Abstract
It is well established that the presence of atrial fibrillation (AF) is associated with an increased risk of stroke; however, the precise role that AF plays in increasing this risk is less well understood. In particular, it is not fully known whether a temporal relationship between AF and stroke exists. Early clinical trials in this field were limited by their rudimentary tools for monitoring of AF recurrences. More recently, studies employing implantable cardiac rhythm devices have brought greater precision to our ability to accurately detect and quantify episodes of AF but have been restricted to patient populations with clinical indications for those types of devices.In the future, new monitoring modalities such as subcutaneous devices and external patches may allow us to extend precise arrhythmia monitoring to the broader AF population. Due to the relatively low rate of clinical events, large clinical trials or registries will be required to fully appreciate the temporal aspects of AF and stroke and alternative metrics for quantifying AF recurrences need to be explored.
Introduction
The incidence of atrial fibrillation (AF) has increased over the past several decades and is projected to continue its growth in the future as the population ages and risk factors for AF become more prevalent.[1] It is well established that the presence of AF is associated with an almost five-fold increased risk of stroke.[2] However, the precise role that AF plays in raising the risk of stroke is less well understood. Is AF merely a marker for other disease processes which predispose a patient to an increased risk of stroke or does a patients risk of stroke increase primarily during and shortly following the occurrence of AF? If a temporal relationship between AF and stroke does exist, it may be possible to improve stroke prevention strategies by determining when patients might benefit most from oral anticoagulation. In this review article, we will present some of the early clinical trials and their limitations, explore the current landscape of studies which have incorporated more sophisticated monitoring of AF, and discuss potential future directions for clinical research on this important topic.
Early Efforts
As early as 1983, Wolf and colleagues asserted that the "onset of AF is clearly temporally related to imminence of stroke".[3] This was based on their finding from the Framingham study that nearly one-quarter of AF-related stroke patients were in AF at the time of stroke onset. Other studies have also confirmed that AF is frequently diagnosed for the first time upon presentation for stroke.[4] If we expand the definition of “temporally related” to also include AF episodes that occur shortly prior to the stroke (e.g., within 30 days), this proportion of strokes occurring near periods of AF will obviously increase. Furthermore, it is estimated that up to 40% of ischemic strokes have an undetermined cause (i.e., cryptogenic) and many of these could be caused by undiagnosed, intermittent AF.[5] Since stroke recurrences in the next 6 months are more than twice as likely if the first stroke was associated with AF,[3] it is important to accurately discern the presence of AF in order for appropriate therapy to be administered.
Oral anticoagulation is a highly effective therapy which can reduce the risk of AF-related stroke by more than 60% relative to placebo in high risk patients.[6] However, oral anticoagulation use is also associated with an increased risk of bleeding complications. Therefore it is important that the use of these therapies be limited to AF patients with additional stroke risk factors in whom the stroke reduction benefits outweigh the bleeding risks. These additional stroke risk factors include congestive heart failure, hypertension, age over 75 years, diabetes, and a prior stroke or transient ischemic attack (TIA), forming the basis of the well-established CHADS2 score.[7] In the CHADS2 scheme, one point is assigned for each risk factor with the exception of prior stroke/TIA which is assigned two points.It has been shown that the risk of stroke increases monotonically as the score becomes elevated. More recently, the CHA2DS2-VASc score has been proposed and includes additional risk factors for vascular disease, female gender, and age between 65-74 years.[8]
In the Framingham era, the tools available for monitoring AF were largely limited to patient symptoms and intermittent in-office screening using external recordings. These rudimentary tools allowed AF to be classified as paroxysmal (self-terminating episodes lasting less than 7 days), persistent (episodes lasting longer than 7 days or requiring termination via cardioversion),or permanent (when attempts at restoring sinus rhythm are unsuccessful or have been abandoned).[9] There is some debate as to whether the risk of stroke is the same for patients with paroxysmal vs. persistent or permanent forms of AF.An early study from Hart et al. suggested that the risk was similar.[10] However, it is important to consider how paroxysmal (or intermittent) AF was defined in that study.Their definition of paroxysmal AF meant that sinus rhythm was documented at some point in the 3-12 months prior to study entry. It is likely that paroxysmal AF patients in that study had a very high burden of AF and were quite different from patients in whom AF is only discovered via more aggressive monitoring techniques. The authors also acknowledge that they were unable to accurately determine the frequency and duration of AF episodes in this study.
In a more recent analysis from the Stockholm Cohort of Atrial Fibrillation, Friberg showed that the rates of ischemic stroke were significantly higher in non-anticoagulated patients with permanent compared to paroxysmal AF (defined as episodes which terminate spontaneously within 7 days and most often within 24 hours).[11] This study suggests that the type or amount of AF may matter in determining a patient's risk of stroke. However to more fully appreciate the complex temporal relationship between AF and stroke, AF must be quantified more precisely than afforded by assignment to one of three possible classifications.
