Abstract
Radiofrequency catheter ablation is an increasingly adopted strategy for difficult-to-manage patients with atrial fibrillation. Echocardiography is the key imaging modality to assess left atrialstructure and function. In this review, the role of echocardiography in atrial fibrillation ablationbefore, during and after ablation is discussed. Currently established roles of echocardiography inpatient selection pre-ablation and peri-procedural guidance, as well as newer echocardiographic techniques including the assessment of atrial mechanics are reviewed in the context of atrial fibrillation ablation.
Introduction
Atrial fibrillation (AF) is a common arrhythmia associated with significant morbidity and mortality. In recent years, radiofrequency catheter ablation with the electrical isolation of the pulmonary veins is commonly performed for patients withparoxysmal and persistent AF who continue tobe symtomatic despite at least one Class I or III antiarrhythmic medication.[01] Restoration of sinus rhythm after AF ablation significantly improvedsymptoms, exercise capacity, quality of life and left ventricular (LV) function, even when concurrent heart disease and ventricular rate control had been adequate before ablation.[02-03]
Multimodality imaging is often employed to assess patients undergoing ablation. However, echocardiography remains integral in the assessment of left atrial (LA) structure and function. This review discusses the role of echocardiography in AF ablation from pre-ablation, during and post-ablation. This includes the initial evaluation and patient selection, pre-procedural screening for LA and LA appendage (LAA) thrombus, direct visualization of anatomic landmarks during ablation, assessment of ablation complications, assessment of LA mechanics post-ablation and risk stratification for thromboembolism.
Pre-Ablation
Transthoracic echocardiography (TTE) is essential for the initial evaluation of patients with AF, in most cases, before AF ablation is even consideredas a treatment option. The overall management strategy of AF depends on a variety of clinical factors, including the type and duration of AF, severity of symptoms, patient age, associatedcardiovascular disease and other concurrentmedical conditions. TTE provides information on the etiologies and predisposing factorsfor the AF, effect on the ventricular function,as well as prognostic information on the risk of recurrence and thromboembolic risk ([Table 1]).
Table 1. The role of TTE and TEE in the Pre-Ablation Assessment of Patients with AF .
| Transthoracic Echocardiogram | Transesophageal Echocardiogram |
|---|---|
| Underlying causes of AF: • Valvular heart disease • Ischemic heart disease • Hypertensive heart disease • Infiltrative disease • Other cardiomyopathies • Pericardial disease • Congenital heart disease | Exclusion of LA appendage thrombus: • Prior to cardioversion • Prior to AF ablation |
| Effect of AF on the LV: • Tachycardia-induced cardiomyopathy | Pulmonary vein anatomy and function: • Variant PV anatomy • Pulmonary vein stenosis |
| Guidance of treatment option: • Rate control vs. rhythm control • Anticoagulation | |
| Prognostic information: • Left atrial size |
Information on LV function impacts the choice of appropriate pharmacological agents for both rate- and rhythm-control strategies. Agents such as beta-blockers including sotalol, and nondihydropyridine calcium channel antagonists should be administered with caution in patients with severe LV dysfunction and heart failure. Impaired LV systolic function is also an independent echcardiographic predictor of stroke in patients withAF, even after adjusting for other clinical features.[04]
Impact of LA Size and LV Function on Patient Selection for AF Ablation
Accurate assessment of both LA size and LV function provides essential information for patient slection and is an importantdeterminant of successful AF ablation
LA Size
Marked LA dilation is associated with a lower success rate of maintaining sinus rhythm after AF ablation compared to patients with structurally normal hearts.[05] As a result, the lack of LA enlargement is an important component of the currentguideline recommendations for the use of AF ablation as an alternative to pharmacologic therapy in symptomic patients.[03,06] Hence, accurate measurement of LA size is crucial for the decision-making on suitability for AF ablation.
LA size measurement is routinely performed by TTE. LA anteroposterior dimension can be measured by M-mode or 2D echo in the parasternal long axis view. This method is convenient and has been widely adopted in routine clinical practice. However, LA volume measured by either the ellipsoid model or the Simpson’s method is a more reliable measure of true LA size than Mmode LA dimension[07] and is the recommended method for the accurate assessment of LA size.[08]
To improve the accuracy of LA size measurement, 3-dimensional echocardiography (3DE), cardiac computed tomography (CT) and cardiac magnetic resonance imaging (CMR) have been studied. The 3DE measurements demonstrate favorable test-retest variability 9 and good agreement with CMR. [09-11] When these techniques are applied in the context of AF ablation, LA size measurements by 3DE ,[12-13] cardiac CT,[14] and CMR[15-16]also show good correlation with subsequent proceduralsuccess. Among the newer techniques, 3DE shows the most promise of adoption in routine clinical practice as it is non-invasive, readily available,and can be added onto the routinely performed post-ablation 2DE examination. As will be discussed later, 3DE also offers the possibility of measuring LA volumes at different phases of the cardiac cycle, yielding information on LA phasic function. Nevertheless, it is worthwhile to note that LA size measurements made by 2DE tend to be lower than those of 3DE,[9,17] cardiac CT[18] and CMR.[19] The relative strengths and weaknesses of various imaging modalities in the valuation of LA size are outlined in ([Table 2])
Table 2. Relative strengths and weaknesses of LA size assessment by various imaging modalities.
| Echocardiography | Cardiac computed tomogra- phy (CT) | Cardiac magnetic resonance (CMR) | |
|---|---|---|---|
| Strengths | • Real-time imaging • Widely available and low in cost • Assessment of LA phasic volumes | • Accurate assessment of true LA volume • Short scan duration | • Accurate assessment of true LA volume • Assessment of LA phasic volumes |
| Weaknesses | • True LA volumes not obtained (unless 3D echo) • Image quality limited by acoustic window | • Radiation risk | • Limited availability in many centers • Longer scan duration |
LV Function
When AF ablation is first adopted, patients with normal LV systolic function are initially selected. However,there is increasing evidence that AF ablation benefits patients with impaired LV systolicfunction.[02,20-22] Currently, task force consensus guidelines suggest that selected symptomatic patients with heart failure and/or reduced ejection fraction could be considered for catheter AF abltion.[01] In the aforementioned studies,the aveage pre ablation ejection fraction rangedfrom 33% to 41%. Several important observationscould be made including the fact that catheter AFablation is feasible without an increase in proceduralcomplication and that the efficacy of theprocedure in patients with impaired systolicfunc-tion is lower than in those with normal ventricularfunction with a higher recurrence rate. Nevertheless,AF ablation results in significant symptomaticrelief, improvement in quality of life, as wellas some recovery of cardiac function. Future studiesare likely to further clarify the relative efficacyand clinical benefits of ablation in patients withsignificant LA dilation and LV systolic dysfunction.
