparison to healthy men (−5.7 ± 3.8 mg/ dl), Figure 3. A linear correlation between postprandial non-LDL cholesterol and ΔLDL-C (r = −0.4605; p<0.01) was shown. Also, the dependence between postprandial remnant cholesterol and ΔLDL-C (r = −0.4201; p<0.05) was observed. In all studied groups, the postprandial decrease in LDL-C was associated with a small decrease in apo-B concentration, Table 2.
Figure 2.
Postprandial changes (mean ± standard error) in triglycerides (delta TG) in men diagnosed with metabolic syndrome (MS) in comparison to healthy men (control I group) and men diagnosed with mixed hyperlipemia (control II group) before- (delta TG) and after (delta TG-3) a three month hypolipidemic treatment
* p<0.05 in comparison to delta TG in fat tolerance test performed before the treatment
Table 2.
Fasting and postprandial lipids and other parameters measured before beginning of the hypolipidemic treatment
| Group | ||||||
|---|---|---|---|---|---|---|
| MetSy 12 men | Control I: 14 normolipemic men | Control II: 10 men with mixed Hyperlipemia | ||||
| mg/dl | mmol/l | mg/dl | mmol/l | mg/dl | mmol/l | |
| T-C: | ||||||
| fasting | 219.91 ± 8.67 | 5.67 ± 0.22 | 174.35 ± 6.38*** | 4.48 ± 0.16 | 240.60 ± 7.24 | 5.52 ± 0.19 |
| postprandial | 218.50 ± 11.03 | 5.62 ± 0.28 | 178.00 ± 7.26** | 4.59 ± 0.18 | 249.30 ± 6.69* | 6.42 ± 0.17 |
| TG: | ||||||
| fasting | 275.41 ± 21.93 | 3.11 ± 0.25 | 100.00± 6.85*** | 1.13 ± 0.08 | 188.70 ± 24.23* | 2.1 ± 0.27 |
| postprandial | 350.83 ± 30.25 | 3.97 ± 0.34 | 182.85± 23.68*** | 2.06 ± 0.27 | 292.20 ± 49.35 | 3.3 ± 0.55 |
| f-p difference | * | ** | ** | |||
| LDL-C: | ||||||
| fasting | 134.10 ± 12.31 | 3.46 ± 0.32 | 108.83 ± 7.42* | 2.79 ± 0.19 | 149.30 ± 7.11*** | 3.84 ± 0.18 |
| postprandial | 108.60 ± 8.60 | 2.79 ± 0.22 | 102.42 ± 7.51 | 2.63 ± 0.19 | 141.50 ± 9.63* | 3.64 ± 0.25 |
| f-p difference | ** | |||||
| Non LDL-C: | ||||||
| fasting | 94.33 ± 5.01 | 2,43 ± 0.13 | 66.64 ± 2.3*** | 1.73 ± 0.06 | 91.40 ± 3.10 | 2.35 ± 0.08 |
| postprandial | 110.10 ± 6.22 | 2.84 ± 0.16 | 80.34 ± 4.3** | 2.07 ± 0.11 | 107.80 ± 8.82 | 2.79 ± 0.23 |
| f-p difference | ** | ** | * | |||
| HDL-C: | ||||||
| fasting | 39.38 ± 1.84 | 1.00 ± 0.05 | 46.64 ± 1.99* | 1.12 ± 0.05 | 53.50 ± 3.28*** | 1.37 ± 0.09 |
| postprandial | 39.93 ± 2.55 | 1.03 ± 0.07 | 43.98 ± 2.27 | 1.13 ± 0.06 | 49.60 ± 2.70* | 1.29 ± 0.07 |
| HDL2-C: | ||||||
| asting | 7.35 ± 1.18 | 0.19 ± 0.03 | 9.00 ± 1.45 | 0.23 ± 0.04 | 12.52 ± 2.76 | 0.32 ± 0.07 |
| postprandial | 7.26 ± 1.46 | 0.18 ± 0.04 | 10.65 ± 1.64 | 0.27 ± 0.04 | 8.51 ± 1.10 | 0.22 ± 0.03 |
| HDL3-C: | ||||||
| fasting | 32.00 ± 1.75 | 0.82 ± 0.05 | 38.06 ± 1.84* | 0.98 ± 0.05 | 42.03 ± 2.36** | 1.06 ± 0.07 |
| postprandial | 32.08 ± 1.59 | 0.82 ± 0.04 | 39.60 ± 2.01** | 1.00 ± 0.05 | 41.33 ± 2.65** | 1.08 ± 0.06 |
| Non HDL-C: | ||||||
| fasting | 180.53 ± 7.94 | 4.64 ± 0.20 | 127.71 ± 6.17*** | 3.30 ± 0.16 | 187.10 ± 8.22 | 4.82 ± 0.21 |
| postprandial | 178.56 ± 10.09 | 4.59 ± 0.26 | 134.22 ± 7.74*** | 3.45 ± 0.19 | 199.70 ± 7.31 | 5.16 ± 0.19 |
| R-C: | ||||||
| fasting | 46.53 ± 10.23 | 1.42 ± 0.11 | 20.70 ± 1.4*** | 0.49 ± 0.05 | 38.00 ± 4.92** | 0.98 ± 0.13 |
| postprandial | 138.63 ± 9.71 | 1.81 ± 0.17 | 90.01 ± 8.7*** | 0.85 ±0.09 | 149.57 ± 9.81 | 1.49 ± 0.25 |
| f-p difference | *** | *** | *** | |||
| Glucose: | ||||||
