Abstract
Atrial fibrillation (AF) is the most common sustained atrial arrhythmia conferring a higher morbidity and mortality. Despite the increasing incidence of AF; available therapies are far from perfect. Dietary fish oils, containing omega 3 fatty acids, also called polyunsaturated fatty acid [PUFA] have demonstrated beneficial electrophysiological, autonomic and anti-inflammatory effects on both atrial and ventricular tissue. Multiple clinical trials, focusing on various subsets of patients with AF, have studied the role of PUFA and their potential role in reducing the incidence of this common arrhythmia. While PUFA appears to have a beneficial effect in the primary prevention of AF in the elderly with structural heart disease, this benefit has not been universally observed. In the secondary prevention of AF, PUFA seems to have a greater impact in the reducing AF in patients with paroxysmal or persistent AF, stages of AF associated with less atrial fibrosis and negative structural remodeling. However, AF suppression has not been consistently demonstrated in clinical trials. In patients undergoing heart surgery, increasing PUFA intake has yielded mixed results in terms of AF prevention post-operatively; however, increased PUFA has been associated with a reduction in hospital stay. Therefore recommending the use of PUFA for the purpose of AF reduction remains controversial. This is in part attributable to the complexity of AF. Other conflicting variables include: heterogeneous patient populations studied; variable dosing; duration of follow-up; comorbidities; and, concomitant pharmacotherapy. This review article reviews in detail available basic and clinical research studies of fish oil in the treatment of AF, and its role in the treatment of this common disorder.
Abbreviations
AF=Atrial fibrillation, CHS=Cardiovascular Health Study,CABG=Coronary artery bypass surgery, d=Day, DHA=Docosahexaenoic acid, EPA=Eicosapentaenoic acid, ERP= Effective refractory period, g=Gram, PAF= Paroxysmal atrial fibrillation, PeAF= Persistent atrial fibrillation PUFA= Polyunsaturated fatty acid.
Keywords: Omega 3 fatty acid, PUFA, Atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common sustained atrial arrhythmia conferring a higher morbidity and mortality.[1,2] As a result of an aging population with an increasing incidence of both obesity and diabetes, the number of projected cases of AF is expected to increase.[3] It is estimated that by the year 2050, twelve million Americans will be diagnosed with AF.[3]
The exact mechanism of AF is not fully understood and therefore, a unifying pathogenicmechanism is lacking. Similarly, the treatment of rate or rhythm control in AF remains imperfect. Antiarrhythmic drugs often have side effects and show limited efficacy (approximately 5%-40%) in providing freedom from AF.Recently radiofrequency ablation, focusing on isolation of the pulmonary veins has emerged as a promising treatment of AF, especially in younger symptomatic patients with early stages of AF.
Fish oil and omega-3 fatty acids, have been found to exert a beneficial effect by reducing triglycerides and increasing high-density lipoproteins in patients with marked hypertriglyceridemia. Both bench research and clinical trials have shown anti-arrhythmic benefit associated with omega-3 fatty acid intake. In addition, omega-3 fatty acids have been shown to have a beneficial effect on various aspects of the cardiovascular system including heart rate, blood pressure, and autonomic tone. Recent studies have shown that a diet rich in omega 3 fatty acids reduces the incidence of both fatal coronary heart disease (CHD) and sudden cardiac death.[4–7] Due to a large burden of AF, lack of effective pharmacological agents and the relative safety of omega 3 fatty acid, there is a widespread interest in omega-3 fatty acid in the prevention of AF.
Omega 3 Fatty Acids
Omega 3 fatty acids are long chain hydrocarbons (ranging from 18-22 carbon atoms) containing multiple double bonds, referred to as polyunsaturated fatty acids (PUFA). Omega 3 fatty acids have the first double bond located on the 3rd carbon from the –CH3 terminus (ω carbon). Omega 3 fatty acids (n-3 PUFA), include: alfa-linoleic acid (ALA; 18:3n-3) commonly found in flaxseed, canola, walnuts, soybeans;eicosapentaenoic acid (EPA) (20:5n-3) and docosahexaenoic acid (DHA) (22:6n-3) commonly found in fish products. Humans synthesize relatively little EPA and even less DHA, therefore seafood consumption provides the major source of PUFA.8 Docosapentaenoic acid (DPA) (22:5n-3), metabolite of EPA, can be found in smaller amounts in fish.
Effects of Omega 3 Fatty Acid on Atrial Fibrillation
One of the most intriguing effects of omega-3 fatty acid appears to be its antiarrhythmic effect. To date, research findings have been mixed, with some trials demonstrating a lower risk of arrhythmias and others showing no significant effect[9–16] While, in vitro studies, animal experiments, and some human studies suggest an anti-arrhythmic effect of n-3 PUFA in AF, clinical data has remained elusive. It is also unclear whether such benefits, if present, are due to direct effects on myocyte electrophysiology or more indirect influences such as improvements in myocardial efficiency, autonomic tone, local inflammatory responses, and the like. In order to fully understand the potential role of omega-3 fatty acids in the prevention of AF one has to understand the pathogenesis. This may prove challenging due to the complex mechanisms of AF.
Subtypes of Atrial Fibrillation
AF is classified as paroxysmal (PAF), persistent (PeAF), chronic, and permanent. PAF is atrial fibrillation which exhibits salvos of AF lasting less than 7 days. PeAF are episodes lasting longer than 7 days, while chronic AF persists for greater than one year. Permanent AF is chronic when it has persisted for longer than one year and is resistant to any form of cardioversion. Clinically, the chronic and permanent subtypes of AF respond poorly to both pharmacologic and ablative therapy. Omega-3 fatty acids appear to have a differential effect on the various subtypes of AF, showing more of an effect in the suppression of PAF as compared to the more persistent subtypes.
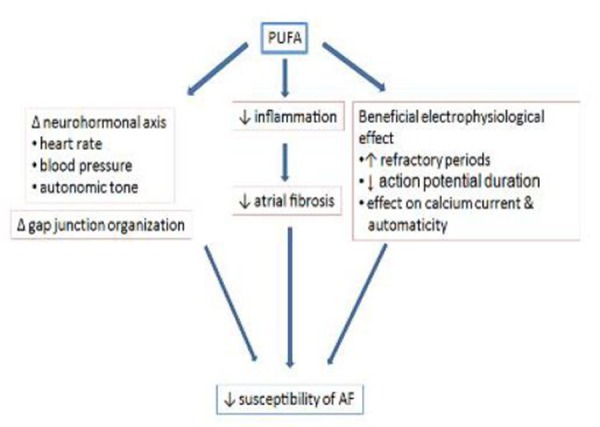
The 3 clinical subtypes seem to possess different pathophysiological mechanisms. PAF, by definition self-terminating, results predominantly from focal mechanisms, most probably in combination with local reentrant circuits originating from specific regions such as the pulmonary veins. PeAF requires a substrate capable of accomodating multiple micro-reentrant circuits. In the long term, as AF and underlying cardiac conditions progress; structural remodeling leads to a complex, fixed substrate that is very resistant to therapy, rendering AF permanent. Atrial remodeling favors the transition towards more-advanced clinical forms of AF, with distinct types of remodeling promoting structural over functional substrates.[17] The following section would describe major mechanisms and the role of omega 3 polyunsaturated fatty acid (referred to as simply PUFA hereafter) as an antifibrillatory agent.[18] The effect of PUFA on various physiological variables, are summarized in [Table 1] and the mechanism which PUFA reduces AF is given in [Figure 1].