TEE and Exclusion of LA/LAA Thrombus
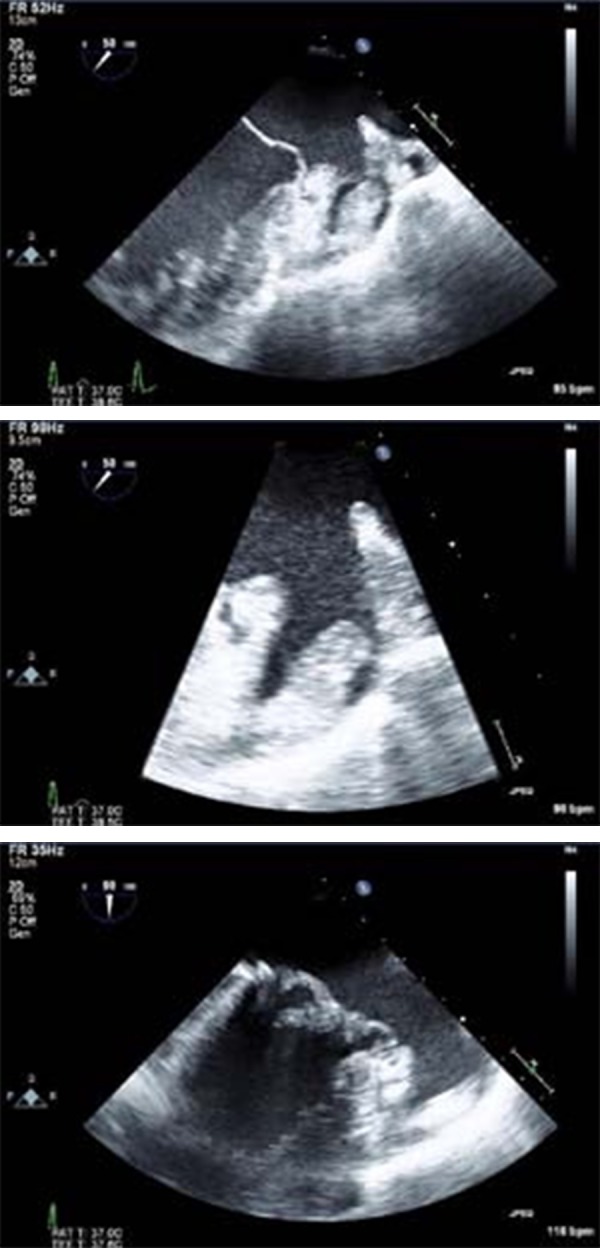
The pathophysiology of AF is complex and is iTransesophageal echocardiography (TEE) is a sensitiveand specific technique for detection of LAand LA appendage (LAA) thrombus[23] and is currently the gold standard investigation for excluding thrombus prior to elective cardioversion and AF ablation[24] [Fig.1]. The sensitivity and the specificity of TEE for detecting LA thrombi are 93-100% and 99-100% respectively.[25-26]
Figure 1. Use of transesophageal echocardiography in diagnosis of left atrial appendage thrombus. A 49-year-old patient with severe mitral regurgitation from mitral valve prolapse. LAA thrombus (green arrow) is found on TEE (A, B), with surrounding spontaneous echo contrast (red asterisk) in the LAA (C).
TEE features associated with thromboembolism include the finding of a LA or LAA thrombus, reduced LAA flow velocity, severe spontaneous echo contrast in the LA or LAA, and atheromatousdisease of the aorta.[27-28] The finding of severe spontaneous echo contrast, which is seen as echogenic swirling blood flow, reflects red cell and clottingfactor aggregation with slow moving bloodwithin the atrium. This, by itself, is not an absolutecontraindication to cardioversionorAFablation,[29] althoughitisassociatedwithLAthrombusfomation,ahigherrisk ofthromboembolism,and increased cardiovascular mortality.[30]
LA and LAA thrombus is an especially impor- tant issue for AF ablation because the procedure not only involves manipulation of multiple cathetersinside the LA with the potential of dislodging in situ thrombus, but also leads to substantial areas of denuded LA endothelium that may becomea nidus for thrombus formation in the days or weeks post-ablation. A recent study found a prevalence of LA thrombus and sludge of 0.6% and 1.5% respectively on routine of pre-ablation TEE. The prevalence of spontaneous echo contrast was as high as 35%. In this population, the predictors of LA thrombus were found to be high CHADS 2score, history of congestive heart failure,and left ventricular ejection fraction <35%.[31] While it remains contentious whether TEEshould be routinely performed in all patients because of the low incidence of thrombus,[32] the recent task force consensus guidelines stated that patients with persistent AF who are in AF at the time of ablation should have a TEE performed to screen for LA/LAA thrombus, regardless of the adequacy of pre-ablation anticoagulation.[01]
Since cardiac CT is commonly performed immediatelybefore AF ablation to use the 3D dataset in image integration with real-time electroanatomicdata during ablation, attempts have been made to use CT to screen for LA thrombus. Retrospective single centre trials have suggested that a negative CT has a high negative predictive value making it a potential alternative for excludingLAA thrombi before ablation.[33-35] This issue will need to be clarified in future studies.
TEE and Pulmonary Venous Anatomy
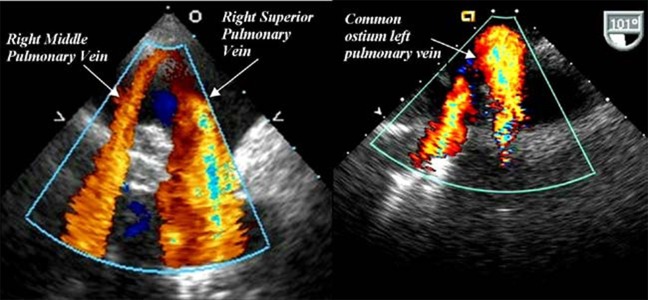
The accurate imaging of LA and pulmonary venous(PV) anatomy is important for understandingthe anatomic relationships between the PVs,LA and LAA. The most commonly seen pattern of PV anatomy is that of two separate right PVs and 2 separate left PVs. The right middle PV drains into the right superior PV before entering the LA.However, variations in PV anatomy are common.Supernumerary right PVs have an incidence of 1829%.[36-41]Common antrum of the left PVs results in a broad PV-LA junction and is found in6-35% of patients.[42-43] Moreover,morphological remodeling of the PVs and LA can also be observed in patients with AF.StudieshavefoundthatPVostia are larger in AF versus non-AF patientsand those with persistent versus paroxysmal AF.[36,40,44] The accurate uerstanding of these anatomicvariations is importantforlocalizationofthePV-LAinterfaceand the ridge between the PV and the LA appendage, so that variations in PV anato-my do not result in a higher recurrence risk.[45]
Cardiac CT and CMR are the gold standard investigationsfor accurate imaging of LA and PV anatomy. TEE is not the first-line investigation for this purpose mainly due to patient comfort, although TEEdoes excel in that it lacks radiationexposure and has a lower cost. Nevertheless, whenever TEE is performed pre-ablation for another reason, valuable information on PV anatomy and its variations could be gained, and all PVs should be interrogated in detail as baseline information. 46-48 While some studies report that TEE can only visualize two-thirds of superior and inferior veins with experienced operators,[49] the superior and inferior PVs can be identified in over 94% of cases.[47-48] The identification of PV anatomical variations, such as common left PV antrum and supernumerary right PVs, is slightly more challenging compared to cardiac CT.[47] In our experience, careful rotation of the probe with the veins in view should permit the visualization of most veins. Useful techniques include imaging the right PVs at 45-60° with a clockwise rotation of the transducer and imaging the left PVs at 110° with a counterclockwise transducer rotation.[50]
Figure 2. Examples of variant PV anatomy shown on TEE. Separate ostium for the rightmiddle and superior PV are noted in (a). Common ostium of the left superior and inferiorpulmonary veins is noted in (b). Reproduced with permission from Gabriel and Klein.[75].