| fasting | 114.83 ± 10.23 | 6.40 ± 0.57 | 92.14 ± 3.29* | 5.11 ± 0.18 | 90.03 ± 1.81* | 5.01 ± 0.10 |
| postprandial | 121.66 ± 9.04 | 6.82 ± 0.50 | 93.01 ± 2.90** | 5.17 ± 0.16 | 96.78 ± 4.10* | 5.30 ± 0.23 |
|
| ||||||
| LPO (nmol/ml): | ||||||
| fasting | 3.75 ± 0.46 | 2.75 ± 0.37 | 2.10 ± 0.34* | |||
| postprandial | 2.73 ± 0.29 | 3.50 ± 0.41 | 2.61 ± 0.28 | |||
| f-p difference | * | |||||
| hsCRP (mg/l): | ||||||
| fasting | 2.36 ± 0.51 | 1.10 ± 0.27* | 1.71 ± 0.54 | |||
| postprandial | 2.28 ± 0.53 | 0.94 ± 0.22* | 1.6 ± 0.56 | |||
| f-p difference | ||||||
| ApoA-I (g/l): | ||||||
| fasting | 1.44 ± 0.10 | 1.49 ± 0.04 | 1.56 ± 0.08 | |||
| postprandial | 1.47 ± 0.08 | 1.43 ± 0.07 | 1.52 ± 0.08 | |||
| ApoB (g/l): | ||||||
| fasting | 1.11 ± 0.12 | 1.06 ± 0.04 | 1.28 ± 0.06 | |||
| postprandial | 1.09 ± 0.12 | 0.99 ± 0.05 | 1.18 ± 0.06 | |||
| ApoB/ApoA-I: | ||||||
| fasting | 0.78 ± 0.06 | 0.72 ± 0.03 | 0.83 ± 0.05 | |||
| postprandial | 0.73 ± 0.07 | 0.71 ± 0.03 | 0.79 ± 0.05 | |||
Results are presented as mean ± standard error (SE). T-C: total cholesterol; TG: triglycerides; LDL-C: LDL cholesterol; HDL- (HDL2-; HDL3-) C: HDL (HDL2-; HDL3-) cholesterol; R-C: remnant cholesterol; LPO: lipid peroxides; hsCRP: high sensitivity C-reactive protein C; apoA-I (B): apolipoprotein A-I (B)
f: fasting, p: postprandial, f-p difference: the difference between the values measured in the fasting and postprandial state; *p<0.05; **p<0.01; ***p<0.001
differences statistically significant in comparison to MetSy group: *p<0.05; **p<0.01; ***p<0.001
Figure 3.
Postprandial changes (mean ± standard error)in low density lipoprotein (LDL)-cholesterol in men diagnosed with metabolic syndrome (MS) in comparison to healthy men (control I) and men with mixed hyperlipemia (control II) before-(delta LDL) and after (delta LDL-3) a three month hypolipidemic treatment
* p<0.05 in comparison to delta LDL in the control I group
3.1 The postprandial changes in lipid peroxides
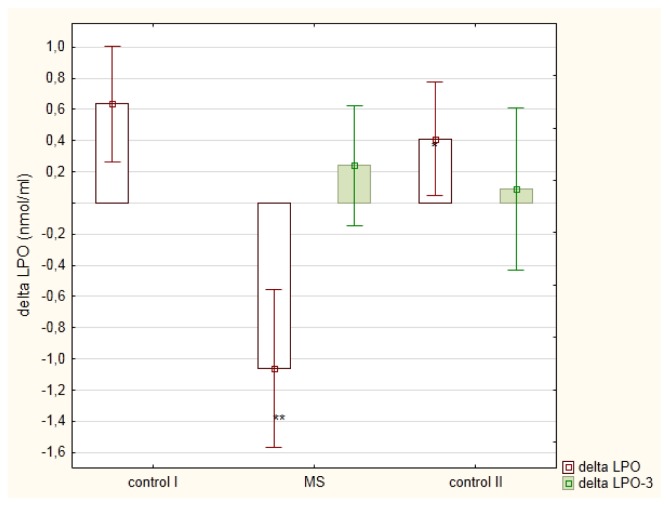
Men with MetSy displayed postprandial reduction in LPO concentration, whereas in healthy men (control I group) and men with mixed hyperlipemia (control II group), LPO level measured after the meal was higher than the one measured at fasting state, Figure 4. Similarly to postprandial changes in TG or LDL-C, meal-induced LPO changes were dependent on the postprandial non-LDL-C (r = −0.4469; p<0.05) and R-C (r = −0.4059; p<0.05). The mean fasting and postprandial remnant cholesterol concentrations were significantly greater in MetSy men in comparison to healthy subjects (p<0.001), Table 2.
Figure 4.
Postprandial changes (mean ± standard error) in lipid peroxides (LPO) in men diagnosed with metabolic syndrome (MS) in comparison to healthy men (control I) and men with mixed hyperlipemia (control II) before-(delta LPO) and after (delta LPO-3) a three month hypolipidemic treatment
* p<0.05 in comparison to delta LPO in the control I group
** p<0.01 in comparison to delta LPO in the control I group
3.2 The postprandial changes in HDL cholesterol, apolipoprotein A and hsCRP
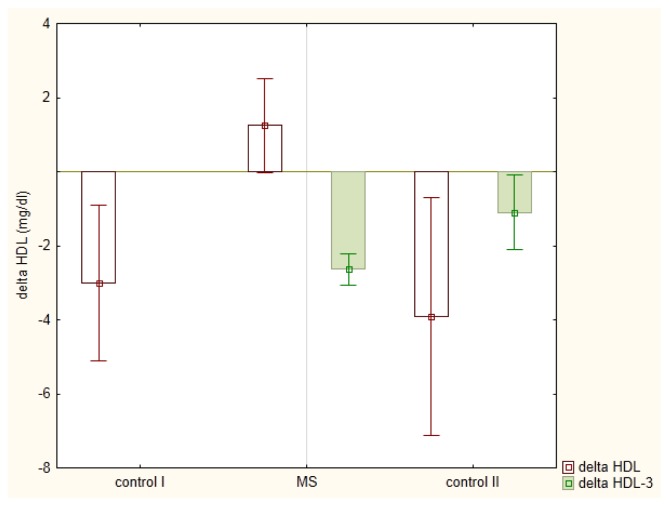
The meal-induced HDL-C changes in studied groups were presented in the Figure 5. They were linearly dependent on fasting HDL2-C (r = −0.6268; p<0.01). Meal induced changes in apolipoproteins A-I and hsCRP were statistically insignificant, Table 2.