Table 1. The table shows the putative mode of action of PUFA at various biological levels as they relate to mechanism of AF.
RMP=resting membrane potential, AP=action potential, Ito=transient outward potassium current, RP=refractory period, APD=action potential duration, PUFA=polyunsaturated fatty acid, AF=atrial fibrillation, RA=right atrium, LA=left atrium, PV=pulmonary vein, ERP=effective refractory period
| Site of Action | Putative Antifibrillatory Action |
|---|---|
| Ion channels | |
| Cell membrane | |
| Myocyte |
|
| Electrical properties | |
| Neurohormonal axis |
Figure 1. The Figure shows the major mechanisms by which PUFA reduces susceptibility of AF. 1. Δ=change, 2. Decreases 3. Increases.
Pathogenesis of AF and Role of Omega 3 PUFA
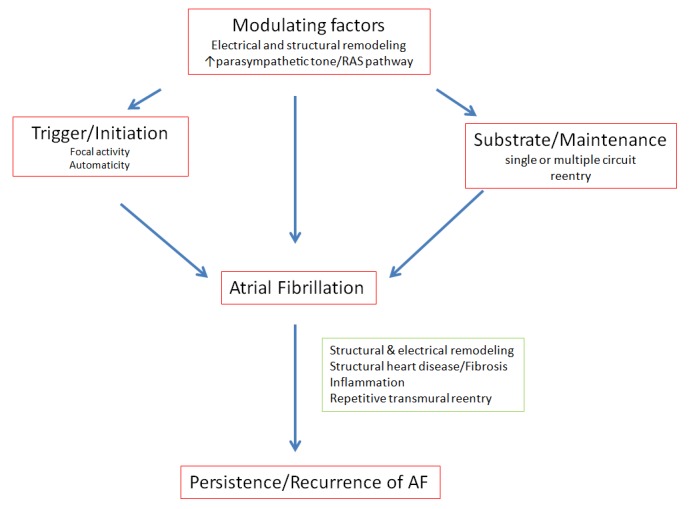
Factors which increase an individual's propensity to develop AF include: increase in atrial mass; decrease in conduction velocity; decrease in atrial refractoriness and increase in the atrial dispersion of refractoriness. In addition, tissue inflammation and autonomic tone serve to facilitate the initiation and propagation of AF. [Figure 2] AF is commonly associated with structural heart disease, but in the absence of structural heart disease (lone AF), genetic factors resulting in abnormal channel function have been implicated. Non-cardiac disorders (e.g. hyperhyperthyroidism or pulmonary disease) can result in either atrial pressure or volume overload resulting in atrial distension with fibrotic changes and eventual remodeling. Interstitial fibrosis disrupts the atrial architecture, affecting the intercellular connexin density, reducing conduction velocity, decreasing atrial refractoriness while increasing the dispersion of refractoriness. These structural changes are the contributing factors which modify atrial tissue resulting in a substrate which can predispose to AF.
Figure 2. Schematic representation of mechanism causing persistent or recurrence of Atrial fibrillation. Trigger in terms of focal activity or automaticity and Substrate in terms of reentry are essential for AF. Both of these factors are influenced by modulating factors. Fibrosis and inflammation either from structural heart disease or AF itself can create a setting for persistence or recurrence of AF.
Atrial Fibrosis
Recently Kitamura et al has shown in a rabbit model that PUFA (EPA) can attenuate atrial fibrosis and AF induced by rapid ventricular pacing. The attenuation of AF is associated with changes in anti-inflammatory markers, suggesting that PUFA attenuates fibrosis by its anti-inflammatory action.[19] However, a direct effect on mitigating fibrosis in humans has not been shown.
Atrial Stretch
One of the mechanisms of AF is a sudden increase in pressure in the atria leading to AF (i.e. acute myocardial infarction or acute mitral regurgitation). Ninio et al.[20] has demonstrated the beneficial role of PUFA on stretch induced AF in rabbits. This study evaluated the effects of a standard diet versus tuna fish oil (contains PUFA) or sunflower oil (n-6 fatty acid) in rabbits. In perfused hearts, intraatrial pressures were increased in a stepwise manner and rapid burst pacing was applied to induce AF. Increased atrial pressure resulted in a reduction in the atrial refractory period and a propensity for induction of sustained AF. Higher pressures were required to induce and sustain AF in the fish oil group compared to the other groups. The stretch induced drop in the atrial refractory period was also attenuated in the fish oil group.[20]
Genetic Factors
Studies have shown that human AF may be associated with genes encoding for the gap junctional protein connexin-40.[21] Sarrazin et al[22] has shown that a daily intake of fish oil decreases the incidence of AF in dogs and is associated with lower connexin-40 levels. Ramadeen et al[23] reported that dogs receiving PUFA had reduction in genetic markers associated with fibrosis, hypertrophy and inflammation. The authors suggested that PUFA-mediated prevention of AF may be due to attenuation of adverse remodeling at the genetic level in response to mechanical stress. At this time the role of PUFA in modulation of genetic factors responsible for AF in humans remains unknown.