These AF classifications, while helpful, are limited by being based on incomplete information. Several studies have shown that patient symptoms are not a reliable surrogate for AF recurrence. Page et al. showed that for every episode of symptomatic paroxysmal AF, patients were likely to experience 12 episodes of asymptomatic AF.[12] A later study with implantable pacemakers confirmed that approximately 94% of stored atrial tachycardia/AF (AT/AF) episodes were asymptomatic.[13] This study and others further demonstrated that symptoms believed by the patient to be caused by AF actually correlated with atrial tachyarrhythmia episodes in approximately only 20% of cases.[13,14] Thus patients frequently do not realize when they are having AF and when they believe they are in AF, they are very often incorrect. Consequently, external monitors have been used to both search for asymptomatic AF as well as corroborate the presence of AF when the patient complains of symptoms.
These external monitoring systems are useful but suffer from several major limitations.One inherent limitation is the use of intermittent recordings to monitor for the presence of a disease which can also be intermittent.It has been well established that intermittent monitoring is highly inaccurate for identifying the presence of AF in patients with paroxysmal AF.[15] For example, performing quarterly 24-hour Holter monitors over the course of a year is likely to identify only about half of the patients who actually have AF. Increasing the frequency or duration of monitoring sessions will improve the sensitivity to detecting AF but comes at the cost of decreased patient compliance. Even in the context of a clinical trial, it has been shown that the average compliance rate with more aggressive external monitoring regimens was only 42%.[16] In an elegant analysis, Charitos et al. illustrated that it is difficult to conclude with high certainty from a series of negative intermittent rhythm examinations that the burden of AF is low.[17] For example, a series of four negative 24-hour Holter monitors performed at random only provides 90% confidence that a patient's AF burden is below 24% (an average of almost 6 hours per day).
Consequently, accurate assessments of the temporal relationship between AF and stroke will be severely hampered if the measurement of AF is imprecise.
Current Status
The advent of implantable cardiac rhythm devices such as pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices introduced the unique opportunity to continuously monitor arrhythmias such as AT/AF for prolonged periods of time. These devices have been shown to accurately detect the presence of AT/AF and quantify the amount of AT/AF in patients who are indicated for cardiac rhythm devices.[18,19] Examples of the diagnostics available from these implantable devices are shown in Figure 1 and include a) the percentage of time spent in AT/AF (i.e., AT/AF burden), b) daily trends of AT/AF recurrences, c) time of day of episode initiation, and d) histogram of episode duration.
Figure 1. Examples of Atrial Tachyarrhythmia/Atrial Fibrillation (AT/AF) Diagnostics Available in Implantable Devices.
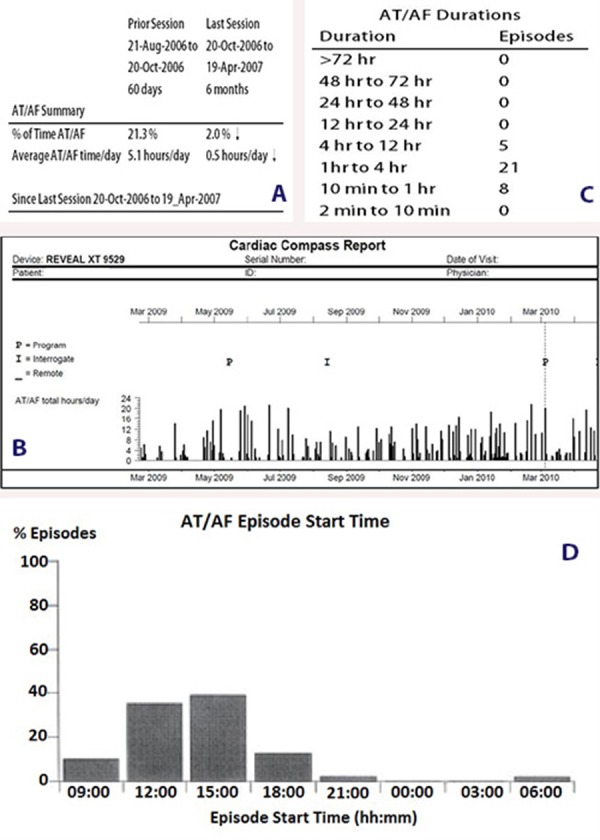
Device diagnostics from implantable devices have been used to quantify the treatment effects of both non-pharmacologic and pharmacologic therapies. In their seminal paper, Martinek et al. described the use of continuous monitoring via pacemakers to quantify the effect of pulmonary vein isolation on AT/AF burden and temporal recurrence.[20]
More recently, the PASCAL study utilized implantable pacemakers to both demonstrate a dose response and quantify the AT/AF burden reduction observed during the trial of a new antiarrhythmic drug.[21] The monitoring capabilities of these devices have also been used in recent years to more precisely understand the relationship between AF and stroke.