Figure 5.
Postprandial changes (mean ± standard error) in high density lipoprotein (HDL) - cholesterol in men diagnosed with metabolic syndrome (MS) in comparison to healthy men (control I) and men with mixed hyperlipemia (control II) before-(delta HDL) and after (delta HDL-3) a three month hypolipidemic treatment
3.3 The postprandial changes in lipids, apolipoproteins and other parameters in men with MetSy or mixed hyperlipidemia after a three-month hypolipidemic therapy
The parameters measured before and after three month hypolipidemic treatment, were compared using paired Student t test and checked by paired Wilcoxon test and sign test. It was shown that after a three-month treatment, men diagnosed with MetSy and mixed hyperlipemia displayed significantly lower, in comparison to initial concentrations, serum total cholesterol, TG, non-LDL, non-HDL and postprandial remnant cholesterol levels, Table 3.
Table 3.
Comparison between lipids and other parameters measured before and after a three month hypolipidemic treatment
| Group | ||||
|---|---|---|---|---|
| MetS (12 men) | Control II (10 men) | |||
| mg/dl | mmol/l | mg/dl | mmol/l | |
| T-C: | ||||
| fasting before the treatment | 219.91 ± 8.67 | 5.67 ± 0.22 | 240.60 ± 7.24 | 5.52 ± 0.19 |
| fasting after the treatment | 197.00 ± 11.78* | 5.08 ± 0.31* | 192.20 ± 10.37*** | 4.95 ± 0.26*** |
| postprandial before the treatment | 218.50 ± 11.03 | 5.62 ± 0.28 | 249.30 ± 6.69 | 6.42 ± 0.17 |
| postprandial after the treatment | 196.54 ± 11.92* | 5.06 ± 0.31* | 189.10 ± 9.91*** | 4.88 ± 0.26*** |
|
| ||||
| TG: | ||||
| fasting before the treatment | 275.41 ± 21.93 | 3.11 ± 0.25 | 188.70 ± 24.23 | 2.1 ± 0.27 |
| fasting after the treatment | 176.09 ± 24.65*** | 1.99 ± 0.28*** | 118.80 ± 15.48* | 1.33 ± 0.17* |
| postprandial before the treatment | 350.83 ± 30.25 | 3.97 ± 0.34 | 292.20 ± 49.35 | 3.3 ± 0.55 |
| postprandial after the treatment | 201.72 ± 27.95*** | 2.28 ± 0.32*** | 159.90 ± 26.32** | 1.80 ± 0.29** |
|
| ||||
| LDL-C: | ||||
| fasting before the treatment | 134.10 ± 12.31 | 3.46 ± 0.32 | 149.30 ± 7.11 | 3.84 ± 0.18 |
| fasting after the treatment | 116.81 ± 12.33 | 3.00 ± 0.31 | 107.60 ± 10.76** | 2.79 ± 0.28** |
| postprandial before the treatment | 108.60 ± 8.60 | 2.79 ± 0.22 | 141.50 ± 9.63 | 3.64 ± 0.25 |
| postprandial after the treatment | 113.90 ± 12.70 | 2.94 ± 0.33 | 104.30 ± 9.45* | 2.68 ± 0.24* |
|
| ||||
| Non-LDL-C : | ||||
| fasting before the treatment | 94.33 ± 5.01 | 2.43 ± 0.13 | 91.40 ± 3.10 | 2.35 ± 0.08 |
| fasting after the treatment | 80.18 ± 3.19 *** | 2.10 ± 0.08*** | 84.60 ± 6.75* | 2.1 ± 0.17* |
| postprandial before the treatment | 110.10 ± 6.22 | 2.84 ± 0.16 | 107.80 ± 8.82 | 2.79 ± 0.23 |
| postprandial after the treatment | 82.63 ± 4.74*** | 2.10 ± 0.12*** | 84.80 ± 3.74* | 2.1 ± 0.09* |
|
| ||||
| HDL-C: | ||||
| fasting before the treatment | 39.38 ± 1.84 | 1.00 ± 0.05 | 53.50 ± 3.28 | 1.37 ± 0.09 |
| fasting after the treatment | 45.09 ± 3.44 | 1.16 ± 0.09 | 54.00 ± 4.24 . | 1.39 ± 0.11 |
| postprandial before the treatment | 39.93 ± 2.55 | 1.03 ± 0.07 | 49.60 ± 2.70 | 1.29 ± 0.07 |
| postprandial after the treatment | 42.45 ± 3.70 | 1.08 ± 0.09 | 52.90 ± 4.60 | 1.37 ± 0.12 |
|
| ||||
| HDL2-C: | ||||
| fasting before the treatment | 7.35 ± 1.18 | 0.19 ± 0.03 | 12.52 ± 2.76 | 0.32 ± 0.07 |
| fasting after the treatment | 10.82 ± 1.89 | 0.28 ± 0.05 | 12.82 ± 1.79 | 0.33 ± 0.05 |
| postprandial before the treatment | 7.28 ± 1.46 | 0.18 ± 0.04 | 8.51 ± 1.10 | 0.22 ± 0.03 |
| postprandial after the treatment | 7.37 ± 1.28 | 0.19 ± 0.03 | 9.44 ± 2.11 | 0.24 ± 0.05 |
|
| ||||
| HDL3-C: | ||||
| fasting before the treatment | 32.00 ± 1.75 | 0.82 ± 0.05 | 42.03 ± 2.36 | 1.06 ± 0.07 |
| fasting after the treatment | 34.26 ± 2.33 | 0.88 ± 0.06 | 41.04 ± 3.41 | 1.06 ± 0.09 |
| postprandial before the treatment | 32.08 ± 1.59 | 0.82 ± 0.04 | 41.33 ± 2.65 | 1.08 ± 0.06 |