Pulmonary Vein Ectopy
Pulmonary vein (PV) ectopy is critical in the initiation, and propagation of PAF.[24] In the landmark study by Haissaguerre et al,[24] 95% of patients with recurrent AF had ectopic foci in PVs. The depolarization of these foci preceded atrial ectopic beats. AF was initiated by a sudden burst of rapid depolarizations. A local depolarization could be recognized during sinus rhythm and abolished by radiofreqency ablation. In addition, the PVs of patients with PAF exhibit distinct electrophysiological properties (shorter effective and functional refractory period, decremental conduction, and anisotropic cellular orientation) that may form the "substrate" for re-entry.[25,26] In patients without structural heart disease or clinical AF, chronic fish oil supplementation prolongs both right atrial and coronary sinus refractoriness and decreases vulnerability to development of AF.[27] Recently it has been shown that the ingestion of high dose fish oil (>6 g/d for ≥1 month) in humans (with PAF) resulted in a prolongation of PV and LA effective refractory periods (ERPs) compared to controls. There was less dispersion of refractoriness in the PVs of patients receiving fish oil. This study revealed lower ERPs in the control group resulted in a higher incidence of PV-initiated AF in controls compared to patients on fish oil (71% vs. 33%, p 0.02). The cycle length of first induced AF in PVs was significantly longer as well in patients using fish oil compared to controls (209 ms vs. 133 ms, p 0.002). The authors suggested that, as a direct consequence of higher PV refractoriness, patients on fish oil had decreased susceptibility to PV-initiated AF and longer cycle length AF in PVs.[28]
Autonomic Tone
The role of the autonomic nervous system in the initiation of AF has been extensively studied. PAF can be initiated in the context of both, enhanced vagal or sympathetic tone. Approximately 25% of patients with paroxysmal AF have "vagotonic AF", in which AF is initiated in the setting of high vagal tone, typically in the evening when the patient is relaxing or during sleep. In theory, drugs that have a vagotonic effect (such as digitalis) may aggravate vagally mediated AF. "Adrenergic AF" occurs in approximately 10%-15% of patients with paroxysmal AF in the setting of high sympathetic tone, for example, during strenuous exertion. Most patients have a mixed or random form of paroxysmal AF, with no consistent pattern of onset.[29] Vagal stimulation shortens refractoriness and sympathetic stimulation increases calcium loading and automaticity. In combination the autonomic nervous system may cause pause-induced triggered activity in both PV and atrial tissue. Foci of triggered activity may be explained by the combination of very short action potential duration and increased calcium release during atrial systole. Fish oils have demonstrable effects on calcium current[30] and cytosolic calcium[31] and electrical automaticity and this may explain its antiarrhythmic effect.[32] Sarrazin et al concluded that oral treatment with fish oil can reduce vulnerability to vagally induced AF in dog models.[22]
Structural and Electrical Remodeling
Both structural and electrical remodeling of the atria alters the substrate, creating the appropriate milieu for the maintenance of AF. On a microscopic level, AF causes atrial fibrosis, while the myocytes experience de-differentiation (regressing to a fetal morphology). De-differentiation includes an increase in cell size, accumulation of glycogen, myolysis, alterations in connexin expression, changes in mitochondrial shape, and fragmentation of the sarcoplasmic reticulum.[33] However, such changes are not uniformly observed throughout the atria contributing substantially to the electrical instability. Electrical remodeling parallels the structural abnormalities and the degree of fibrosis observed in AF. In addition, progressive shortening and dispersion of atrial refractory periods are the major changes occurring during AF.[34] Acute PUFA treatment (in dogs) has been shown to reduce the degree of shortening of atrial ERPs especially at high atrial rate, which may minimize paroxysms of AF.[35] Lau and associates[36] have shown that in sheep, PUFA prevents the development of heart failure related left atrial enlargement and is associated with reduction in atrial interstitial fibrosis. Kumar et al[14] have shown that chronic PUFA therapy improves LA appendageal function, by improving the emptying velocity, emptying fraction, reducing the incidence of spontaneous echocardiographic contrast and atrial mechanical stunning, in patients undergoing cardioversion of PeAF or atrial flutter.
Inflammation
Clinical studies provide evidence for a role of inflammation as a contributing factor in pathophysiology of AF, particularly in patients with persistent AF.[37] PUFA supplementation has been associated with lower levels of eicosanoids (leuotriene E4), Interleukin 1-beta, tumor necrosis factor-alfa and oxidative stress (as measured by F2-isoprostane).[38–41] In contrast, Madsen et al, showed that dietary supplementation of PUFA (2-7 g/d for 12 weeks) had no effect on the serum concentration of C-reactive protein (a marker of low grade inflammation), in humans.[42] Yusof et al[43] investigated the effect of moderate doses of EPA (1.8 g/d) and DHA (0.3 g/d) administered for 8 weeks to healthy males. The change in plasma soluble intercellular adhesion molecule (an inflammatory marker) was inversely related to change in DHA levels but less to change in EPA. At these doses PUFA demonstrated no marked effect on plasma lipids or inflammatory markers. There was a stronger association with reduction of inflammatory molecules with DHA compared to EPA suggesting that DHA may have more potent anti-inflammatory properties.[43]
Primary Prevention of AF
Various studies have reported on the primary prevention of AF with PUFA, [Table 2]. Most of the data was derived from epidemiological studies and these have produced inconsistent results. The first study by Mozaffarian et al[44] reported on (Cardiovascular Health Study group [CHS]), a prospective cohort study that enrolled 4,815 adults (men and women) of ≥65 years (mean 73 years) age. Food frequency questionnaires were administered at baseline to assess fish intake (tuna fish, other broiled or baked fish and fried fish or fish sandwich). Approximately 12% were smokers; 20% had coronary heart disease; 25% had diabetes and 45% had hypertension. AF was assessed during annual follow-up and from hospital discharge records. During a 12 year follow up, 20% developed AF. Consumption of tuna and other broiled or baked fish were inversely associated with AF, with a 35% lower risk associated with fish intake of ≥5 times per week as compared to <1 time per month. However, similar analyses from other population-based studies have not corroborated the findings.[45–47] The Danish diet, cancer and health study,[45] a prospective cohort of 47,949 participants (age 50-60 years) also looked at the effect of dietary fish intake on AF. Dietary intake was obtained from a detailed semi-quantitative questionnaire and AF was assessed from the national registry of hospital discharges. Approximately 36% of enrollees were smokers and 11% were hypertensive with no prior history of cardiovascular disease. Consumption of fatty fish was not associated with a reduction in risk of AF or flutter, when comparing patients with high and low fatty fish consumption at 5.7 years of follow-up.
Table 2. Summary of studies in primary prevention of AF.