During Ablation
Use of ICE During Ablation
During AF ablation, intraoperative TEE dramatically improves the visualization of anatomic landmarks over that of fluoroscopy. However,it is limited by patient discomfort and more importantly, the need for airway management during a prolonged procedure.[51-53] Advances in intracardiac echocardiography (ICE) performed by electrophysiologists have improved both the efficacy and safety of the procedure ([Table 3]).
Table 3. The role of Intracardiac Echocardiography during AF Ablation.
| Intracardiac Echocardiography |
|---|
| Identification of key anatomic locations: • Guidance of transseptal puncture • Diagnosis of variant PV anatomy Optimization of ablation catheter placement: • Enhanced catheter-tissue interface • Avoidance of tissue damage • Visualization of the relationship between catheter tip ad esophagus Diagnosis of intra-procedural complications: • Cardiac perforation and tamponade • Thrombus formation • Early signs of Pulmonary vein stenosis |
Available ICE systems include those with mechanicalsingle-element transducers and phased-arraymulti-element transducers. A mechanical transducercontains a rotational single-element and produces high quality images but only at shallow depths. To visualize LA structures, the transducer has to be inside the LA. Phased-array multi-elementtransducers image at frequencies from 5.5-10MHz,providing 2D images with a deeper penetrationand allowing an RA-located ICE probe to imagethe LA without an additional transseptal puncture.
During an ablation procedure, ICE accurately identifies key anatomic locations, such as the fossa ovalis, LAA, valve apparatus, pulmonary veins and extracardiac structures. It facilitates transseptal puncture, which is often challenging in clinical scenarios, such as large septal aneurysm, lipomatous atrial septal hypertrophy, double membrane septum, prior cardiac surgery distorting anatomy, and previous surgical or percutaneousclosure of atrial septal defect or patent foramen ovale. It determines the exact position of the transseptal sheath by the tenting of the interatrial septum and confirms access to the LA by the injectionof agitated saline. With ICE guidance, it is possible to aim for a transseptal puncture in the posterior region of the fossa ovalis. This is believed to be safer than the more anterior portions as the pulmonary veins are posterior structures.[54] ICE provides real-time images of PV anatomy and is far more sensitive to small movements of the circular mapping and the ablation catheters than fluoroscopy alone. Tissue contact is traditionally monitored by stability of the ablation catheter on fluoroscopy and stability of the electrical recording.The detection of microbubbles during ablation with ICE indicates tissue superheating and has been used to optimize ablation catheter placement.This strategy has been used to prevent tissue damage and scar formation, reduce the risk of tissue superheating, optimize radiofrequency energy delivery, and increase the number of lesions with optimal contact and energy delivery.[55] Recent development in open irrigation platforms has lessened the importance of ICE in this regard.[56-57] However, recent research has investigated using ICE to monitor the relationship between the catheter tip and adjacent structures, such as the esophagus. This strategy may reduce the incidence of esophageal injury.[58-59] ICE is able to detect intra-procedural complica-tions promptly, similar to intraoperative TEE; however, potential complications include cardiac perforation and tamponade, thrombus formation on the transseptal sheath and other catheters, as well as pulmonary vein stenosis (PVS), which maybe predicted by an increase in PV flow velocitywith Doppler measured during the procedure.[55]
Post-Ablation
Patients are followed clinically with varying use ofroutine imaging studies post-ablation amongst institutions.A summary of the role of TTE and TEE in thepost-ablation patients is outlined in ([Table 4]).PVS is routinely screened for in some practices by cardiacCT,CMR,and/orTEE.TTEisalsosometimes performed to document the degree of atrial remodeling and changes in LV function post-ablation.
Table 4. Role of Transthoracic and Transesophageal Echocardiography in the Post-Ablation Assessment of Patients with Atrial Fibrillation.
| Transthoracic Echocardiogram | Transesophageal Echocardiogram |
|---|---|
| Atrial mechanics: • Prediction of AF recurrence • Assessment of post-ablation thromboembolic risk | Pulmonary vein stenosis: • Anatomical diagnosis • Functional diagnosis – detection of turbulence, increased flow velocities |
TEE and the Diagnosis of PVS
Thermal injury to PV musculature results in PV stenosis. The incidence of post-ablation severe PV stenosis has been reported to be 3.4%.60 Symptoms of PV stenosis include shortness of breath, cough, hemoptysis, chest pain, and recurrent lung infections.[60-61] With the evolution of techniques, the incidence of PV stenosis has declined due to the avoidance of delivering radiofrequency energy within the PV, together with the increasing use of ICE and complementary image integration systems with pre-ablation cardiac CT and real-time electroanatomical data.
While some institutions routinely screen for PVS post-ablation, others perform imaging tests when symptoms dictate them. It remains unclear whether early diagnosis and treatment of asymptomatic PVS provide long-term advantage, although asymp-tomatic PVS has the potential to cause progressivehypoplasia of the entire pulmonary vein proximal to the stenosis.[62] Such pulmonary vascular occlusivedamage may not be fully reversible and may lead to difficulties with subsequent percutaneous treatment should symptoms develop in the future.
The diagnosis of PVS is most commonly made by tomographic modalities such as cardiac CT or CMR, because they quantify the degree of PVS withexcellent reproducibility and demonstrate the relationship of the stenosis to the rest of the PV anatomyso percutaneous treatment can be planned.TEE, on the other hand, plays a supplementaryrole in the diagnosis of PVS and offers both anatomicaland functional information.PVscanbevisualizedin the great majority of patients studied,and PV ostial diameters at the venoatrial junctioncan be determined and compared with reference vessel diameters to quantify the degree of narrowing.[47] PV stenosis severity is defined according to the percentage reduction in luminal diameter, with a >70% luminal diameter reduction commonlyconsideredseverePVS.[1] Anabsolutediameter of<7mm mayalsobe sufficient to diagnosesignificant PVS on TEE. When comparing TEE and cardiac CT,two important aspects arenotable.Firstly,PVsare elliptical in shape with a larger diameterin the cranio-caudal axis than the transaxial axis;this is not as readily recognized by TEE. Secondly,TEE has the tendency to systematically underestimateostial diameters compared to CT. These aspects should be taken into account when serialstudies across different modalities are compared.
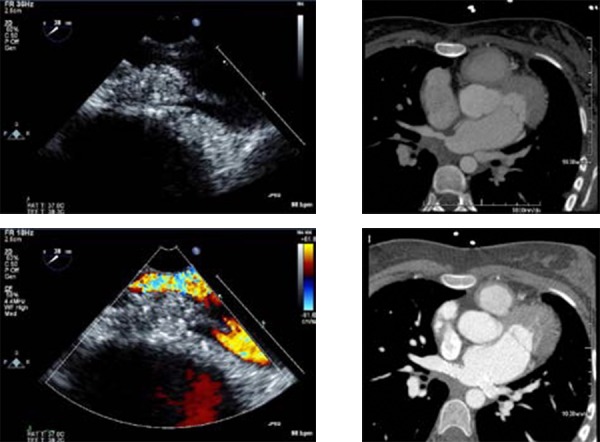
The unique feature of TEE in the diagnosis of PVS is that it provides functional assessment of the PVs. The use of color and pulsed Doppler assessment of PV flow confirm the presence of hemodynamicallysignificant stenosis by detecting turbulence and aliasing of the color Doppler signal as well as an increase in pulsed wave Doppler diastolic flow velocities ([Fig.3]). The optimal cutoff velocity for defining stenosis is currently unknown, althoughstudies have shown that a peak diastolic velocity of>100cm/s has a 86% sensitivity and 95% specificity for diagnosing PVS compared to the gold standard investigation of cardiac CT.[63] It is important toremember that such comparison may not be valid,as functional information from TEE is not equivlent to anatomical information from cardiac CT,andthetwo modalities may supply incremental valuein selected cases. For instance, functional informationmay be important in assessing patients withequivocal symptoms and a moderate degree of stenosis.Information on the functional significance of stenosis may also be helpful over that of sizealone in determining the necessity of intervention.