One of the first studies to exploit the diagnostic features of implantable devices was a sub-analysis of the MOde Selection Trial (MOST).[22] In this sub-study, the presence of atrial high rate episodes (AHRE) from 312 pacemaker patients was correlated with clinical outcomes. The investigators found that the presence of any AHRE (>5 minutes in duration) was an independent predictor of death or non-fatal stroke with a hazard ratio of 2.79 [1.51, 5.15], p=0.0011. Due to technical limitations of the devices employed in this early study, it was not possible to report or analyze the duration of AHRE with greater precision. Furthermore, the composite endpoint of death or non-fatal stroke was dominated by death (n=44) rather than stroke (n=10). However, this study was critical in bringing the issue of device-detected AHRE and stroke risk to our attention.
Subsequently, the Italian AT500 Registry Investigators reported data from 725 patients with pacemakers having specialized atrial arrhythmia diagnostic capabilities.[23] After adjusting for known risk factors, they found that patients with device-detected AT/AF episodes >24 hours in duration had an increased risk of embolism with a hazard ratio of 3.1 [1.1, 10.5], p=0.44. As with the MOST sub-analysis, this study reported on an association between device-detected atrial arrhythmias and stroke but did not fully explore the temporal relationship between them.
The TRENDS trial analyzed data from 2486 patients with pacemakers, ICDs, or CRT devices.[24] In this study, 30-day rolling windows were evaluated for the presence of AT/AF.The day with maximal AT/AF burden was tabulated within each 30-day window and categorized as high burden (≥5.5 hours of AT/AF), low burden (<5.5 hours of AT/AF), or no burden.In order to maximize statistical power, the cut-off between the high and low burden subgroups was selected as the median of the maximum day of burden among those 30-day windows with any AT/AF. The annualized risk of thromboembolism (stroke, TIA, and systemic embolism) was 2.4% for the high burden subgroup and 1.1% for both the low burden and no burden subgroups.Compared to the no burden subgroup, the adjusted hazard ratios for thromboembolism were 0.98 [0.34, 2.82], p=0.97 in the low burden subgroup and 2.20 [0.96, 5.05], p=0.06 in the high burden subgroup.By using a time-varying exposure which was updated daily, this represented one of the first attempts to more fully understand the temporal relationship between AF and stroke in patients with long-term continuous arrhythmia monitoring.
Using similar techniques, Shanmugam et al. prospectively analyzed data from 560 heart failure patients with CRT devices.[25] The median value of daily AHRE burden among those with AHRE was 3.8 hours, resulting in three population subsets: zero AHRE burden, low AHRE burden (<3.8 hours), and high AHRE burden (>3.8 hours). After adjusting for known stroke risk factors, the high AHRE burden group was independently associated with thromboembolic events compared to the zero burden group with a hazard ratio of 9.4 [1.8-47.0], p=0.006. They also reported that 73% of patients did not exhibit a temporal association between their AHRE and the thromboembolic event. However, their definition of “temporal association” required that the patient be in an atrial arrhythmia at the time of thromboembolism diagnosis. This strict definition precludes the possibility that a patient could have had a long AHRE which terminated shortly before the onset of stroke symptoms or prior to presentation at the clinic. One could argue that such a scenario should be treated as temporally related, especially since the spontaneous conversion from AF to sinus rhythm may be an important factor in precipitating some thromboembolic events.[26]
The ASSERT trial enrolled 2580 patients who were ≥65 years old with hypertension and no history of AF.[27] The vast majority of patients (95%) had recently been implanted with a pacemaker while the remaining 5% received an ICD. During the first 3 months of the study, device data was analyzed and patients were classified as either having subclinical atrial tachyarrhythmias of at least 6 minutes in duration (10% of patients) or not (90% of patients). Patients remained fixed in these categories for the remainder of the trial (mean follow-up duration = 2.5 years) even if atrial tachyarrhythmias were subsequently detected in those without early episodes. Patients classified as having subclinical atrial tachyarrhythmias in the initial 3 months had an increased risk of ischemic stroke or systemic embolism with a hazard ratio of 2.49 [1.28, 4.85], p=0.007. However, there was no requirement for the atrial arrhythmia and the ischemic event to be temporally related. In fact, a patient could have a single AT/AF episode detected during the first month and then have no further episodes for the duration of the trial. If this patient had a stroke at month 30, it would have been considered to be associated with that much earlier arrhythmic episode. Conversely, a different patient might not have had any AT/AF episodes in the first 3 months but could have had many long episodes in month 4 which were followed almost immediately by a stroke and this event would not be considered to be associated with the arrhythmia. In addition, more than twice as many patients (24%) were discovered to have subclinical AT/AF episodes occurring only after the initial 3 month categorization period than during this initial 3 month period (10%) yet these patients were included in the no AT/AF group for analysis. The 3 month categorization period likely served the effective purpose of stratifying those patients with a high burden of AT/AF from those patients with a low or no burden of AT/AF. As shown in prior studies, patients who are most likely to be identified during relatively brief periods of monitoring are those patients with the greatest arrhythmic burden.[15] Indeed, in a secondary analysis of the ASSERT data which included all device-detected episodes over the entire follow-up period, the investigators found that the annualized rate of stroke or systemic embolism was greatest in patients having episode durations in the longest quartile. Thus while the ASSERT study was instrumental in furthering our understanding of the relationship between subclinical AT/AF and stroke in patients without a prior history of AF, the published results to date have added relatively little to our knowledge regarding the temporal aspects of AF and stroke.