N=number of patients, Yr=year, F/U=follow up, a/w=associated with, hx=history, cvd=cardiovascular disease, AF=atrial fibrillation
| Study (Year) | Design (N) | Active Group | Reference Group | Age (Yr) | F/U (Yr) | Outcome | Favors PUFA |
|---|---|---|---|---|---|---|---|
| Mozaffarian[44] (2004) | Prospective, cohort, (4,815) | tuna and broiled/baked fish 1-4 times/wk | 1 time/month | 73 | 12 | 28% lower risk with intake 1-4 times/wk, 31% with ≥5x/wk | Yes |
| The Danish Diet, Cancer, and Health Study[45] (2005) | Prospective, cohort, (47,949) | quintile 2-5 | quintile 1 | 56 | 5.7 | Consumption of fish was not a/w reduction of risk of AF/flutter | No |
| Physician Health Study[70] (2006) | Prospective, cohort, no hx of cvd, (17,679) | Ate fish≥5 meals/wk | Ate <1 time/mo | 15 | 7.1% developed AF during f/u, Fish consumption a/w risk of AF | No | |
| The Rotterdam Study[46] (2006) | Prospective, cohort, (5,148) | tertile 3 | tertile 1 | 67 | 6.4 | No association of EPA, DHA or fish to AF, >20 g/d fish no effect vs. no fish | No |
| Macchia[71] (2008) | Population study, (2,239,205) | PUFA use | no PUFA use | 65 | 1 | PUFA reduced both all cause mortality and incidence of AF in pt hospitalized with MI | Yes |
| Kuopio Ischemic Heart Disease Risk Factor Study[16] (2009) | Prospective, cohort, (2,174) | quartile 4 | quartile 1 | 42-60 | 17.7 | Increased PUFA levels protect against AF, relationship stronger if no CHF/MI, only DHA a/w risk of AF | Yes |
| The Women's Health Initiative[47] (2010) | Cohort, (44,720) | quartile 4 | quartile 1 | 63 | 6 | No evidence of association with fish or PUFA and incident AF | No |
These inconsistencies are attributed to comparison of studies, recruiting patients with unmatched demographic profile, wide range of recruitment ages, underestimation of AF (i.e. AF estimation by annual check or hospital records), socio-economic and lifestyle differences (i.e. frequency of smoking and alcohol use), dietary changes during follow-up, and incidental differences in underlying heart disease. Studies that showed no benefit from high PUFA intake on AF included younger and healthier individuals with a lower prevalence of hypertension and diabetes and no significant cardiovascular disease, whereas the CHS enrolled an older population of ≥65 years with a greater prevalence of cardiovascular disease and a greater incidence of subsequent AF (20%) compared with a significantly lower incidence of AF in the Rotterdam Study (6%; mean age 67 years) or The Danish Study (1.1%; mean age 56 years). It is possible that anti-fibrotic and anti-inflammatory effects of PUFAs have a greater protective effect in older patients with structural heart disease, whereas the ability of PUFAs to increase parasympathetic tone may be proarrhythmic in younger individuals with normal hearts who are more likely to have vagally mediated AF.[48]
Post-operative AF in Patients Undergoing Coronary Artery Bypass Surgery
AF is a common complication of coronary artery bypass surgery (CABG). In a prospective [12] randomized clinical trial, 160 patients undergoing CABG (mean age 66 years, males 136), were randomized to either control (n 81, usual care) or intervention group (n 79, usual care + PUFA, 2g/d for at least 5 days prior to CABG and continued until the day of discharge). All patients were in sinus rhythm upon enrollment and randomization. The PUFA group exhibited a 65% reduction in post-operative AF.[12] suggesting an anti-inflammatory action of PUFA in the prevention of AF. Smaller trials have evaluated the role of peri-operative PUFA supplementation and its effect on post-operative AF, following cardiac surgery.[10,11,13,15] One trial found benefit,[11] one had mixed results[15] and the other two[10,13] showed no effect, however, the trials incorporated a smaller sample size (n 102 to 200) with differing study design limiting any unifying conclusion. Recently, Sandesara et al[49] conducted a multicenter study randomizing 260 patients to PUFA (EPA and DHA) versus placebo (corn oil) administered at least 24 hours prior to surgery (CABG with or without valve surgery). The patients were continued on the therapy for 14 days post-operatively. The patients were followed up to 30 days post-surgery, and there was no significant difference in the incidence of AF between the two groups.[49] Recently, Armaganijan et al[50] published a meta-analysis evaluating 538 patients from 4 studies. The patients were predominantly male with an average age of 62 years with normal LA size and left ventricular ejection fraction. The association of PUFA with a reduction of post-operative atrial fibrillation did not reach statistical significance.[50]
Despite the lack of effect on the incidence of AF, the use of PUFA in the peri-operative setting has been associated with a shorter hospital stay,[15] shorter stay in the intensive care unit[11] and a reduction in post-operative complications.[12] In a study published by Heidersdottir et al[13], which included patients who underwent valve repair, the use of PUFA did not significantly reduce the incidence of post-operative AF. In this study the authors noted that elevated levels of C-reactive protein correlated with a higher incidence of post-operative AF.[13] Although these studies suggest that tissue inflammation increases the incidence of AF, despite bench research suggesting an anti-inflammatory effect with PUFA, PUFA does not significantly affect the incidence of post-operative AF. Therefore, the routine use of PUFA to prevent post-operative AF is not universally supported by the evidence at this time. Currently, we await the results of the OPERA (Omega-3 Fatty Acids for the Prevention of Post-operative Atrial Fibrillation) trial.This randomized, placebo controlled clinical trial will enroll 1,516 patients scheduled for CABG comparing high dose PUFA consumption (10 g 3-5 days before surgery then followed by 2 g/d for 10 days post-operatively) versus placebo. The primary end-point will look at the incidence of AF up to 10 days post surgery.
Secondary Prevention of AF
Several studies have evaluated the use of PUFA on the recurrence of AF. In these studies most of the patients had PAF and some had PeAF. The reason that permanent AF is under-represented is that PUFA may have a minimal impact on atria that have undergone irreversible remodeling. These studies are summarized in [Table 3]. Kumar et al[28] studied 36 patients without structural heart disease. The patients underwent electrophysiological testing after therapy with PUFA (2 g/d for ≥ 1 month). The investigators showed that patients treated with PUFA were more resistant to inducible AF possibly attributable to prolongation in ERP, reduction in ERP dispersion and a prolonged mean AF cycle length. Additional studies by Biscione et al[52] and Patel et al[53] also showed the value of PUFA in the treatment of AF. While the former studies showed benefit, most other randomized trials have not supported these findings.[54–58]
Table 3. Summary of clinical trials in secondary prevention of AF.