| postprandial after the treatment | 35.18 ± 2.82 | 0.90 ± 0.07 | 43.36 ± 2.95 | 1.11 ± 0.07 |
|
| ||||
| Non-HDL-C : | ||||
| fasting before the treatment | 180.53 ± 7.94 | 4.64 ± 0.20 | 187.10 ± 8.22 | 4.82 ± 0.21 |
| fasting after the treatment | 151.90 ± 10.39** | 3.92 ± 0.27** | 131.66 ± 17.23*** | 3.40 ± 0.44*** |
| postprandial before the treatment | 178.56 ± 10.09 | 4.59 ± 0.26 | 199.70 ± 7.31 | 5.16 ± 0.19 |
| postprandial after the treatment | 154.09 ± 10.93* | 3.97 ± 0.28* | 129.36 ± 17.07*** | 3.33 ± 0.44*** |
|
| ||||
| R-C: | ||||
| fasting before the treatment | 46.53 ± 10.23 | 1.42 ± 0.11 | 38.00 ± 4.92 | 0.98 ± 0.13 |
| fasting after the treatment | 35.09 ± 4.94 | 0.91 ± 0.12 | 30.58 ± 8.88 | 0.81 ± 0.13 |
| postprandial before the treatment | 138.63 ± 9.71 | 1.81 ± 0.17 | 149.57 ± 9.81 | 1.49 ± 0.25 |
| postprandial after the treatment | 40.27 ± 5.66*** | 1.03 ± 0.15*** | 31.90 ± 5.18*** | 0.83 ± 0.14*** |
|
| ||||
| Glucose: | ||||
| fasting before the treatment | 114.83 ± 10.23 | 6.40 ± 0.57 | 90.03 ± 1.81 | 5.01 ± 0.10 |
| fasting after the treatment | 105.63 ± 7.58 | 5.89 ± 0.42 | 93.00 ± 2.84 | 5.17 ± 0.16 |
| postprandial before the treatment | 121.66 ± 9.04 | 6.82 ± 0.50 | 96.78 ± 4.10 | 5.30 ± 0.23 |
| postprandial after the treatment | 109.90 ± 7.38 | 6.11 ± 0.41 | 99.40 ± 5.01 | 5.50 ± 0.28 |
|
| ||||
| LPO (mmol/ml): | ||||
| fasting before the treatment | 3.75 ± 0.46 | 2.10 ± 0.34 | ||
| fasting after the treatment | 2.35 ± 0.34 | 2.09 ± 0.32 | ||
| postprandial before the treatment | 2.73 ± 0.29 | 2.61 ± 0.28 | ||
| postprandial after the treatment | 2.33 ± 0.43 | 2.30 ± 0.46 | ||
|
| ||||
| hsCRP (mg/l): | ||||
| fasting before the treatment | 2.36 ± 0.51 | 1.71 ± 0.54 | ||
| fasting after the treatment | 2.59 ± 0.71 | 0.61 ± 0.14 | ||
| postprandial before the treatment | 2.28 ± 0.53 | 1.6 ± 0.56 | ||
| postprandial after the treatment | 2.47 ± 0.66 | 0.71 ± 0.24 | ||
|
| ||||
| ApoA-I (g/l): | ||||
| fasting before the treatment | 1.44 ± 0.10 | 1.56 ± 0.08 | ||
| fasting after the treatment | 1.45 ± 0.07 | 1.54 ± 0.11 | ||
| postprandial before the treatment | 1.47 ± 0.08 | 1.52 ± 0.08 | ||
| postprandial after the treatment | 1.49 ± 0.07 | 1.53 ± 0.08 | ||
|
| ||||
| ApoB (g/l): | ||||
| fasting before the treatment | 1.11 ± 0.12 | 1.28 ± 0.06 | ||
| fasting after the treatment | 1.12 ± 0.06 | 0.93 ± 0.05*** | ||
| postprandial before the treatment | 1.09 ± 0.12 | 1.18 ± 0.06 | ||
| postprandial after the treatment | 1.13 ± 0.06 | 0.97 ± 0.06* | ||
|
| ||||
| ApoB/ApoA-I: | ||||
| fasting before the treatment | 0.78 ± 0.06 | 0.83 ± 0.05 | ||
| fasting after the treatment | 0.78 ± 0.05 | 0.61 ± 0.06* | ||
| postprandial before the treatment | 0.73 ± 0.07 | 0.79 ± 0.05 | ||
| postprandial after the treatment | 0.77 ± 0.05 | 0.66 ± 0.06 | ||
Results are presented as mean ± standard error (SE). T-C: total cholesterol; TG: triglycerides; LDL-C: LDL cholesterol; HDL- (HDL2-; HDL3-) C: HDL (HDL2-; HDL3-) cholesterol; R-C: remnant cholesterol; LPO: lipid peroxides; hsCRP: high sensitivity C-reactive protein C; apoA-I (B): apolipoprotein A-I (B)
difference statistically significant in comparison to values measured before the treatment: *p<0.05; **p<0.01; ***p<0.001 using paired t tests for dependent samples
Fat tolerance test performed in men diagnosed with MetSy after a three month treatment showed significantly lower changes in TG (p<0.05) in comparison to changes induced by meal before the treatment, Figure 2. LDL-C level, which before treatment decreased postprandially, after the treatment was similar before and after a meal; a similar pattern was observed for LPO. On the contrary, HDL-C, and especially HDL2-C, before the treatment remained on a similar level before and after a meal, and after the treatment decreased postprandially. Level of R-C before the treatment increased after a meal; after the treatment did not fluctuate between fasting and non-fasting levels, Tables 2 and 4.