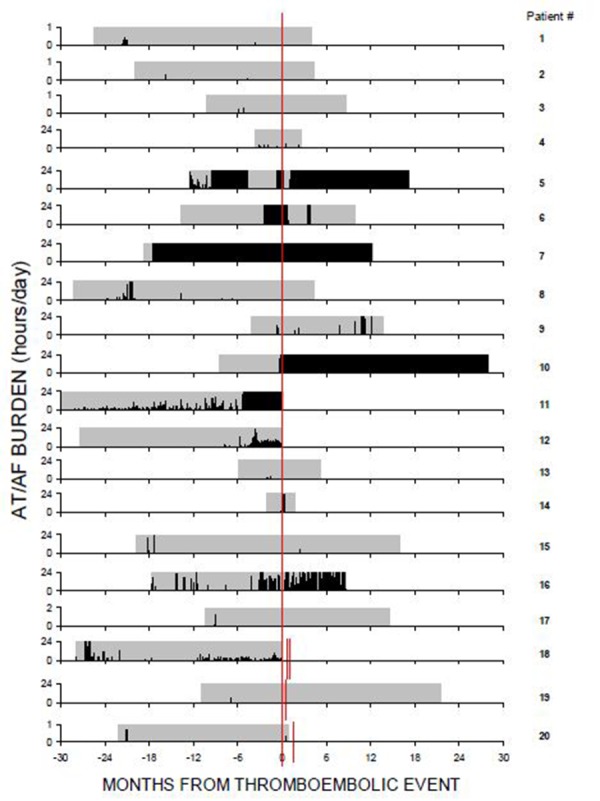
Perhaps the study which most directly addressed the issue of a temporal relationship between AF and stroke was a recent sub-analysis from the TRENDS trial.[28] In this post-hoc analysis, Daoud et al. leveraged the extensive diagnostic capabilities of the implantable devices. Among the 40 patients who experienced a thromboembolic event and had at least 30 days of device data available prior to the event, exactly half (n=20) had at least one episode of AT/AF detected prior to the event (Figure 2). Among these 20 patients, 6 were in AT/AF at the time of thromboembolism diagnosis and another 5 had episodes of AT/AF detected within the 30 days prior to the event. Thus, the majority (11/20 or 55%) of patients who experienced device-documented AT/AF prior to their thromboembolic event had AT/AF episodes within temporal proximity of the event.While the other half of patients (n=20) did not have any episodes of AT/AF detected prior to the thromboembolic event, it is important to note that the duration of preevent monitoring was significantly shorter in these patients without AT/AF detected prior (251±221 days) compared to those patients with AT/AF detected prior (485±273 days). In fact, 35% of patients (7/20) without AT/AF detected by the device prior to their thromboembolism had ≤90 days of device data available. In the TRENDS study, the minimum duration of AT/AF was required to be at least 5 minutes as this duration has been shown to reduce the likelihood of erroneous detection.[29] It is noteworthy however that 6 of the 20 patients (30%) who did not meet this threshold for device-detected AT/AF prior to the event did have shorter episodes of AT/AF recorded by the device prior to the event. Furthermore, 7 thromboembolism patients had a prior history of AF but no episodes longer than 5 minutes documented by the device prior to the event. Taken together, the TRENDS trial suggests that multiple mechanisms are responsible for thromboembolism among patients with implantable devices and AF has a prominent role in a significant proportion of them. However, since this study included a mixture of patients both with and without a prior history of atrial tachyarrhythmias, it is difficult to directly compare the percentage of thromboembolic events that were temporally associated with AF to that reported in other studies.
Figure 2. Full Disclosure of Atrial Tachyarrhythmia/Atrial Fibrillation (AT/AF) Recurrences Relative to Thromboembolic Events [denoted by Red Line(s)].
The ongoing IMPACT study[30] may help to address whether stroke prevention treatments can be safely started and stopped in patients when guided by the continuous arrhythmia monitoring features in their ICD or CRT device. The criteria for both initiation and interruption of oral anticoagulation are based on a combination of CHADS2 scores and the duration of AT/AF recorded over a consecutive 2 day period (Table 1). This is an important study because it will address whether it is safe to discontinue oral anticoagulation in high risk patients who have been free of AT/AF episodes for prolonged periods of time.One could infer that if AF must be temporally related in order to increase stroke risk, this study should be successful. However, if AF is merely a marker for increased risk of ischemic stroke, it may not be prudent to discontinue oral anticoagulation at any time following diagnosis of the arrhythmia.