pcm=pacemaker, atach-fib=atrial tachycardia or fibrillation, N=number of patients, PUFA=polyunsaturated fatty acid, rx=treatment, RCT= randomized controlled trial, db=double blind, tb=triple blind, pc=placebo controlled, cvn= cardioversion, gp=group, a/w=associated with, afib=atrial fibrillation
| Study (Year) | Design (N) | Clinical Setting | PUFA/Dose | Follow up | Outcome | Favors PUFA |
|---|---|---|---|---|---|---|
| Biscione[52] (2005) | observation, prospective, cross over (40) | PAF with dual chamber pcm | 1 g/d | 4 months on rx; 4 months off rx | Powerful effect of PUFA in reduction of atach-fib, reduction in AF burden | Yes |
| Erdogan[54] (2007) | RCT, tb, pc (108) | Persistent afib, post cvn, | PUFA vs. placebo 4 wk before and 1 yr after cvn | 1 year | No significant difference in AF relapse | No |
| Patel[53] (2009) | retrospective, case controlled (258) | paroxysmal 70%, non paroxysmal 30% in PUFA gp both gp; PV ablation | <655 mg of fish oil | 8 wks | lower early and late recurrence of AF in PUFA gp | Yes |
| Kowey[55] (2010) | RCT, db, pc (663) | Paroxysmal AF (542) and Persistent AF (121) | 8 g/d for 7 d then 4 g/d x24 wk | 24 wks | No significant difference in recurrent symptomatic AF/Fl | No |
| Nodari[56] (2011) | RCT, db, pc (199) | persistent AF, at least 1 relapse post cvn | 2 g/d | 1 year | Higher probability of maintenance of SR in PUFA gp | Yes |
| Bianconi[57] (2011) | RCT, db, pc (204) | chronic persistent AF, post cvn | 3 g/m till cvn and 2 g/d for 24 wk | 24 wks | No significant difference in recurrence of AF or mean time to AF recurrence in PUFA gp | No |
| Ozaydin[58] (2011) | RCT (47) | Post cvn, comparison with amiodarone | PUFA & amiodarone vs. amiodarone | 1 year | No significant difference in AF relapse | No |
Kowey et al[55] performed a randomized, double blind, multicenter study, in which they evaluated the impact of PUFA (8 g/d for first 7 days then 4 g/d for 24 weeks) on time to first recurrence of AF in 663 relatively young patients (mean age, 60 years). Inclusion criteria included patients with PAF or PeAF. However, the breakdown analysis of patients enrolled, at the end of the study, showed that the vast majority of patients (82%) had PAF. In the final analysis, symptomatic AF recurrence occurred in 48% of the placebo group compared to 53% of PUFA treated participants (p 0.26). In patients with PeAF, the arrhythmia recurred in 33% of placebo compared to 50% of the PUFA group (p 0.09). Thus, neither PeAF nor PAF responded to PUFA therapy. This was a well-designed double blind, placebo-controlled trial enrolling patients with comparable demographic characteristics. In addition, plasma omega-3 fatty-acid concentrations were measured to document effective supplementation; and intention-to-treat analysis was used. However, the limitations of the study were a: lack of information on dietary sources of PUFA; only symptomatic AF was assessed, and possibility of type II error due to overestimation of AF recurrence. Additional studies from Ergodan et al[54] Nodari et al[56] and Bianconi et al[57] have failed to show a significant difference in AF relapse from PUFA use in patients with PeAF. However, in a retrospective study, Patel et al[53] studied AF recurrence after PV ablation; PUFA supplementation was associated with a lower AF recurrence at 8 weeks of follow up. Therefore, current clinical data is inconsistent regarding the role of PUFA in the secondary prevention of AF. We wait with anticipation for the completion of the several ongoing studies (NCT00597220, NCT01235130, NCT00552084, NCT00791089, NCT00841451 listed in http://clinicaltrials.gov) especially, FORωARD (Fish Oil Research with ω-3 for Atrial Fibrillation Recurrence Delay) study59, which is expected to enroll 1,400 patients with PAF or PeAF. This study will determine if supplementation of 1 g of PUFA can reduce the recurrence of AF in patients who have recovered normal sinus rhythm.
Recommendation
Despite, beneficial effect of PUFA in experimental studies, there is neither consistent nor robust evidence that PUFA reduces either the incidence or recurrence of AF. However, in a clinical setting of older patient with cardiovascular disease, without advanced atrial structural remodeling, on an optimum loading and maintenance dose, with close objective monitoring over a sufficient duration, PUFA may prove to be a useful agent in prevention of AF. We wait with anticipation the results of several large prospective randomized ongoing clinical trials.
Conclusions
AF is a very common arrhythmia and its therapy remains a challenge. Extensive experimental evidence points to potentially beneficial effects of PUFA on AF. Since PUFAs are a natural dietary constituent and have very few adverse effects, any efficacy of PUFAs against AF would have important clinical consequences. However, our extensive literature review does not demonstrate a significant and consistent clinical benefit of PUFA supplementation on AF prevention. The reasons for the discrepancies appear to be multi-factorial. The anti-arrhythmic potential of PUFA may be attributed to its action at the substrate level (e.g. anti-fibrotic and anti-inflammatory) and a direct electrophysiological effect on the ion channels, the potency of which is likely to depend on the clinical situation and AF milieu. Like the renin-angiotensin-aldosterone system inhibitors and statins, PUFAs may produce a differential effect in the remodeled and un-remodeled atria. Despite compelling evidence from experimental models, there has been no study demonstrating reverse remodeling with PUFA.[48] The doses used in clinical trials have been generally lower than those applied in animal experiments, and the duration of treatment might have not been long enough for the antiarrhythmic effect to fully develop. There is suggestion that individual component of PUFAs may be more important than the total PUFA concentration because of the differences in the effects produced by DHA and EPA.[16,43] At this time the optimum dose of fatty acid per day or the relative composition of EPA versus DHA or the specific formulation of PUFA remains to be established. Study[60] has shown that it takes weeks, of oral intake of PUFA, to reach a steady state level in tissues. Therefore, study subjects must be loaded with a sufficient dose and of sufficient duration to achieve a good tissue concentration. The presence of subclinical coronary heart disease, concurrent therapy with beta blockers and renin-angiotensin-aldosterone inhibitor may have limited the perceptible magnitude of benefit. Finally, there are limited large randomized trials to allow for definite recommendation at this time.
Disclosures
No disclosures relevant to this article were made by the author.