Table 4.
Fasting and postprandial lipids and other measured parameters measured after a three month hypolipidemic treatment
| Group | ||||
|---|---|---|---|---|
| MetS (12 men) | Control II (10 men) | |||
| mg/dl | mmol/l | mg/dl | mmol/l | |
| T-C: | ||||
| fasting | 197.00 ± 11.78 | 5.08 ± 0.31 | 192.20 ± 10.37 | 4.95 ± 0.26 |
| postprandial | 196.54 ± 11.92 | 5.06 ± 0.31 | 189.10 ± 9.91 | 4.88 ± 0.26 |
|
| ||||
| TG: | ||||
| fasting | 176.09 ± 24.65 | 1.99 ± 0.28 | 118.80 ± 15.48 | 1.33 ± 0.17 |
| postprandial | 201.72 ± 27.95 | 2.28 ± 0.32 | 159.90 ± 26.32 | 1.80 ± 0.29 |
| f-p difference | ** | |||
|
| ||||
| LDL-C: | ||||
| fasting | 116.81 ± 12.33 | 3.00 ± 0.31 | 107.60 ± 10.76 | 2.79 ± 0.28 |
| postprandial | 113.90 ± 12.70 | 2.94 ± 0.33 | 104.30 ± 9.45 | 2.68 ± 0.24 |
|
| ||||
| Non-LDL-C : | ||||
| fasting | 80.18 ± 3.19 | 2.10 ± 0.08 | 84.60 ± 6.75 | 2.1 ± 0.17 |
| postprandial | 82.63 ± 4.74 | 2.10 ± 0.12 | 84.80 ± 3.74 | 2.1 ± 0.09 |
|
| ||||
| HDL-C: | ||||
| fasting | 45.09 ± 3.44 | 1.16 ± 0.09 | 54.00 ± 4.24 | 1.39 ± 0.11 |
| postprandial | 42.45 ± 3.70 | 1.08 ± 0.09 | 52.90 ± 4.60 | 1.37 ± 0.12 |
| f-p difference | *** | |||
|
| ||||
| HDL2-C: | ||||
| fasting | 10.82 ± 1.89 | 0.28 ± 0.05 | 12.82 ± 1.79 | 0.33 ± 0.05 |
| postprandial | 7.37 ± 1.28 | 0.19 ± 0.03 | 9.44 ± 2.11 | 0.24 ± 0.05 |
| f-p difference | * | * | ||
|
| ||||
| HDL3-C: | ||||
| fasting | 34.26 ± 2.33 | 0.88 ± 0.06 | 41.04 ± 3.41 | 1.06 ± 0.09 |
| postprandial | 35.18 ± 2.82 | 0.90 ± 0.07 | 43.36 ± 2.95 | 1.11 ± 0.07 |
| f-p difference | ** | |||
|
| ||||
| Non-HDL-C : | ||||
| fasting | 151.90 ± 10.39 | 3.92 ± 0.27 | 131.66 ± 17.23 | 3.40 ± 0.44 |
| postprandial | 154.09 ± 10.93 | 3.97 ± 0.28 | 129.36 ± 17.07 | 3.33 ± 0.44 |
|
| ||||
| R-C: | ||||
| fasting | 35.09 ± 4.94 | 0.91 ± 0.12 | 30.58 ± 8.88 | 0.81 ± 0.13 |
| postprandial | 40.27 ± 5.66 | 1.03 ± 0.15 | 31.90 ± 5.18 | 0.83 ± 0.14 |
|
| ||||
| Glucose: | ||||
| fasting | 105.63 ± 7.58 | 5.89 ± 0.42 | 93.00 ± 2.84 | 5.17 ± 0.16 |
| postprandial | 109.90 ± 7.38 | 6.11 ± 0.41 | 99.40 ± 5.01 | 5.50 ± 0.28 |
|
| ||||
| LPO (mmol/ml): | ||||
| fasting | 2.35 ± 0.34 | 2.09 ± 0.32 | ||
| postprandial | 2.33 ± 0.43 | 2.30 ± 0.46 | ||
|
| ||||
| hsCRP (mg/l): | ||||
| fasting | 2.59 ± 0.71 | 0.61 ± 0.14* | ||
| postprandial | 2.47 ± 0.66 | 0.71 ± 0.24* | ||
| f-p difference | * | |||
|
| ||||
| ApoA-I (g/l): | ||||
| fasting | 1.45 ± 0.07 | 1.54 ± 0.11 | ||
| postprandial | 1.49 ± 0.07 | 1.53 ± 0.08 | ||
| f-p difference | * | |||
|
| ||||
| ApoB (g/l): | ||||
| fasting | 1.12 ± 0.06 | 0.93 ± 0.05* | ||
| postprandial | 1.13 ± 0.06 | 0.97 ± 0.06 | ||
|
| ||||
| ApoB/ApoA-I: | ||||
| fasting | 0.78 ± 0.05 | 0.61 ± 0.06* | ||
| postprandial | 0.77 ± 0.05 | 0.66 ± 0.06 | ||
Results are presented as mean ± standard error (SE). T-C: total cholesterol; TG: triglycerides; LDL-C: LDL cholesterol; HDL- (HDL2-; HDL3-) C: HDL (HDL2-; HDL3-) cholesterol; R-C: remnant cholesterol; LPO: lipid peroxides; hsCRP: high sensitivity C-reactive protein C; apoA-I (B): apolipoprotein A-I (B)
difference statistically significant in comparison to group of men with mixed hyperlipidemia: p<0.05
f: fasting, p: postprandial, f-p difference: the difference between the values measured in the fasting and postprandial state; *p<0.05; **p<0.01; ***p<0.001
difference statistically significant in comparison to MetSy group: *p<0.05
Additionally, in men with mixed hyperlipemia, hypolipidemic treatment led to decrease in LDL cholesterol, apo B lipoprotein, and, at fasting state, also apoB/apoA-I ratio. On the contrary, in men with MetSy, there were no significant changes in LDL cholesterol, apo B and apo B/ apo A-I ratio during the three month observation, Table 3.