Figure 3. PV stenosis on TEE. In this 22-year-old patient who underwent pulmonary vein isolation for AF, moderate ostial thickening is identified at the ostium of the right superior pulmonary vein (green arrow). (A) Turbulence is identified on color Doppler imaging suggesting functional significance. (B) Cardiac CT confirms the presence of severe PV stenosis of the right superior pulmonary vein (C), which is subsequently stented (D). In addition, the common antrum of the left pulmonary veins was also stenosed and stented (red arrow).
Atrial Mechanics – Prediction of Recurrence
AF results in electrical and structural remodeling of the atrium [64-66] that can be considered part of a rate-related atrial cardiomyopathy. The termination of arrhythmia may, as a result, lead to a degree of reverse remodeling of the atrial cardiomyopathy. The documentation of atrial reverse remodeling post-ablation may not routinely be performed in clinical practice, but studies have recently suggested a potential role in predicting recurrence postablation and stratifying thromboembolism risk.
Understanding atrial mechanics extends our current interest from simply measuring the maximum LA volume at end-ventricular systole to measuring LA phasic functions ([Table 5]). Analyzing events at various phases of the cardiac cycle can supply information on the dynamic LA reservoir (atrial filling), conduit (passive atrial emptying) and contractile (active atrial contraction) functions Although studies are sparse at the moment,it is likely that AF ablation has varying effects on the different components of LA phasic function.
Table 5. Potentially Useful Measures of LA Mechanics.
| LA phasic volumes | LA maximum volume (end-ventricular systole) LA pre-atrial contraction volume (start of atrial systole) LA minimum volume (end-atrial systole) | ||
|---|---|---|---|
| LA ejection fraction | Total LA emptying fraction (LA reservoir function) Passive LA emptying fraction (LA conduit function) Active LA emptying fraction (LA contractile function) | ||
| Doppler echo | Mitral inflow E velocity Mitral inflow A velocity E’ mitral annulus tissue Doppler velocity A’ mitral annulus tissue Doppler velocity | ||
| Strain (ε) | εtotal (LA reservoir function) εpositive (LA conduit function) εnegative (LA contractile function) | ||
| Strain rate (SR) | SRpositive(LA reservoir function) SRearly negative(LA conduit function) SRlate negative(LA contractile function) |
Marsan et al. studied 57 patients with AF,[43] ofwhom had paroxysmal AF.[12] Atrial volumeswere studied at various phases of the cardiac cycle to assess LA phasic functions. In the patients who maintained sinus rhythm at 3 months, there was asignificant reduction in overall LA volume, with improvement in LA active contractile and resevoir functions. LA conduit function, or passiveemptying, was relatively unchanged, highlighting that LA phasic function analysis can study the effect of AF ablation on the LA in detail. Such changes were not observed in studies performed immediately after AF ablation, but rather took several weeks to occur. In the group that reverted back to AF, the changes of atrial reverse remodeling were not observed, illustrating that changes in atrial mechanics post-ablation could be used to predict future AF recurrence. Other studies using 3D echocardiography[12-13] or CMR[67-68] have also demonstrated post-ablation atrial reverse remodeling. The magnitude of change of the various parameters is in the range of 10-20%. A lack of demonstrated atrial reverse remodeling has been associated with post-ablation recurrence.[12-13,68]
In addition to measuring phasic volumes, LA mechanics could also be studied with Doppler echocardiography. Traditionally, the measurement of pulsed Doppler derived mitral A wave velocity and a’ from mitral annular tissue Doppler velocity gives some insight into LA contractile function.Studies have found that a’ decreases immediately after AF ablation but subsequently improves, suggesting that LA contractile function deterioratesimmediately post-ablation but recovers later.[69]
Recent research applies strain and strain rate imaging to study LA mechanics using either color tissue Doppler imaging techniques or 2-dimensional speckle tracking techniques.[70-72] Using these techniques, changes in LA phasic functions pre- and post-ablation can be accurately quantified. Schneider et al.[72] studied 118 patients with paroxysmal and persistent AF before and 3 months after AF ablation. Color tissue Doppler imagingmeasured the LA strain and strain rates at the reservoir,conduit and contractile phases of the atrial cardiac cycle, and was feasible in 97%. Changes in atrial myocardial properties post-ablation differed significantly between patients with paroxysmal and persistent AF. Recurrence is predicted by a lower post-ablation strain and strain rate during the LA reservoir phase, as well as a lower strain rate during the LA contractile phase. Such difference in atrial mechanics is not detected by conventional parameters of Doppler echocardiography, suggesting that strain and strain rate analysis appears more sensitive in investigating changes in LA mechanics after AF ablation. Studies have used 2-dimensional speckle tracking techniques to measure LA mechanics,[70-71] although they have yet not been applied to the AF ablation population.
Atrial Mechanics – Thromboembolic Risk
The study of atrial mechanics may also be important for the prediction of thromboembolic risk. Currently, studies are sparse and the effect of changes in atrial mechanics post-ablation onthromboembolic risk is uncertain. Many patients may opt for AF ablation as an alternative to longterm anticoagulation with warfarin therapy.[73] However, this strategy cannot be recommended at this stage because the impact of AF ablation on thromboembolic risk remains unknown. While some studies demonstrate an improvement in LA function using 3-dimensional echocardiographic measurements,[12-13] other studies have shown that post-ablation LA reservoir and contractile functions remain significantly impaired, especially when compared to patients undergoing cardioversion and control subjects.[74] Further studies are required both to understand the effect of AF ablation on LA mechanics and how the changes in LA mechanics impact on thromboembolic risk. Such changes are likely to differ between patients with paroxysmal vs. persistent AF, as well as with the number of prior AF ablations.
Current guideline recommendations for anticoagulation rely on pre-ablation risk factors. Postablation LA function changes have not been incorporated into the decision making process due to the lack of evidence. The guideline recommends that discontinuation of anticoagulation with warfarin therapy be avoided in patients with congestive heart failure, history of high blood pressure, age ≥75years, diabetes, prior stroke or transient CHADS2 score ≥2. In those with a CHADS2 score of 1 post AF-ablation, either aspirin or warfarin is thought to be appropriate.[1]
Conclusions
Echocardiography plays a central role in decision making for patients undergoing AF ablation—preablation,during ablation and post-ablation. The role of echocardiography pre-ablation is now well established, in patient selection, screening of patients for LA/LAA thrombus prior to ablation, and the use of ICE in the guidance of catheter ablation.Emerging echocardiographic roles include the identificationof variant pulmonary vein anatomy, diagnosisof PVS, as well as the use of data from atrialmechanics studies in documenting atrial reverse remodeling and in prognosticating for AF recurrence and future thromboembolic events. The role of echocardiography will continue to evolve with the increasing use of AF ablation in AF management.