Table 1. Criteria for Oral Anticoagulation Initiation and Interruption in the IMPACT Study.
ARHE = Atrial high rate episode, TIA = Transient ischemic attack
| Initiation Criteria | Interruption Criteria | |
|---|---|---|
| AHRE Duration (over 48 consecutive hours) | Consecutive days free from AHRE | |
| CHADS2 Score | ||
| 1 and 2 | 48 hours | 30 days |
| 3 and 4 | 24 hours | 90 days |
| 5 and 6 or any prior stroke/TIA | ≤12 hours | Do not discontinue |
The aforementioned device-based studies have several characteristics in common which have limited our ability to fully appreciate the multifaceted temporal relationship between AF and stroke. First, the number of stroke events in these trials has been very low.Even the two largest completed trials (TRENDS which enrolled 3045 patients and ASSERT which enrolled 2580 patients) each reported a total of only 51 ischemic events.Pooling data across different trials is difficult due to differences in inclusion criteria, clinical endpoints, and device capabilities/programming (Table 2).A second factor is that each of these previous studies with continuous arrhythmia monitoring has been limited to populations with a clinical indication for a pacemaker, ICD, or CRT device. While many of these patients do happen to also have AF, only a small percentage of the overall AF population is indicated for one of these types of devices. Thus patients who are most representative of the broader AF population remain unstudied from the perspective of comprehensive AF monitoring.
Table 2. Summary of Trials Using Continuous Arrhythmia Monitoring to Correlate AT/AF and Stroke Risk.
AT/AF = Atrial tachyarrhythmia/atrial fibrillation, C.I. = Confidence interval, CRT = Cardiac resynchronization therapy, ICD = Implantable cardioverter defibrillator, TIA = Transient ischemic attack
| Study Name | Monitoring Device | Prior AT/AF? | AT/AF Burden Threshold | Endpoint Event | Hazard Ratio (C.I.) |
|---|---|---|---|---|---|
| MOST Sub-study22 | 100% Pacemaker | Allowed, but not required | >5 minutes | Death or non-fatal stroke | 2.79 [1.51,5.15] |
| Italian AT500 Registry23 | 100% Pacemaker | History of symptomatic AT | >24 hours | Ischemic stroke, TIA, or peripheral arterial embolism | 3.1 [1.1, 10.5] |
| TRENDS24 | TRENDS24 50% Pacemaker 31% ICD 19% CRT | Allowed, but not required | ≥5.5 hours | Ischemic stroke, TIA, or systemic embolism | 2.20 [0.96, 5.05] |
| Biotronik25 | 100% CRT | Allowed, but not required | ≥3.8 hours | Ischemic stroke, TIA, or peripheral arterial embolism | 9.4 [1.8, 47.0] |
| ASSERT27 | 95% Pacemaker 5% ICD | Excluded | ≥6 minutes | Ischemic stroke or systemic embolism | 2.49 [1.28, 4.85] |
Future Directions
There are several reasons why patients with iplantable cardiac rhythm devices may not be representative of the broader AF population. One reason is that these patients tend to be sicker and have more co-morbidities. Consequently, their risk of stroke may be different compared to non-device patients with similar AF burden profiles due to the presence of these additional risk factors. Secondly, some studies have reported a significantly higher incidence of cerebral ischemic events among patients receiving high amounts of ventricular pacing.[31] In contrast, some have speculated that atrial pacing may be cardio-protective by increasing the left atrial appendage emptying velocity. In either case, the device which is being used for arrhythmia monitoring also has the ability to perturb the cardiac system by delivering pacing therapy.Therefore, the ideal clinical study to assess the temporal relationship between AF and stroke might incorporate a monitoring device which fulfills the following requirements:
Capable of full disclosure arrhythmia monitoring over long periods of time (i.e., years)
Presents minimal patient compliance concerns
Does not perturb the system which it is monitoring (i.e., no pacing)
Able to be used in the broader AF population
Small subcutaneous devices (Figure 3) are now available[32] which meet these goals, can accurately quantify AF,[33] and may facilitate additional research into this broader AF population.Several ongoing or planned studies have incorporated these types of monitoring devices to investigate the relationship between AF and stroke. In the area of secondary stroke prevention, the CRYSTAL-AF study is being conducted to compare subcutaneous monitoring to standard of care monitoring for the detection of AF in patients with a recent cryptogenic stroke.[34] In the area of primary stroke prevention, the REVEAL AF[35] and ASSERT II [36] trials are being planned to explore the incidence of newly diagnosed AF in patients at high risk of stroke. These primary prevention studies, in particular, offer the possibility of providing a new view into the temporal relationship between AF and stroke in the broader population of „at risk patients. External patches capable of monitoring arrhythmias for days to weeks at a time have also recently become available.[37] In order to reliably capture arrhythmias prior to an ischemic stroke however, it may be necessary to frequently re-apply new patches as the device memory becomes filled. This could potentially have an adverse impact on patient compliance as well as cost.