References
- 1.Kannel W B, Wolf P A, Benjamin E J, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am. J. Cardiol. 1998 Oct 16;82 (8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Movsowitz Herman D., Lampert Craig, Jacobs Larry E., Kotler Morris N.. Atrial Fibrillation in the Elderly: A Review. Am J Geriatr Cardiol. 1994 Mar;3 (2):26–40. [PubMed] [Google Scholar]
- 3.Lloyd-Jones Donald, Adams Robert, Carnethon Mercedes, De Simone Giovanni, Ferguson T Bruce, Flegal Katherine, Ford Earl, Furie Karen, Go Alan, Greenlund Kurt, Haase Nancy, Hailpern Susan, Ho Michael, Howard Virginia, Kissela Brett, Kittner Steven, Lackland Daniel, Lisabeth Lynda, Marelli Ariane, McDermott Mary, Meigs James, Mozaffarian Dariush, Nichol Graham, O'Donnell Christopher, Roger Veronique, Rosamond Wayne, Sacco Ralph, Sorlie Paul, Stafford Randall, Steinberger Julia, Thom Thomas, Wasserthiel-Smoller Sylvia, Wong Nathan, Wylie-Rosett Judith, Hong Yuling. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119 (3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Mente Andrew, de Koning Lawrence, Shannon Harry S, Anand Sonia S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009 Apr 13;169 (7):659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 5.Harris William S, Mozaffarian Dariush, Lefevre Michael, Toner Cheryl D, Colombo John, Cunnane Stephen C, Holden Joanne M, Klurfeld David M, Morris Martha Clare, Whelan Jay. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 2009 Apr;139 (4):804S–19S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Chenchen, Harris William S, Chung Mei, Lichtenstein Alice H, Balk Ethan M, Kupelnick Bruce, Jordan Harmon S, Lau Joseph. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am. J. Clin. Nutr. 2006 Jul;84 (1):5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian Dariush, Rimm Eric B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006 Oct 18;296 (15):1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 8.Harris William S, Mozaffarian Dariush, Rimm Eric, Kris-Etherton Penny, Rudel Lawrence L, Appel Lawrence J, Engler Marguerite M, Engler Mary B, Sacks Frank. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009 Feb 17;119 (6):902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer Ingeborg A, Raitt Merritt H, Dullemeijer Carla, Kraemer Dale F, Zock Peter L, Morris Cynthia, Katan Martijn B, Connor William E, Camm John A, Schouten Evert G, McAnulty John. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur. Heart J. 2009 Apr;30 (7):820–6. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saravanan Palaniappan, Bridgewater Ben, West Annette L, O'Neill Stephen C, Calder Philip C, Davidson Neil C. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 11.Heidt M C, Vician M, Stracke S K H, Stadlbauer T, Grebe M T, Boening A, Vogt P R, Erdogan A. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg. 2009 Aug;57 (5):276–80. doi: 10.1055/s-0029-1185301. [DOI] [PubMed] [Google Scholar]
- 12.Calò Leonardo, Bianconi Leopoldo, Colivicchi Furio, Lamberti Filippo, Loricchio Maria Luisa, de Ruvo Ermenegildo, Meo Antonella, Pandozi Claudio, Staibano Mario, Santini Massimo. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J. Am. Coll. Cardiol. 2005 May 17;45 (10):1723–8. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 13.Heidarsdottir Ragnhildur, Arnar David O, Skuladottir Gudrun V, Torfason Bjarni, Edvardsson Vidar, Gottskalksson Gizur, Palsson Runolfur, Indridason Olafur S. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010 Mar;12 (3):356–63. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 14.Kumar Saurabh, Sutherland Fiona, Wheeler Miriam, Heck Patrick M, Lee Geoffrey, Teh Andrew W, Garg Manohar L, Morgan John G, Sparks Paul B. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human atrial mechanical function after reversion of atrial arrhythmias to sinus rhythm: reversal of tachycardia-mediated atrial cardiomyopathy with fish oils. Heart Rhythm. 2011 May;8 (5):643–9. doi: 10.1016/j.hrthm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Farquharson Aaron L, Metcalf Robert G, Sanders Prashanthan, Stuklis Robert, Edwards James R M, Gibson Robert A, Cleland Leslie G, Sullivan Thomas R, James Michael J, Young Glenn D. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am. J. Cardiol. 2011 Sep 15;108 (6):851–6. doi: 10.1016/j.amjcard.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen Jyrki K, Mursu Jaakko, Voutilainen Sari, Tuomainen Tomi-Pekka. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009 Dec 08;120 (23):2315–21. doi: 10.1161/CIRCULATIONAHA.109.852657. [DOI] [PubMed] [Google Scholar]
- 17.Nattel Stanley, Burstein Brett, Dobrev Dobromir. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008 Apr;1 (1):62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 18.Jalife JA, Antzelevitch C, Yoshito L, Jackman W.M, Scheinman M, Chen S.A. Pathophysiology of Atrial fibrillation. In: Natale NR, A., ed. Atrial fibrillation ablation 2011 update, The state of the art based on the VeniceChart International Consensus Document. Venice: Wiley-Blackwell. 2011;0:0–0. [Google Scholar]
- 19.Kitamura Kazuhisa, Shibata Rei, Tsuji Yukiomi, Shimano Masayuki, Inden Yasuya, Murohara Toyoaki. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am. J. Physiol. Heart Circ. Physiol. 2011 May;300 (5):H1814–21. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- 20.Ninio Daniel M, Murphy Karen J, Howe Peter R, Saint David A. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1189–94. doi: 10.1111/j.1540-8167.2005.50007.x. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin Emelia J, Chen Peng-Sheng, Bild Diane E, Mascette Alice M, Albert Christine M, Alonso Alvaro, Calkins Hugh, Connolly Stuart J, Curtis Anne B, Darbar Dawood, Ellinor Patrick T, Go Alan S, Goldschlager Nora F, Heckbert Susan R, Jalife José, Kerr Charles R, Levy Daniel, Lloyd-Jones Donald M, Massie Barry M, Nattel Stanley, Olgin Jeffrey E, Packer Douglas L, Po Sunny S, Tsang Teresa S M, Van Wagoner David R, Waldo Albert L, Wyse D George. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009 Feb 03;119 (4):606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarrazin Jean-Francois, Comeau Genevieve, Daleau Pascal, Kingma John, Plante Isabelle, Fournier Dominique, Molin Franck. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n-3 polyunsaturated fatty acids in dogs. J. Am. Coll. Cardiol. 2007 Oct 09;50 (15):1505–12. doi: 10.1016/j.jacc.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 23.Ramadeen Andrew, Laurent Gabriel, dos Santos Claudia C, Hu Xudong, Connelly Kim A, Holub Bruce J, Mangat Iqwal, Dorian Paul. n-3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm. 2010 Apr;7 (4):520–8. doi: 10.1016/j.hrthm.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 25.Hocini Mélèze, Ho Siew Y, Kawara Tokuhiro, Linnenbank André C, Potse Mark, Shah Dipen, Jaïs Pierre, Janse Michiel J, Haïssaguerre Michel, De Bakker Jacques M T. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation. 2002 May 21;105 (20):2442–8. doi: 10.1161/01.cir.0000016062.80020.11. [DOI] [PubMed] [Google Scholar]