Acknowledgments
Dr. Andrew To acknowledges the support from the Overseas Fellowship Award from the National Heart Foundation of New Zealand. Both authors acknowledge secretarial support from Marie Campbell.
Abbreviations
2DE: 2-dimensional Echocardiography
3DE: 3-dimensional Echocardiography
AF: Atrial Fibrillation
CMR: Cardiac Magnetic Resonance
CT: Computed Tomography
ICE: Intracardiac Echocardiography
LA: Left Atrium / Left Atrial
LAA: Left Atrial Appendage
LV: Left Ventricle / Left Ventricular
PV: Pulmonary Vein(s) / Pulmonary Venous
PVS: Pulmonary Vein Stenosis
TEE: Transesophageal Echocardiography
TTE: Transthoracic Echocardiography
References
- 1.Calkins Hugh, Brugada Josep, Packer Douglas L, Cappato Riccardo, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, Haines David E, Haissaguerre Michel, Iesaka Yoshito, Jackman Warren, Jais Pierre, Kottkamp Hans, Kuck Karl Heinz, Lindsay Bruce D, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Natale Andrea, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Ruskin Jeremy N, Shemin Richard J. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007 Jun;9 (6):335–79. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 2.Hsu Li-Fern, Jaïs Pierre, Sanders Prashanthan, Garrigue Stéphane, Hocini Mélèze, Sacher Fréderic, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Scavée Christophe, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004 Dec 02;351 (23):2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 3.Wann L Samuel, Curtis Anne B, January Craig T, Ellenbogen Kenneth A, Lowe James E, Estes N A Mark, Page Richard L, Ezekowitz Michael D, Slotwiner David J, Jackman Warren M, Stevenson William G, Tracy Cynthia M, Fuster Valentin, Rydén Lars E, Cannom David S, Le Heuzey Jean-Yves, Crijns Harry J, Lowe James E, Curtis Anne B, Olsson S Bertil, Ellenbogen Kenneth A, Prystowsky Eric N, Halperin Jonathan L, Tamargo Juan Luis, Kay G Neal, Wann L Samuel, Jacobs Alice K, Anderson Jeffrey L, Albert Nancy, Hochman Judith S, Buller Christopher E, Kushner Frederick G, Creager Mark A, Ohman Erik Magnus, Ettinger Steven M, Stevenson William G, Guyton Robert A, Tarkington Lynn G, Halperin Jonathan L, Yancy Clyde W. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011 Jan 11;57 (2):223–42. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch. Intern. Med. 1998 Jun 22;158 (12):1316–20. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- 5.Themistoclakis Sakis, Schweikert Robert A, Saliba Walid I, Bonso Aldo, Rossillo Antonio, Bader Giovanni, Wazni Oussama, Burkhardt David J, Raviele Antonio, Natale Andrea. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008 May;5 (5):679–85. doi: 10.1016/j.hrthm.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffery L, Antman Elliott M, Hunt Sharon Ann, Nishimura Rick, Ornato Joseph P, Page Richard L, Riegel Barbara, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm A John, Dean Veronica, Deckers Jaap W, Despres Catherine, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais Joao, Osterspey Ady, Zamorano José Luis. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J. Am. Coll. Cardiol. 2006 Aug 15;48 (4):854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Abhayaratna Walter P, Seward James B, Appleton Christopher P, Douglas Pamela S, Oh Jae K, Tajik A Jamil, Tsang Teresa S M. Left atrial size: physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 2006 Jun 20;47 (12):2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Lang Roberto M, Bierig Michelle, Devereux Richard B, Flachskampf Frank A, Foster Elyse, Pellikka Patricia A, Picard Michael H, Roman Mary J, Seward James, Shanewise Jack S, Solomon Scott D, Spencer Kirk T, Sutton Martin St John, Stewart William J. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18 (12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins Carly, Bricknell Kristen, Marwick Thomas H. Use of real-time three-dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. J Am Soc Echocardiogr. 2005 Sep;18 (9):991–7. doi: 10.1016/j.echo.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Keller A M, Gopal A S, King D L. Left and right atrial volume by freehand three-dimensional echocardiography: in vivo validation using magnetic resonance imaging. Eur J Echocardiogr. 2000 Mar;1 (1):55–65. doi: 10.1053/euje.2000.0010. [DOI] [PubMed] [Google Scholar]
- 11.Artang Ramin, Migrino Raymond Q, Harmann Leanne, Bowers Mark, Woods Timothy D. Left atrial volume measurement with automated border detection by 3-dimensional echocardiography: comparison with Magnetic Resonance Imaging. Cardiovasc Ultrasound. 2009 Mar 31;7 () doi: 10.1186/1476-7120-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsan Nina Ajmone, Tops Laurens F, Holman Eduard R, Van de Veire Nico R, Zeppenfeld Katja, Boersma Eric, van der Wall Ernst E, Schalij Martin J, Bax Jeroen J. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am. J. Cardiol. 2008 Oct 01;102 (7):847–53. doi: 10.1016/j.amjcard.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Delgado Victoria, Vidal Bàrbara, Sitges Marta, Tamborero David, Mont Lluís, Berruezo Antonio, Azqueta Manuel, Paré Carles, Brugada Josep. Fate of left atrial function as determined by real-time three-dimensional echocardiography study after radiofrequency catheter ablation for the treatment of atrial fibrillation. Am. J. Cardiol. 2008 May 01;101 (9):1285–90. doi: 10.1016/j.amjcard.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Parikh Sachin S, Jons Christian, McNitt Scott, Daubert James P, Schwarz Karl Q, Hall Burr. Predictive capability of left atrial size measured by CT, TEE, and TTE for recurrence of atrial fibrillation following radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2010 May;33 (5):532–40. doi: 10.1111/j.1540-8159.2010.02693.x. [DOI] [PubMed] [Google Scholar]
- 15.Perea Rosario J, Tamborero David, Mont Lluis, De Caralt Teresa M, Ortiz Jose T, Berruezo Antonio, Matiello Maria, Sitges Marta, Vidal Barbara, Sanchez Marcelo, Brugada Josep. Left atrial contractility is preserved after successful circumferential pulmonary vein ablation in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Apr;19 (4):374–9. doi: 10.1111/j.1540-8167.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- 16.Montefusco Antonio, Biasco Luigi, Blandino Alessandro, Cristoforetti Yvonne, Scaglione Marco, Caponi Domenico, Di Donna Paolo, Boffano Carlo, Cesarani Federico, Coin Daniele, Perversi Jacopo, Gaita Fiorenzo. Left atrial volume at MRI is the main determinant of outcome after pulmonary vein isolation plus linear lesion ablation for paroxysmal-persistent atrial fibrillation. J Cardiovasc Med (Hagerstown) 2010 Aug;11 (8):593–8. doi: 10.2459/JCM.0b013e32833831e4. [DOI] [PubMed] [Google Scholar]