Figure 3. Example of a Subcutaneous Arrhythmia Monitoring Device.
There are additional factors which could make it difficult to fully assess the temporal relationship between AF and stroke. First it must be recognized that only 15- 20% of ischemic strokes are caused by AF[02,38] although the true value might actually be somewhat higher due to the presence of occult AF.Thus for every 5-6 strokes that occur, only one is likely to be attributable to AF.In addition, even patients with documented AF can have strokes caused by other mechanisms such as large or small vessel disease.Secondly, while the association between AF and stroke is well established, it is important to note that AF does not directly cause a stroke.Rather,AF can lead to the formation of a thrombus which in turn can dislodge and actually cause a stroke.We are well aware of when a stroke occurs due to the presence of symptoms.We now also have the ability to accurately detect when AF occurs and quantify its magnitude in patients who have continuous monitoring devices.However, less is known about the time-course surrounding thrombus formation and particularly thrombus dislodgement, which is essential for AF-related stroke. It is likely that many factors contribute to thrombus dislodgement and thus the temporal relationship between AF and stroke is not straight-forward.Both the fact that not all strokes are related to AF and that AF may more directly govern thrombus formation but not necessarily thrombus dislodgment introduces "noise" into our measurement (and thus into our understanding) of the temporal relationship between AF and stroke.
To overcome these limitations, more sophisticated statistical models which consider alternative methods of quantifying AF are needed. But what is the right metric for quantifying paroxysmal AF? Prior studies have used the maximum day of AF burden[24,25] and the duration of the longest episode.[23,27] However, there are a variety of other metrics which could also be considered, including the cumulative AF burden, average AF burden, or the number (i.e., frequency) of AF episodes. Recently, the metric of AF density has been proposed to describe the temporal aggregation of AF burden.[17] This raises the question of whether the pattern of AF recurrences [39] may also have clinical value. For example, there are an infinite number of ways in which a patient can manifest an average AF burden of 50% (Figure 4). It is unlikely that the stroke risk associated with each of these possible AF burden recurrence patterns is identical. Perhaps a certain amount of AF is required for thrombus formation while converting between AF and sinus rhythm states increases the likelihood of thrombus dislodgement.
Figure 4. Examples of Atrial Fibrillation AF Burden Patterns Which Equate to an Average Burden Value of 50%.
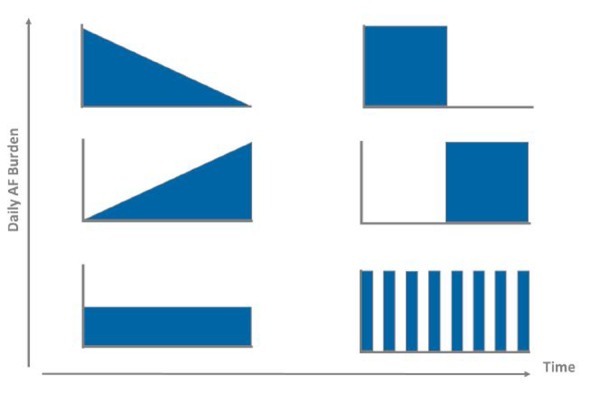
Regardless of the specific AF metric chosen, large numbers of stroke events will be required to permit this sort of modeling. Given that strokes are relatively infrequent and the rates have been decreasing over time,[40] this would require enormous sample sizes if conducted prospectively.Since the primary requirement for such an analysis is to have accurate knowledge of the temporal distribution of AF prior to a stroke, a large registry study among patients with existing implantable monitoring devices may be more practical.
Conclusions
The introduction of implantable devices with continuous monitoring capabilities has dramatically expanded our view into the complex relationship between atrial fibrillation and ischemic stroke. It is becoming clear from recent studies with implantable devices that the amount of AF does impact the risk of stroke and that even a modest amount of AF can increase this risk.Less is understood about the temporal connection between AF and stroke for a variety of reasons. Due to the relatively low rate of clinical events, large clinical trials or registries will be required to better characterize this relationship and determine if stroke risk is transiently increased during or near periods of AF. Alternative metrics for quantifying AF recurrences also need to be explored. Until larger trials or registries are conducted, it is important to follow established guidelines regarding anticoagulation
Disclosures
Paul D. Ziegler is an employee and shareholder of Medtronic, Inc.