- 26.Jaïs Pierre, Hocini Mélèze, Macle Laurent, Choi Kee-Joon, Deisenhofer Isabel, Weerasooriya Rukshen, Shah Dipen C, Garrigue Stéphane, Raybaud Florence, Scavee Christophe, Le Metayer Philippe, Clémenty Jacques, Haïssaguerre Michel. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002 Nov 05;106 (19):2479–85. doi: 10.1161/01.cir.0000036744.39782.9f. [DOI] [PubMed] [Google Scholar]
- 27.Kumar Saurabh, Sutherland Fiona, Rosso Raphael, Teh Andrew W, Lee Geoffrey, Heck Patrick M, Feldman Alexander, Medi Caroline, Watt Shannon, Garg Manohar L, Sparks Paul B. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human atrial electrophysiology. Heart Rhythm. 2011 Apr;8 (4):562–8. doi: 10.1016/j.hrthm.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Kumar Saurabh, Sutherland Fiona, Teh Andrew W, Heck Patrick M, Lee Geoffrey, Garg Manohar L, Sparks Paul B. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human pulmonary vein and left atrial electrophysiology in paroxysmal atrial fibrillation. Am. J. Cardiol. 2011 Aug 15;108 (4):531–5. doi: 10.1016/j.amjcard.2011.03.082. [DOI] [PubMed] [Google Scholar]
- 29.Morady F, Zipes D.P, Bonow M. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia. Elsevier Saunders. 0;825:0–0. [Google Scholar]
- 30.Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1994 Sep 13;91 (19):8832–6. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinaldi B, Di Pierro P, Vitelli M R, D'Amico M, Berrino L, Rossi F, Filippelli A. Effects of docosahexaenoic acid on calcium pathway in adult rat cardiomyocytes. Life Sci. 2002 Jul 19;71 (9):993–1004. doi: 10.1016/s0024-3205(02)01792-7. [DOI] [PubMed] [Google Scholar]
- 32.Kang J X, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur. J. Pharmacol. 1996 Feb 15;297 (1-2):97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- 33.Ausma J, Wijffels M, Thoné F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997 Nov 04;96 (9):3157–63. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 34.Wijffels M C, Kirchhof C J, Dorland R, Allessie M A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 01;92 (7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 35.da Cunha D N Q, Hamlin R L, Billman G E, Carnes C A. n-3 (omega-3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br. J. Pharmacol. 2007 Feb;150 (3):281–5. doi: 10.1038/sj.bjp.0706977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau Dennis H, Psaltis Peter J, Carbone Angelo, Kelly Darren J, Mackenzie Lorraine, Worthington Michael, Metcalf Robert G, Kuklik Pawel, Nelson Adam J, Zhang Yuan, Wong Christopher X, Brooks Anthony G, Saint David A, James Michael J, Edwards James, Young Glenn D, Worthley Stephen G, Sanders Prashanthan. Atrial protective effects of n-3 polyunsaturated fatty acids: a long-term study in ovine chronic heart failure. Heart Rhythm. 2011 Apr;8 (4):575–82. doi: 10.1016/j.hrthm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Aviles Ronnier J, Martin David O, Apperson-Hansen Carolyn, Houghtaling Penny L, Rautaharju Pentti, Kronmal Richard A, Tracy Russell P, Van Wagoner David R, Psaty Bruce M, Lauer Michael S, Chung Mina K. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003 Dec 16;108 (24):3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 38.Mickleborough Timothy D, Murray Rachael L, Ionescu Alina A, Lindley Martin R. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am. J. Respir. Crit. Care Med. 2003 Nov 15;168 (10):1181–9. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 39.Accinni R, Rosina M, Bamonti F, Della Noce C, Tonini A, Bernacchi F, Campolo J, Caruso R, Novembrino C, Ghersi L, Lonati S, Grossi S, Ippolito S, Lorenzano E, Ciani A, Gorini M. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr Metab Cardiovasc Dis. 2006 Mar;16 (2):121–7. doi: 10.1016/j.numecd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Seierstad S L, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, Frøyland L, Arnesen H. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur. J. Clin. Invest. 2005 Jan;35 (1):52–9. doi: 10.1111/j.1365-2362.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- 41.Mori Trevor A, Woodman Richard J, Burke Valerie, Puddey Ian B, Croft Kevin D, Beilin Lawrence J. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic. Biol. Med. 2003 Oct 01;35 (7):772–81. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 42.Madsen Trine, Christensen Jeppe H, Blom Mogens, Schmidt Erik B. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br. J. Nutr. 2003 Apr;89 (4):517–22. doi: 10.1079/BJN2002815. [DOI] [PubMed] [Google Scholar]
- 43.Yusof Hayati M, Miles Elizabeth A, Calder Philip. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot. Essent. Fatty Acids. 2008 Mar;78 (3):219–28. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Mozaffarian Dariush, Psaty Bruce M, Rimm Eric B, Lemaitre Rozenn N, Burke Gregory L, Lyles Mary F, Lefkowitz David, Siscovick David S. Fish intake and risk of incident atrial fibrillation. Circulation. 2004 Jul 27;110 (4):368–73. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost Lars, Vestergaard Peter. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am. J. Clin. Nutr. 2005 Jan;81 (1):50–4. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- 46.Brouwer Ingeborg A, Heeringa Jan, Geleijnse Johanna M, Zock Peter L, Witteman Jacqueline C M. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am. Heart J. 2006 Apr;151 (4):857–62. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Berry Jarett D, Prineas Ronald J, van Horn Linda, Passman Rod, Larson Joseph, Goldberger Jeffrey, Snetselaar Linda, Tinker Lesley, Liu Kiang, Lloyd-Jones Donald M. Dietary fish intake and incident atrial fibrillation (from the Women's Health Initiative). Am. J. Cardiol. 2010 Mar 15;105 (6):844–8. doi: 10.1016/j.amjcard.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savelieva Irene, Kakouros Nicholaos, Kourliouros Antonios, Camm A John. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011 May;13 (5):610–25. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- 49.Sandesara CM. Fish-oil capsules don’t stop post-CABG atrial fib in randomized trial. Heart Rhythm Society Annual Meeting. 2010;0:0–0. [Google Scholar]