4 Discussion
A fasting TG level ≤ 150 mg/dl [21], a non-fasting TG level (measured at any time within up to 8 hour after any normal meal) ≤ 180 mg/dl, as well as a postprandial TG level (measured at a fixed time within up to 8 hour of a standardized fat tolerance test; FTT) ≤ 220 mg/dl [18], should be considered as desirable serum TG concentrations. In the context of these recommendations, only in the group of healthy, normolipemic men, a fasting TG level and postprandial response were physiological (Table 2). However, in men diagnosed with mixed hyperlipidemia, after a three-month hypolipidemic treatment, fasting and postprandial TG levels decreased to normal levels. Simultaneously, the three-month treatment decreased fasting and postprandial TG concentrations to levels close to normal also in men diagnosed with MetSy. The decrease in TG concentrations after a hypolipidemic therapy is usually observed as a result of the action of peroxisome proliferator-activated receptors alpha (PPAR-alpha) agonists [22]. Not fully satisfactory effect of hypolipidemic treatment in patients diagnosed with MetSy has been observed also in other studies [23,24].
The difference between men with MetSy and mixed hyperlipemia was the lack of hypolipidemic treatment effect on apo B and apo B/apo A-I ratio in men with MetSy (Table 3). Contrarily, in men with mixed hyperlipemia, significant decreases in apo B and apo B/apo A-I ratio at fasting state were observed. The value of the apo B/ apoA-I ratio is a particularly effective parameter for risk assessment of atherosclerosis: the greater the value of the ratio, the greater the risk of cardiovascular disease [25]. Some studies, (i.e. Copenhagen City Heart Study, LIPID or AMORIS) showed that apo B has a stronger capacity as a predictor of cardiovascular risk than both LDL-C and non-HDL-C [26,27]. In AMORIS study, fatal myocardial infarction was related to increased values of apo B/apo A-I [28]. Simultaneously, apo B may be more closely associated with metabolic syndrome than either LDL-C, or non-HDL-C [29]. This study showed that in men with MetSy, a three-month hypolipidemic treatment was efficient with regard to lipid profile (reduced the TG, total and non-HDL cholesterol) but did not reduce cardiovascular risk determined by apo B/apo A-I ratio.
In some randomized multicenter studies on cardiovascular risk assessment, non-fasting lipid levels are determined. Correction of non-fasting serum TG to fasting values in Finnish population under the National FINRISK 2007 Study reduced miscalculation of high LDL-C in population from 5.1 to 1.6%, and the occurrence of metabolic syndrome from 4.2 to 2.1% [30]. This study showed a lower, in comparison to fasting values, LDL cholesterol after three hours of the fat tolerance test in men diagnosed with MetSy. Also in other studies, performed in patients with diabetes mellitus type 2, LDL cholesterol, measured by various methods, was postprandially reduced [31]. A decrease in LDL-C after a meal may occur in all people, including a healthy population (LDL-C tendency to decrease postprandially was observed also in normolipemic men in our study), and may not be in any way connected with disturbances in lipid metabolism. All the same, as LDL-C level is the most often used predictor of cardiovascular diseases, lower levels of non-fasting LDL cholesterol may cause the underestimation of cardiovascular risk, which could result in a shift of studied populations to lower-risk groups.
Our study has also shown that postprandial LDL-C reduction was greater in men with MetSy, comparing to healthy men and men diagnosed with mixed hyperlipemia (Figure 3). This difference was not caused by different fasting levels, which were similar in all groups of men. Simultaneously, postprandial LDL-C changes, similarly to changes in TG and LPO, were dependent on postprandial non-LDL cholesterol and remnant cholesterol levels. Postprandial remnants, originating from chylomicrons and VLDLs, may reflect intestinal absorption or utilization of exogenous cholesterol.
One could suspect that the absorption of cholesterol in men with MetSy is higher in comparison to healthy men, as their postprandial remnant cholesterol is significantly greater than in controls (Table 2). However, meal-induced R-C changes in all studied groups (men with MetSy, healthy men and men with mixed hyperlipemia) were similar. Thus, if the absorption of exogenous cholesterol was similar in all groups, the reason for the different decreases in LDL cholesterol may be a different degree of its utilization. Postprandial utilization of LDL, deriving mainly from VLDLs, is associated with the accumulation of cholesterol in peripheral tissues, including the vessel wall. A very small, but noticeable, decrease in apo B, as well as a decrease in lipid peroxides after a meal in men with metabolic syndrome may result from the utilization of low density lipoproteins. It is quite possible that a higher decrease in LDL-cholesterol is a consequence of an increased inflow of cholesterol into vessel wall. It would present a strong atherogenic mechanism in men with MetSy, not only because of an increased cholesterol inflow into vessel wall, but also because of a less efficient reverse cholesterol transport cleaning mechanism, as men with MetSy have lower HDL levels. Also, postprandial decrease in HDL-C (namely HDL2-C) after a three-month hypolipidemic treatment in men with MetSy (or mixed hyperlipemia) may indicate a weakened HDL defense response.
The presence of a linear relationship between delta LDL-C (also delta LPO and delta TG) and postprandial non-LDL cholesterol (and remnant cholesterol) indicates that the mechanisms limiting (allowing) meal-induced lipid changes are associated with lipoproteins such as VLDLs, IDLs, HDLs and chylomicrones [32–34]. These mechanisms are included in residual risk existing in people with metabolic syndrome, despite normal or even low LDL-C level [35].
In this study, metabolic syndrome (similar to mixed hyperlipemia) was associated also with higher, in comparison to state of health, non-HDL-cholesterol. At present, the non-HDL-C was established as a secondary treatment target, whereas a non-HDL-C reporting as a factor improving non-HDL-C goal attainment [36].