- 17.Maddukuri Prasad V, Vieira Marcelo L C, DeCastro Stefano, Maron Martin S, Kuvin Jeffrey T, Patel Ayan R, Pandian Natesa G. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr. 2006 Aug;19 (8):1026–32. doi: 10.1016/j.echo.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Maddukuri Prasad V, Vieira Marcelo L C, DeCastro Stefano, Maron Martin S, Kuvin Jeffrey T, Patel Ayan R, Pandian Natesa G. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr. 2006 Aug;19 (8):1026–32. doi: 10.1016/j.echo.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith H J, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging. 1999 Oct;15 (5):397–410. doi: 10.1023/a:1006276513186. [DOI] [PubMed] [Google Scholar]
- 20.Chen Michael S, Marrouche Nassir F, Khaykin Yaariv, Gillinov A Marc, Wazni Oussama, Martin David O, Rossillo Antonio, Verma Atul, Cummings Jennifer, Erciyes Demet, Saad Eduardo, Bhargava Mandeep, Bash Dianna, Schweikert Robert, Burkhardt David, Williams-Andrews Michelle, Perez-Lugones Alejandro, Abdul-Karim Ahmad, Saliba Walid, Natale Andrea. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004 Mar 17;43 (6):1004–9. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Tondo Claudio, Mantica Massimo, Russo Giovanni, Avella Andrea, De Luca Lucia, Pappalardo Augusto, Fagundes Rafael Lopes, Picchio Edo, Laurenzi Francesco, Piazza Vito, Bisceglia Irma. Pulmonary vein vestibule ablation for the control of atrial fibrillation in patients with impaired left ventricular function. Pacing Clin Electrophysiol. 2006 Sep;29 (9):962–70. doi: 10.1111/j.1540-8159.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- 22.Lutomsky Boris A, Rostock Thomas, Koops Andreas, Steven Daniel, Müllerleile Kai, Servatius Helge, Drewitz Imke, Ueberschär Denis, Plagemann Thorsten, Ventura Rodolfo, Meinertz Thomas, Willems Stephan. Catheter ablation of paroxysmal atrial fibrillation improves cardiac function: a prospective study on the impact of atrial fibrillation ablation on left ventricular function assessed by magnetic resonance imaging. Europace. 2008 May;10 (5):593–9. doi: 10.1093/europace/eun076. [DOI] [PubMed] [Google Scholar]
- 23.Aschenberg W, Schlüter M, Kremer P, Schröder E, Siglow V, Bleifeld W. Transesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J. Am. Coll. Cardiol. 1986 Jan;7 (1):163–6. doi: 10.1016/s0735-1097(86)80275-3. [DOI] [PubMed] [Google Scholar]
- 24.Pearson A C, Labovitz A J, Tatineni S, Gomez C R. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J. Am. Coll. Cardiol. 1991 Jan;17 (1):66–72. doi: 10.1016/0735-1097(91)90705-e. [DOI] [PubMed] [Google Scholar]
- 25.Maddukuri Prasad V, Vieira Marcelo L C, DeCastro Stefano, Maron Martin S, Kuvin Jeffrey T, Patel Ayan R, Pandian Natesa G. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr. 2006 Aug;19 (8):1026–32. doi: 10.1016/j.echo.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Manning W J, Weintraub R M, Waksmonski C A, Haering J M, Rooney P S, Maslow A D, Johnson R G, Douglas P S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995 Dec 01;123 (11):817–22. doi: 10.7326/0003-4819-123-11-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Zabalgoitia M, Halperin J L, Pearce L A, Blackshear J L, Asinger R W, Hart R G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J. Am. Coll. Cardiol. 1998 Jun;31 (7):1622–6. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 28.Fatkin D, Kuchar D L, Thorburn C W, Feneley M P. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for "atrial stunning" as a mechanism of thromboembolic complications. J. Am. Coll. Cardiol. 1994 Feb;23 (2):307–16. doi: 10.1016/0735-1097(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 29.Black I W, Hopkins A P, Lee L C, Walsh W F. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J. Am. Coll. Cardiol. 1991 Aug;18 (2):398–404. doi: 10.1016/0735-1097(91)90592-w. [DOI] [PubMed] [Google Scholar]
- 30.Bernhardt Peter, Schmidt Harald, Hammerstingl Christoph, Lüderitz Berndt, Omran Heyder. Patients with atrial fibrillation and dense spontaneous echo contrast at high risk a prospective and serial follow-up over 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J. Am. Coll. Cardiol. 2005 Jun 07;45 (11):1807–12. doi: 10.1016/j.jacc.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 31.Zabalgoitia M, Halperin J L, Pearce L A, Blackshear J L, Asinger R W, Hart R G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J. Am. Coll. Cardiol. 1998 Jun;31 (7):1622–6. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 32.Calvo N, Mont L, Vidal B, Nadal M, Montserrat S, Andreu D, Tamborero D, Pare C, Azqueta M, Berruezo A, Brugada J, Sitges M. Usefulness of transoesophageal echocardiography before circumferential pulmonary vein ablation in patients with atrial fibrillation: is it really mandatory? Europace. 2011 Apr;13 (4):486–91. doi: 10.1093/europace/euq456. [DOI] [PubMed] [Google Scholar]
- 33.Jaber Wael A, White Richard D, Kuzmiak Stacie A, Boyle Janet M, Natale Andrea, Apperson-Hansen Carolyn, Thomas James D, Asher Craig R. Comparison of ability to identify left atrial thrombus by three-dimensional tomography versus transesophageal echocardiography in patients with atrial fibrillation. Am. J. Cardiol. 2004 Feb 15;93 (4):486–9. doi: 10.1016/j.amjcard.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Achenbach S, Sacher D, Ropers D, Pohle K, Nixdorff U, Hoffmann U, Muschiol G, Flachskampf F A, Daniel W G. Electron beam computed tomography for the detection of left atrial thrombi in patients with atrial fibrillation. Heart. 2004 Dec;90 (12):1477–8. doi: 10.1136/hrt.2003.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Yuli Y, Klein Allan L, Halliburton Sandra S, Popovic Zoran B, Kuzmiak Stacie A, Sola Srikanth, Garcia Mario J, Schoenhagen Paul, Natale Andrea, Desai Milind Y. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am. Heart J. 2007 Dec;154 (6):1199–205. doi: 10.1016/j.ahj.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Kato Ritsushi, Lickfett Lars, Meininger Glenn, Dickfeld Timm, Wu Richard, Juang George, Angkeow Piamsook, LaCorte Jennifer, Bluemke David, Berger Ronald, Halperin Henry R, Calkins Hugh. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003 Apr 22;107 (15):2004–10. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- 37.Lickfett Lars, Kato Ritsushi, Tandri Harikrishna, Jayam Vinod, Vasamreddy Chandrasekhar R, Dickfeld Timm, Lewalter Thorsten, Luderitz Berndt, Berger Ronald, Halperin Henry, Calkins Hugh. Characterization of a new pulmonary vein variant using magnetic resonance angiography: incidence, imaging, and interventional implications of the "right top pulmonary vein". J. Cardiovasc. Electrophysiol. 