References
- 1.Miyasaka Yoko, Barnes Marion E, Gersh Bernard J, Cha Stephen S, Bailey Kent R, Abhayaratna Walter P, Seward James B, Tsang Teresa S M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114 (2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991 Aug;22 (8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P A, Kannel W B, McGee D L, Meeks S L, Bharucha N E, McNamara P M. Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke. 1983 Sep 1;14 (5):664–7. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- 4.Lin H J, Wolf P A, Benjamin E J, Belanger A J, D'Agostino R B. Newly diagnosed atrial fibrillation and acute stroke. The Framingham Study. Stroke. 1995 Sep;26 (9):1527–30. doi: 10.1161/01.str.26.9.1527. [DOI] [PubMed] [Google Scholar]
- 5.Sacco R L, Ellenberg J H, Mohr J P, Tatemichi T K, Hier D B, Price T R, Wolf P A. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann. Neurol. 1989 Apr;25 (4):382–90. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 6.Hart R G, Benavente O, McBride R, Pearce L A. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 1999 Oct 05;131 (7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gage B F, Waterman A D, Shannon W, Boechler M, Rich M W, Radford M J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285 (22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 8.Lip Gregory Y H, Nieuwlaat Robby, Pisters Ron, Lane Deirdre A, Crijns Harry J G M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137 (2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Fuster Valentin, Rydén Lars E, Cannom Davis S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Kay G Neal, Le Huezey Jean-Yves, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann L Samuel. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011 Mar 15;57 (11):e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Hart R G, Pearce L A, Rothbart R M, McAnulty J H, Asinger R W, Halperin J L. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J. Am. Coll. Cardiol. 2000 Jan;35 (1):183–7. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 11.Friberg Leif, Hammar Niklas, Rosenqvist Mårten. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur. Heart J. 2010 Apr;31 (8):967–75. doi: 10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]
- 12.Page R L, Wilkinson W E, Clair W K, McCarthy E A, Pritchett E L. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994 Jan;89 (1):224–7. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 13.Strickberger S Adam, Ip John, Saksena Sanjeev, Curry Ken, Bahnson Tristram D, Ziegler Paul D. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005 Feb;2 (2):125–31. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Quirino Gianluca, Giammaria Massimo, Corbucci Giorgio, Pistelli Paolo, Turri Elena, Mazza Antonio, Perucca Antonello, Checchinato Catia, Dalmasso Maurizio, Barold S Serge. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009 Jan;32 (1):91–8. doi: 10.1111/j.1540-8159.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler Paul D, Koehler Jodi L, Mehra Rahul. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006 Dec;3 (12):1445–52. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Vasamreddy Chandrasekhar R, Dalal Darshan, Dong Jun, Cheng Alan, Spragg David, Lamiy Sameh Z, Meininger Glenn, Henrikson Charles A, Marine Joseph E, Berger Ronald, Calkins Hugh. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2006 Feb;17 (2):134–9. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 17.Charitos Efstratios I, Stierle Ulrich, Ziegler Paul D, Baldewig Malte, Robinson Derek R, Sievers Hans-Hinrich, Hanke Thorsten. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012 Aug 14;126 (7):806–14. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]
- 18.Purerfellner Helmut, Gillis Anne M, Holbrook Reece, Hettrick Douglas A. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004 Jul;27 (7):983–92. doi: 10.1111/j.1540-8159.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 19.Passman Rod S, Weinberg Kenneth M, Freher Mark, Denes Pable, Schaechter Andi, Goldberger Jeffrey J, Kadish Alan H. Accuracy of mode switch algorithms for detection of atrial tachyarrhythmias. J. Cardiovasc. Electrophysiol. 2004 Jul;15 (7):773–7. doi: 10.1046/j.1540-8167.2004.03537.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinek Martin, Aichinger Josef, Nesser Hans-Joachim, Ziegler Paul D, Purerfellner Helmut. New insights into long-term follow-up of atrial fibrillation ablation: full disclosure by an implantable pacemaker device. J. Cardiovasc. Electrophysiol. 2007 Aug;18 (8):818–23. doi: 10.1111/j.1540-8167.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz Michael D, Nagarakanti Rangadham, Lubinski Andrzej, Bandman Olga, Canafax Daniel, Ellis David J, Milner Peter G, Ziola Margaret, Thibault Bernard, Hohnloser Stefan H. A randomized trial of budiodarone in paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2012 Jun;34 (1):1–9. doi: 10.1007/s10840-011-9636-3. [DOI] [PubMed] [Google Scholar]