Increased pro-oxidative and pro-inflammatory activities are further risk factors. At a fasting state, men diagnosed with metabolic syndrome, in comparison to normolipemic men, displayed elevated high sensititivity C-reactive protein and slightly increased lipid peroxides. The slightly increased fasting hsCRP level was observed also in men diagnosed with mixed hyperlipemia (Table 1). A pro-oxidative and inflammatory state is commonly present in persons with metabolic syndrome [14,16,37]. Multiple mechanisms can lead to elevation of LPO and/or CRP, e.g. obesity, resistance to insulin and hypertriglyceridemia [38]. However, in our study, like in other studies [39], lower LDL-C after a meal were accompanied by a decrease in LPO. The relation between LDL and LPO seems to be strict after a meal, as an exogenous oxidized cholesterol esters are directly incorporated into lipoproteins and transferred via the LDL into endothelial cells [40].
Of course, this study has some limitations. Only men who regularly ate fat-rich foods agreed to take part in the study (hence the small number of volunteers). We measured lipid levels before and 3 hours after the standardized high-fat (approximately 50% fat) meal, although in other studies, the maximal postprandial lipid changes were observed 4 to 6 hours after a meal. It depended on the composition of fats and also on the method of measurement. However, modified β quantification test, as recommended by NCEP, demands the observation after 3hours [31]. An advantage of this study is the fact that the studied group consisted only of men with newly diagnosed MetSy, who had not been treated before.
5 Conclusion
The present study shows postprandial decreases in LDL cholesterol in men diagnosed with metabolic syndrome. This decrease may cause underestimation of cardiovascular risk. On the other hand, in men with MetSy, postprandial decrease in LDL cholesterol was not observed after three months of hypolipidemic treatment. Simultaneously, the apoB/apoA-I ratio remained the same, which suggests that in those patients, the reduction in risk of cardiovascular event was only partial.
Acknowledgments
This study is part of a research project funded by the Polish Minister Science and Higher Education under statute activities no. 447/2010-13.
List of abbreviations
- BMI
body mass index
- LDL-C
low density lipoprotein cholesterol
- MetSy
metabolic syndrome
- LPO
lipid peroxides
- hsCRP
high-sensitivity C-reactive protein
- TG
triglycerides
- apoB
apolipoprotein B
- apo A-I
apolipoprotein A-I
- HDL-C
high density lipoprotein cholesterol
- HDL3 (HDL2) – C
high density lipoprotein 3 (2) cholesterol
- HTG
hypertriglyceridemia
- FTT
fast tolerance test
- TC
total cholesterol
- R-C
remnant cholesterol
- SE
standard error
- LIPID study
Long-Term Intervention with Pravastatin in Ischaemic Disease study
- AMORIS study
Apolipoprotein-Related Mortality Risk study
- FINRISK study
Finland Cardiovascular Risk Study
- NCEP
National Cholesterol Education Program
- VLDLs
very low density lipoproteins
- IDLs
intermediate density lipoprotein cholesterol
Footnotes
Conflict of interest: The authors state that they have no conflicts of interest.
References
- 1.Reaven GM. The Metabolic Syndrome: Requiescat in Pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Achimastos AD, Efstathiou SP, Christoforatos T, Panagiotou TN, Stergiou GS, Mountokalakis TD. Arterial stiffness: determinants relationship to the metabolic syndrome. Angiology. 2007;58:11–20. doi: 10.1177/0003319706295477. [DOI] [PubMed] [Google Scholar]
- 4.Beijers HJ, Henry RM, Bravenboer B, Ferreira I, Dekker JM, Nijpels G, Stehouwer CD. Metabolic Syndrome in Nondiabetic Individuals Associated With Maladaptive Carotid Remodeling: The Hoorn Study. Am J Hypertens. 2011 Jan 6; doi: 10.1038/ajh.2010.256. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Tzou WS, Douglas PS, Srinivasan SR, Chen W, Berenson G, Stein JH. Advanced lipoprotein testing does not improve identification of subclinical atherosclerosis in young adults: the Bogalusa Heart Study. ANN Intern Med. 2005;142:742–50. doi: 10.7326/0003-4819-142-9-200505030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk. A systematic review and meta-analysis. J Am CollCardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Hua Q, Lerman LO, Lerman A. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968–75. doi: 10.1016/j.mayocp.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nofer JR, Kehre B, Fobker M, Levkau B, Assman G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 11.Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur Heart J Suppl. 2005;7:F4–F8. [Google Scholar]
- 12.Andrews KL, Moore XL, Chin-Dusting JP. Anti-atherogenic effects of high-density lipoprotein on nitric oxide synthesis in the endothelium. Clin Exp Pharmacol Physiol. 2010;37:736–42. doi: 10.1111/j.1440-1681.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- 13.Onat A, Can G, Yüksel H. Dysfunction of high-density lipoprotein and its apolipoproteins: New mechanisms underlying cardiometabolic risk in the population at large Turk Kardiyol Dern Ars. 2012;40:368–85. doi: 10.5543/tkda.2012.55490. [DOI] [PubMed] [Google Scholar]