2004 May;15 (5):538–43. doi: 10.1046/j.1540-8167.2004.03499.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin W S, Prakash V S, Tai C T, Hsieh M H, Tsai C F, Yu W C, Lin Y K, Ding Y A, Chang M S, Chen S A. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000 Mar 21;101 (11):1274–81. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 39.Mansour Moussa, Holmvang Godtfred, Sosnovik David, Migrino Raymond, Abbara Suhny, Ruskin Jeremy, Keane David. Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004 Apr;15 (4):387–93. doi: 10.1046/j.1540-8167.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwartzman David, Lacomis Joan, Wigginton William G. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J. Am. Coll. Cardiol. 2003 Apr 16;41 (8):1349–57. doi: 10.1016/s0735-1097(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 41.Tsao H M, Wu M H, Yu W C, Tai C T, Lin Y K, Hsieh M H, Ding Y A, Chang M S, Chen S A. Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2001 Dec;12 (12):1353–7. doi: 10.1046/j.1540-8167.2001.01353.x. [DOI] [PubMed] [Google Scholar]
- 42.Mlcochová Hanka, Tintera Jaroslav, Porod Václav, Peichl Petr, Cihák Robert, Kautzner Josef. Magnetic resonance angiography of pulmonary veins: implications for catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2005 Oct;28 (10):1073–80. doi: 10.1111/j.1540-8159.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 43.Jongbloed Monique R M, Bax Jeroen J, Lamb Hildo J, Dirksen Martijn S, Zeppenfeld K, van der Wall Ernst E, de Roos Albert, Schalij Martin J. Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head-to-head comparison. J. Am. Coll. Cardiol. 2005 Feb 01;45 (3):343–50. doi: 10.1016/j.jacc.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 44.Knackstedt Christian, Visser Laurent, Plisiene Jurgita, Zarse Markus, Waldmann Matthias, Mischke Karl, Koch Karl-Christian, Hoffmann Rainer, Franke Andreas, Hanrath Peter, Schauerte Patrick. Dilatation of the pulmonary veins in atrial fibrillation: a transesophageal echocardiographic evaluation. Pacing Clin Electrophysiol. 2003 Jun;26 (6):1371–8. doi: 10.1046/j.1460-9592.2003.t01-1-00196.x. [DOI] [PubMed] [Google Scholar]
- 45.Hof Irene, Chilukuri Karuna, Arbab-Zadeh Armin, Scherr Daniel, Dalal Darshan, Nazarian Saman, Henrikson Charles, Spragg David, Berger Ronald, Marine Joseph, Calkins Hugh. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J. Cardiovasc. Electrophysiol. 2009 Sep;20 (9):1005–10. doi: 10.1111/j.1540-8167.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 46.Jander Nikolaus, Minners Jan, Arentz Thomas, Görnandt Lothar, Fürmaier Rudolf, Kalusche Dietrich, Neumann Franz Josef. Transesophageal echocardiography in comparison with magnetic resonance imaging in the diagnosis of pulmonary vein stenosis after radiofrequency ablation therapy. J Am Soc Echocardiogr. 2005 Jun;18 (6):654–9. doi: 10.1016/j.echo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 47.To Andrew C Y, Gabriel Ruvin S, Park Margaret, Lowe Boris S, Curtin Ronan J, Sigurdsson Gardar, Sherman Minerva, Wazni Oussama M, Saliba Walid I, Bhargava Mandeep, Lindsay Bruce D, Klein Allan L. Role of Transesophageal Echocardiography Compared to Computed Tomography in Evaluation of Pulmonary Vein Ablation for Atrial Fibrillation (ROTEA study). J Am Soc Echocardiogr. 2011 Sep;24 (9):1046–55. doi: 10.1016/j.echo.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Toffanin Gianluca, Scarabeo Virginia, Verlato Roberto, De Conti Fabio, Zampiero Aldo Antonio, Piovesana Piergiuseppe. Transoesophageal echocardiographic evaluation of pulmonary vein anatomy in patients undergoing ostial radiofrequency catheter ablation for atrial fibrillation: a comparison with magnetic resonance angiography. J Cardiovasc Med (Hagerstown) 2006 Oct;7 (10):748–52. doi: 10.2459/01.JCM.0000247322.57536.04. [DOI] [PubMed] [Google Scholar]
- 49.Wood Mark A, Wittkamp Michael, Henry Daniel, Martin Robert, Nixon J V, Shepard Richard K, Ellenbogen Kenneth A. A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am. J. Cardiol. 2004 Jan 01;93 (1):49–53. doi: 10.1016/j.amjcard.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Tabata Tomotsugu, Thomas James D, Klein Allan L. Pulmonary venous flow by doppler echocardiography: revisited 12 years later. J. Am. Coll. Cardiol. 2003 Apr 16;41 (8):1243–50. doi: 10.1016/s0735-1097(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 51.Marrouche Nassir F, Martin David O, Wazni Oussama, Gillinov A Marc, Klein Allan, Bhargava Mandeep, Saad Eduardo, Bash Dianna, Yamada Hirotsugu, Jaber Wael, Schweikert Robert, Tchou Patrick, Abdul-Karim Ahmad, Saliba Walid, Natale Andrea. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation. 2003 Jun 03;107 (21):2710–6. doi: 10.1161/01.CIR.0000070541.83326.15. [DOI] [PubMed] [Google Scholar]
- 52.Ren Jian-Fang, Marchlinski Francis E, Callans David J. Real-time intracardiac echocardiographic imaging of the posterior left atrial wall contiguous to anterior wall of the esophagus. J. Am. Coll. Cardiol. 2006 Aug 01;48 (3):594; author reply 594–5. doi: 10.1016/j.jacc.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Wazni Oussama M, Rossillo Antonio, Marrouche Nassir F, Saad Eduardo B, Martin David O, Bhargava Mandeep, Bash Dianna, Beheiry Salwa, Wexman Mark, Potenza Domenico, Pisano Ennio, Fanelli Raffaele, Bonso Aldo, Themistoclakis Sakis, Erciyes Demet, Saliba Walid I, Schweikert Robert A, Brachmann Johannes, Raviele Antonio, Natale Andrea. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J. Cardiovasc. Electrophysiol. 2005 Jun;16 (6):576–81. doi: 10.1111/j.1540-8167.2005.40480.x. [DOI] [PubMed] [Google Scholar]
- 54.Saliba Walid, Thomas James. Intracardiac echocardiography during catheter ablation of atrial fibrillation. Europace. 2008 Nov;10 Suppl 3 ():iii42–7. doi: 10.1093/europace/eun233. [DOI] [PubMed] [Google Scholar]
- 55.Kalman J M, Fitzpatrick A P, Olgin J E, Chin M C, Lee R J, Scheinman M M, Lesh M D. Biophysical characteristics of radiofrequency lesion formation in vivo: dynamics of catheter tip-tissue contact evaluated by intracardiac echocardiography. Am. Heart J. 1997 Jan;133 (1):8–18. doi: 10.1016/s0002-8703(97)70242-4. [DOI] [PubMed] [Google Scholar]