- 22.Glotzer Taya V, Hellkamp Anne S, Zimmerman John, Sweeney Michael O, Yee Raymond, Marinchak Roger, Cook James, Paraschos Alexander, Love John, Radoslovich Glauco, Lee Kerry L, Lamas Gervasio A. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003 Apr 01;107 (12):1614–9. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 23.Capucci Alessandro, Santini Massimo, Padeletti Luigi, Gulizia Michele, Botto GianLuca, Boriani Giuseppe, Ricci Renato, Favale Stefano, Zolezzi Francesco, Di Belardino Natale, Molon Giulio, Drago Fabrizio, Villani Giovanni Q, Mazzini Elena, Vimercati Marco, Grammatico Andrea. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J. Am. Coll. Cardiol. 2005 Nov 15;46 (10):1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Glotzer Taya V, Daoud Emile G, Wyse D George, Singer Daniel E, Ezekowitz Michael D, Hilker Christopher, Miller Clayton, Qi Dongfeng, Ziegler Paul D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009 Oct;2 (5):474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugam Nesan, Boerdlein Annegret, Proff Jochen, Ong Peter, Valencia Oswaldo, Maier Sebastian K G, Bauer Wolfgang R, Paul Vince, Sack Stefan. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012 Feb;14 (2):230–7. doi: 10.1093/europace/eur293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigner M J, Caulfield T A, Danias P G, Silverman D I, Manning W J. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann. Intern. Med. 1997 Apr 15;126 (8):615–20. doi: 10.7326/0003-4819-126-8-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Healey Jeff S, Connolly Stuart J, Gold Michael R, Israel Carsten W, Van Gelder Isabelle C, Capucci Alessandro, Lau C P, Fain Eric, Yang Sean, Bailleul Christophe, Morillo Carlos A, Carlson Mark, Themeles Ellison, Kaufman Elizabeth S, Hohnloser Stefan H. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012 Jan 12;366 (2):120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 28.Daoud Emile G, Glotzer Taya V, Wyse D George, Ezekowitz Michael D, Hilker Christopher, Koehler Jodi, Ziegler Paul D. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011 Sep;8 (9):1416–23. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Pollak W M, Simmons J D, Interian A, Atapattu S A, Castellanos A, Myerburg R J, Mitrani R D. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001 Apr;24 (4 Pt 1):424–9. doi: 10.1046/j.1460-9592.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 30.Ip John, Waldo Albert L, Lip Gregory Y H, Rothwell Peter M, Martin David T, Bersohn Malcolm M, Choucair Wassim K, Akar Joseph G, Wathen Mark S, Rohani Pooyan, Halperin Jonathan L. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: Rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am. Heart J. 2009 Sep;158 (3):364–370.e1. doi: 10.1016/j.ahj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Saccomanno G, Fraticelli A, Marini M, Spazzafumo L, Paciaroni E. Permanent ventricular and dual chamber cardiac stimulation: role of pacing mode in relation to chronic atrial fibrillation risk and stroke development. Arch Gerontol Geriatr. 2004 Sep 18;29 (1):61–74. doi: 10.1016/s0167-4943(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar Shantanu, Ritscher David, Mehra Rahul. A detector for a chronic implantable atrial tachyarrhythmia monitor. IEEE Trans Biomed Eng. 2008 Mar;55 (3):1219–24. doi: 10.1109/TBME.2007.903707. [DOI] [PubMed] [Google Scholar]
- 33.Hindricks Gerhard, Pokushalov Evgueny, Urban Lubos, Taborsky Milos, Kuck Karl-Heinz, Lebedev Dmitry, Rieger Guido, Pürerfellner Helmut. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):141–7. doi: 10.1161/CIRCEP.109.877852. [DOI] [PubMed] [Google Scholar]
- 34.Sinha Anil-Martin, Diener Hans-Christoph, Morillo Carlos A, Sanna Tommaso, Bernstein Richard A, Di Lazzaro Vincenzo, Passman Rod, Beckers Frank, Brachmann Johannes. Cryptogenic Stroke and underlying Atrial Fibrillation (CRYSTAL AF): design and rationale. Am. Heart J. 2010 Jul;160 (1):36–41.e1. doi: 10.1016/j.ahj.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Incidence of AF in high risk patients (REVEAL AF). http://www.clinicaltrials.gov. 0;0:0–0. [Google Scholar]
- 36.Prevalence of sub-clinical atrial fibrillation using an implantable cardiac monitor (ASSERT-II) http://www.clinicaltrials.gov/ct2/show/ NCT01694394. 0;0:0–0. [Google Scholar]
- 37.Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. J Am Coll Cardiol. Eur J Cardiothorac Surg. 2011;58:1741–1749. doi: 10.1016/j.jacc.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Bogousslavsky J, Cachin C, Regli F, Despland P A, Van Melle G, Kappenberger L. Cardiac sources of embolism and cerebral infarction--clinical consequences and vascular concomitants: the Lausanne Stroke Registry. Neurology. 1991 Jun;41 (6):855–9. doi: 10.1212/wnl.41.6.855. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar S, Koehler J, Ziegler P, Mehra R. Dominant temporal patterns in long term continuous monitoring of atrial tachyarrhythmia burden. Heart Rhythm. Eur J Cardiothorac Surg. 2006;0:0–0. [Google Scholar]
- 40.Oberg AL, Ferguson JA, McIntyre LM, Horner RD. Incidence of stroke and season of the year: evidence of an association. Am J Epidemiol. Eur J Cardiothorac Surg. 2000;0:0–0. doi: 10.1093/aje/152.6.558. [DOI] [PubMed] [Google Scholar]