- 14.Calabro P, Yeh ETH. Intra-abdominal adiposity, inflammation, and cardiovascular risk: New insight into global cardiometabolic risk. Current Hypertension Rep. 2008;10:32–38. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- 15.Yuan G, Al-Shali KZ, Hegele R. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansel B, Giral P, Nobecourt E, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 17.Bai H, Liu BW, Deng ZY, Shen T, Fang DZ, Zhao YH, Liu Y. Plasma very-low-density lipoprotein, low-density lipoprotein, and high-density lipoprotein oxidative modification induces procoagulant profiles in endogenous hypertriglyceridemia. Free Radic Biol Med. 2006;40:1796–803. doi: 10.1016/j.freeradbiomed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 19.Nordestgaard BG, Freiberg JJ. Clinical relevance of non-fasting and postprandial hypertriglyceridemia and remnant cholesterol. CurrVascPharmacol. 2011;9:281–6. doi: 10.2174/157016111795495585. [DOI] [PubMed] [Google Scholar]
- 20.Salazar MR, Carbajal HA, Espeche WG, LeivaSisnieguez CE, Balbín E, Dulbecco CA, Aizpurúa M, Marillet AG, Reaven GM. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am J Cardiol. 2012;109:1749–53. doi: 10.1016/j.amjcard.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM National Cholesterol Education Program (NCEP)-The National Cholesterol Guidelines in 2001, Adult Treatment Panel (ATP) III. Approach to lipoprotein management in 2001 National Cholesterol Guidelines. Am J Cardiol. 2002;90:11i–21i. doi: 10.1016/s0002-9149(02)02631-0. [DOI] [PubMed] [Google Scholar]
- 22.Fruchart JC, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today. 2006;42:39–64. doi: 10.1358/dot.2006.42.1.963528. [DOI] [PubMed] [Google Scholar]
- 23.Stone NJ, Saxon D. Approach to treatment of the patient with metabolic syndrome: lifestyle therapy. Am J Cardiol. 2005;96:15E–21E. doi: 10.1016/j.amjcard.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Goodson BL, Wung SF, Archbold KH. Obstructive sleep apnea hypopnea syndrome and metabolic syndrome: A synergistic cardiovascular risk factor. J Am Acad Nurse Pract. 2012;24:695–703. doi: 10.1111/j.1745-7599.2012.00771.x. [DOI] [PubMed] [Google Scholar]
- 25.Walldius G, Aastveit AH, Jungner I. Stroke mortality and the apoB/apoA-I ratio: results of the AMORIS prospective study. J Intern Med. 2006;259:259–66. doi: 10.1111/j.1365-2796.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 26.Jungner I, Sniderman AD, Furberg C, Aastveit AH, Holme I, Walldius G. Does low-density lipoprotein size add to atherogenic particle number in predicting the risk of fatal myocardial infarction? Am J Cardiol. 2006;97:943–6. doi: 10.1016/j.amjcard.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 27.Contois JH, Warnick GR, Sniderman AD. Reliability of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B measurement. J Clin Lipidol. 2011;5:264–72. doi: 10.1016/j.jacl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Walldius G. Apolipoprotein B (apoB) more closely related to subclinical atherosclerosis than non-HDL cholesterol and LDL cholesterol. J Intern Med. 2010;268:549–51. doi: 10.1111/j.1365-2796.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 29.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. CircCardiovascQual Outcomes. 2011;4:337–452. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 30.Sundvall J, Leiviskä J, Laatikainen T, Peltonen M, Salomaa V, Vanhala M, Korpi-Hyövälti E, Lauronen J, Alfthan G. The use of fasting vs. non-fasting triglyceride concentration for estimating the prevalence of high LDL-cholesterol and metabolicsyndrome in population surveys. BMC Med Res Methodol. 2011;11:63. doi: 10.1186/1471-2288-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund SS, Petersen M, Frandsen M, Smidt UM, Parving HH, Vaag AA, Jensen T. Agreement between fasting and postprandial LDL cholesterol measured with 3 methods in patients with type 2 diabetes mellitus. Clin Chem. 2011;57:298–308. doi: 10.1373/clinchem.2009.133868. [DOI] [PubMed] [Google Scholar]
- 32.Liskum L. Cholesterol biosynthesis. New Comprehensive Biochemistry. 2002;36:409–432. [Google Scholar]
- 33.Ruge T, Sukonina V, Kroupa O, Makoveichuk E, Lundgren M, Svensson MK, Olivecrona G, Eriksson JW. Effects of hyperin-sulinemia on lipoprotein lipase, angiopoietin-like protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metabolism. 2012;61:652–60. doi: 10.1016/j.metabol.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Zélie J, Fournier N, Bellanger N, Chapman MJ, Goff WL, Guerin M. Postprandial lipemiaenhances the capacity of large HDL2 particles to mediate free cholesterol efflux via SR-BI and ABCG1 pathways in type IIB hyperlipidemia. J Lipid Res. 2010;51:3350–3358. doi: 10.1194/jlr.P009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR. Cardiovascular Morbidity and Mortality Associated With the Metabolic Syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 36.Virani SS, Wang D, Woodard LD, Chitwod SS, Landrum CR, Zieve FJ, Ballantyne CM, Petersen LA. Non–high-density lipoprotein cholesterol reporting and goal attainment in primary care. J Clin Lipidol. 2012;6:545–552. doi: 10.1016/j.jacl.2012.04.080. [DOI] [PubMed] [Google Scholar]
- 37.Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J ClinNutr. 2011;94:422–430. doi: 10.3945/ajcn.111.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pravenec M, Kajiya T, Zídek V, Landa V, Mlejnek P, Simáková M, Silhavý J, Malínská H, Oliyarnyk O, Kazdová L, Fan J, Wang J, Kurtz TW. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension. 2011;57:731–737. doi: 10.1161/HYPERTENSIONAHA.110.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazloom Z, Hejazi N, Dabbaghmanesh MH, Tabatabaei HR, Ahmadi A, Ansar H. Effect of vitamin C supplementation on postprandial oxidative stress and lipid profile in type 2 diabeticpatients. Pak J Biol Sci. 2011;14:900–904. doi: 10.3923/pjbs.2011.900.904. [DOI] [PubMed] [Google Scholar]
- 40.Spiteller G. The relation of lipid peroxidation processes with atherogenesis: a new theory on atherogenesis. Mol Nutr Food Res. 2005;49:999–1013. doi: 10.1002/mnfr.200500055. [DOI] [PubMed] [Google Scholar]