- 50.Armaganijan Luciana, Lopes Renato D, Healey Jeff S, Piccini Jonathan P, Nair Girish M, Morillo Carlos A. Do omega-3 fatty acids prevent atrial fibrillation after open heart surgery? A meta-analysis of randomized controlled trials. Clinics (Sao Paulo) 2011;66 (11):1923–8. doi: 10.1590/S1807-59322011001100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biscione Francesco, Totteri Alessandro, De Vita Antonio, Lo Bianco Francesco, Altamura Giuliano. [Effect of omega-3 fatty acids on the prevention of atrial arrhythmias]. Ital Heart J Suppl. 2005 Jan;6 (1):53–9. [PubMed] [Google Scholar]
- 53.Patel Dimpi, Shaheen Mazen, Venkatraman Preeti, Armaganijan Luciana, Sanchez Javier E, Horton Rodney P, Di Biase Luigi, Mohanty Prasant, Canby Robert, Bailey Shane M, Burkhardt J David, Gallinghouse G Joseph, Zagrodzky Jason D, Kozeluhova Marketa, Natale Andrea. Omega-3 polyunsaturated Fatty Acid supplementation reduced atrial fibrillation recurrence after pulmonary vein antrum isolation. Indian Pacing Electrophysiol J. 2009 Nov 01;9 (6):292–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Erdogan A, Bayer M, Kollath D, Greiss H. Omega AF study: polyunsaturated fatty acids (PUFA) for prevention of atrial fibrillation relapse after successful external cardioversion. Heart Rhythm. 2007;4:0–0. [Google Scholar]
- 55.Kowey Peter R, Reiffel James A, Ellenbogen Kenneth A, Naccarelli Gerald V, Pratt Craig M. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010 Dec 01;304 (21):2363–72. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 56.Nodari Savina, Triggiani Marco, Campia Umberto, Manerba Alessandra, Milesi Giuseppe, Cesana Bruno M, Gheorghiade Mihai, Dei Cas Livio. n-3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation. 2011 Sep 06;124 (10):1100–6. doi: 10.1161/CIRCULATIONAHA.111.022194. [DOI] [PubMed] [Google Scholar]
- 57.Bianconi Leopoldo, Calò Leonardo, Mennuni Mauro, Santini Luca, Morosetti Paolo, Azzolini Paolo, Barbato Giuseppe, Biscione Francesco, Romano Paolo, Santini Massimo. n-3 polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011 Feb;13 (2):174–81. doi: 10.1093/europace/euq386. [DOI] [PubMed] [Google Scholar]
- 58.Ozaydın Mehmet, Erdoğan Doğan, Tayyar Senol, Uysal Bayram Ali, Doğan Abdullah, Içli Atilla, Ozkan Emel, Varol Ercan, Türker Yasin, Arslan Akif. N-3 polyunsaturated fatty acids administration does not reduce the recurrence rates of atrial fibrillation and inflammation after electrical cardioversion: a prospective randomized study. Anadolu Kardiyol Derg. 2011 Jun;11 (4):305–9. doi: 10.5152/akd.2011.080. [DOI] [PubMed] [Google Scholar]
- 59.Macchia Alejandro, Varini Sergio, Grancelli Hugo, Nul Daniel, Laffaye Nicolas, Ferrante Daniel, Tognoni Gianni, Doval Hernan C. The rationale and design of the FORomegaARD Trial: A randomized, double-blind, placebo-controlled, independent study to test the efficacy of n-3 PUFA for the maintenance of normal sinus rhythm in patients with previous atrial fibrillation. Am. Heart J. 2009 Mar;157 (3):423–7. doi: 10.1016/j.ahj.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Metcalf Robert G, James Michael J, Gibson Robert A, Edwards James Rm, Stubberfield John, Stuklis Robert, Roberts-Thomson Kurt, Young Glenn D, Cleland Leslie G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007 May;85 (5):1222–8. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y F, Wright S N, Wang G K, Morgan J P, Leaf A. Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc. Natl. Acad. Sci. U.S.A. 1998 Mar 03;95 (5):2680–5. doi: 10.1073/pnas.95.5.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leaf A. The electrophysiologic basis for the antiarrhythmic and anticonvulsant effects of n-3 polyunsaturated fatty acids: heart and brain. Lipids. 2001;36 Suppl ():S107–10. doi: 10.1007/s11745-001-0691-y. [DOI] [PubMed] [Google Scholar]
- 63.Kang J X, Leaf A. The cardiac antiarrhythmic effects of polyunsaturated fatty acid. Lipids. 1996 Mar;31 Suppl ():S41–4. doi: 10.1007/BF02637049. [DOI] [PubMed] [Google Scholar]
- 64.Jahangiri A, Leifert W R, Patten G S, McMurchie E J. Termination of asynchronous contractile activity in rat atrial myocytes by n-3 polyunsaturated fatty acids. Mol. Cell. Biochem. 2000 Mar;206 (1-2):33–41. doi: 10.1023/a:1007025007403. [DOI] [PubMed] [Google Scholar]
- 65.Fiaccavento Roberta, Carotenuto Felicia, Minieri Marilena, Masuelli Laura, Vecchini Alba, Bei Roberto, Modesti Andrea, Binaglia Luciano, Fusco Angelo, Bertoli Aldo, Forte Giancarlo, Carosella Luciana, Di Nardo Paolo. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am. J. Pathol. 2006 Dec;169 (6):1913–24. doi: 10.2353/ajpath.2006.051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLennan P L, Bridle T M, Abeywardena M Y, Charnock J S. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in marmoset monkeys. Am. J. Clin. Nutr. 1993 Nov;58 (5):666–9. doi: 10.1093/ajcn/58.5.666. [DOI] [PubMed] [Google Scholar]
- 67.McLennan P L, Bridle T M, Abeywardena M Y, Charnock J S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am. Heart J. 1992 Jun;123 (6):1555–61. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 68.O'Keefe James H, Abuissa Hussam, Sastre Antonio, Steinhaus David M, Harris William S. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am. J. Cardiol. 2006 Apr 15;97 (8):1127–30. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 69.Kenny D, Warltier D C, Pleuss J A, Hoffmann R G, Goodfriend T L, Egan B M. Effect of omega-3 fatty acids on the vascular response to angiotensin in normotensive men. Am. J. Cardiol. 1992 Nov 15;70 (15):1347–52. doi: 10.1016/0002-9149(92)90773-r. [DOI] [PubMed] [Google Scholar]
- 70.Aizer A, Gaziano J.M, Manson J>E, Buring J.E, Albert C.M. Relationship between fish consumption and the development of atrial fibrillation in men. Heart Rhythm. 2006;0:0–0. [Google Scholar]
- 71.Macchia Alejandro, Monte Simona, Pellegrini Fabio, Romero Marilena, Ferrante Daniel, Doval Hernán, D'Ettorre Antonio, Maggioni Aldo Pietro, Tognoni Gianni. Omega-3 fatty acid supplementation reduces one-year risk of atrial fibrillation in patients hospitalized with myocardial infarction. Eur. J. Clin. Pharmacol. 2008 Jun;64 (6):627–34. doi: 10.1007/s00228-008-0464-z. [DOI] [PubMed] [Google Scholar]