- 56.Marrouche Nassir F, Guenther Jens, Segerson Nathan M, Daccarett Marcos, Rittger Harald, Marschang Harald, Schibgilla Volker, Schmidt Martin, Ritscher Guido, Noelker Georg, Brachmann Johannes. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrum isolation: procedural parameters, outcomes, and the effect on esophageal injury. J. Cardiovasc. Electrophysiol. 2007 Jun;18 (6):583–8. doi: 10.1111/j.1540-8167.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 57.Kanj Mohamed H, Wazni Oussama, Fahmy Tamer, Thal Sergio, Patel Dimpi, Elay Claude, Di Biase Luigi, Arruda Mauricio, Saliba Walid, Schweikert Robert A, Cummings Jennifer E, Burkhardt J David, Martin David O, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Forleo Giovanni, Natale Andrea. Pulmonary vein antral isolation using an open irrigation ablation catheter for the treatment of atrial fibrillation: a randomized pilot study. J. Am. Coll. Cardiol. 2007 Apr 17;49 (15):1634–41. doi: 10.1016/j.jacc.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 58.Helms Adam, West J Jason, Patel Amit, Mounsey J Paul, DiMarco John P, Mangrum J Michael, Ferguson John D. Real-time rotational ICE imaging of the relationship of the ablation catheter tip and the esophagus during atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2009 Feb;20 (2):130–7. doi: 10.1111/j.1540-8167.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 59.Kenigsberg David N, Lee Benjamin P, Grizzard John D, Ellenbogen Kenneth A, Wood Mark A. Accuracy of intracardiac echocardiography for assessing the esophageal course along the posterior left atrium: a comparison to magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2007 Feb;18 (2):169–73. doi: 10.1111/j.1540-8167.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 60.Saad Eduardo B, Rossillo Antonio, Saad Cynthia P, Martin David O, Bhargava Mandeep, Erciyes Demet, Bash Dianna, Williams-Andrews Michelle, Beheiry Salwa, Marrouche Nassir F, Adams James, Pisanò Ennio, Fanelli Raffaele, Potenza Domenico, Raviele Antonio, Bonso Aldo, Themistoclakis Sakis, Brachmann Joannes, Saliba Walid I, Schweikert Robert A, Natale Andrea. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation. 2003 Dec 23;108 (25):3102–7. doi: 10.1161/01.CIR.0000104569.96907.7F. [DOI] [PubMed] [Google Scholar]
- 61.Dong Jun, Vasamreddy Chandrasekhar R, Jayam Vinod, Dalal Darshan, Dickfeld Timm, Eldadah Zayd, Meininger Glenn, Halperin Henry R, Berger Ronald, Bluemke David A, Calkins Hugh. Incidence and predictors of pulmonary vein stenosis following catheter ablation of atrial fibrillation using the anatomic pulmonary vein ablation approach: results from paired magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2005 Aug;16 (8):845–52. doi: 10.1111/j.1540-8167.2005.40680.x. [DOI] [PubMed] [Google Scholar]
- 62.Yang Hui-Min, Lai Chi K, Patel Jignesh, Moore John, Chen Peng-Sheng, Shivkumar Kalyanam, Fishbein Michael C. Irreversible intrapulmonary vascular changes after pulmonary vein stenosis complicating catheter ablation for atrial fibrillation. Cardiovasc. Pathol. 2007 Jan 16;16 (1):51–5. doi: 10.1016/j.carpath.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdsson Gardar, Troughton Richard W, Xu Xiao-Fang, Salazar Holger P, Wazni Oussama M, Grimm Richard A, White Richard D, Natale Andrea, Klein Allan L. Detection of pulmonary vein stenosis by transesophageal echocardiography: comparison with multidetector computed tomography. Am. Heart J. 2007 May;153 (5):800–6. doi: 10.1016/j.ahj.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 64.Morillo C A, Klein G J, Jones D L, Guiraudon C M. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995 Mar 01;91 (5):1588–95. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 65.Wijffels M C, Kirchhof C J, Dorland R, Power J, Allessie M A. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997 Nov 18;96 (10):3710–20. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 66.Wijffels M C, Kirchhof C J, Dorland R, Allessie M A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 01;92 (7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 67.Jayam Vinod K, Dong Jun, Vasamreddy Chandrasekhar R, Lickfett Lars, Kato Ritsushi, Dickfeld Timm, Eldadah Zayd, Dalal Darshan, Blumke David A, Berger Ronald, Halperin Henry R, Calkins Hugh. Atrial volume reduction following catheter ablation of atrial fibrillation and relation to reduction in pulmonary vein size: an evaluation using magnetic resonance angiography. J Interv Card Electrophysiol. 2005 Jul;13 (2):107–14. doi: 10.1007/s10840-005-0215-3. [DOI] [PubMed] [Google Scholar]
- 68.Tsao Hsuan-Ming, Wu Mei-Han, Huang Bien-Hsien, Lee Shih-Huang, Lee Kun-Tai, Tai Ching-Tai, Lin Yung-Kuo, Hsieh Ming-Hsiung, Kuo Jen-Yuan, Lei Meng-Huan, Chen Shih-Ann. Morphologic remodeling of pulmonary veins and left atrium after catheter ablation of atrial fibrillation: insight from long-term follow-up of three-dimensional magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2005 Jan;16 (1):7–12. doi: 10.1046/j.1540-8167.2005.04407.x. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues Ana Clara Tude, Scannavacca Mauricio I, Caldas Márcia Azevedo, Hotta Viviane Tiemi, Pisani Cristiano, Sosa Eduardo A, Mathias Wilson. Left atrial function after ablation for paroxysmal atrial fibrillation. Am. J. Cardiol. 2009 Feb 01;103 (3):395–8. doi: 10.1016/j.amjcard.2008.09.094. [DOI] [PubMed] [Google Scholar]
- 70.Saraiva Roberto M, Demirkol Sayit, Buakhamsri Adisai, Greenberg Neil, Popović Zoran B, Thomas James D, Klein Allan L. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010 Feb;23 (2):172–80. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Vianna-Pinton Rita, Moreno Carlos A, Baxter Christy M, Lee Kwan S, Tsang Teresa S M, Appleton Christopher P. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009 Mar;22 (3):299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Schneider Carsten, Malisius Rainer, Krause Korff, Lampe Friedrun, Bahlmann Edda, Boczor Sigrid, Antz Matthias, Ernst Sabine, Kuck Karl-Heinz. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur. Heart J. 2008 Jun;29 (11):1397–409. doi: 10.1093/eurheartj/ehn168. [DOI] [PubMed] [Google Scholar]
- 73.Oral Hakan, Chugh Aman, Ozaydin Mehmet, Good Eric, Fortino Jackie, Sankaran Sundar, Reich Scott, Igic Petar, Elmouchi Darryl, Tschopp David, Wimmer Alan, Dey Sujoya, Crawford Thomas, Pelosi Frank, Jongnarangsin Krit, Bogun Frank, Morady Fred. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006 Aug 22;114 (8):759–65. doi: 10.1161/CIRCULATIONAHA.106.641225. [DOI] [PubMed] [Google Scholar]
- 74.Boyd Anita C, Schiller Nelson B, Ross David L, Thomas Liza. Differential recovery of regional atrial contraction after restoration of sinus rhythm after intraoperative linear radiofrequency ablation for atrial fibrillation. Am. J. Cardiol. 2009 Feb 15;103 (4):528–34. doi: 10.1016/j.amjcard.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Gabriel Ruvin S, Klein Allan L. Managing catheter ablation for atrial fibrillation: the role of echocardiography. Europace. 2008 Nov;10 Suppl 3 ():iii8–13. doi: 10.1093/europace/eun226. [DOI] [PubMed] [Google Scholar]