Abstract
Pre-procedure X-ray computed tomography (CT) and magnetic resonance imaging (MRI) angiography are commonly used to delineate the complex and variable relationship of the left atrium, pulmonary veins, and surrounding structures. 3D CT and MR angiography are routinely incorporated into electroanatomic mapping systems to guide ablation lesion placement in the context of patient specific anatomy. Post-procedure CT and MRI have also proven useful for evaluating complications such as pulmonary vein stenosis. In the future, these imaging modalities may be used to visualize more detailed tissue characteristics such as atrial fibrosis and ablation lesions. This could improve selection of patients for different treatment strategies and perhaps guide more effective ablation. This review will discuss current and emerging applications of CT and MRI before and after radiofrequency catheter ablation of atrial fibrillation.
Introduction
Recognition that the triggering foci for atrial fibrillation (AF) frequently arise from the pulmonary veins (PVs) has lead to largely anatomic catheter ablation strategies to isolate these triggers from the atrial tissue.[1–04] 3D CT and MRI have helped understand the variable nature of PV anatomy in relation to surrounding structures. These modalities are now commonly used to plan and guide AF ablation procedures. Post-procedure, CT and MRI are used to evaluate complications such as pulmonary vein stenosis and esophageal injury. In its current state, AF ablation appears superior to pharmacologic therapy for controlling AF.[5] However, ablation success rates remain in the range of 70% with worse outcomes reported for more persistent atrial fibrillation and with repeat procedures commonly required to achieve successful treatment.[6] MRI and CT of detailed atrial tissue characteristics, such as fibrosis, are being studied to better understand the patient-specific atrial su bstrate that contributes to the success and failure of AF ablation. It is also recognized that ablation procedure failure is commonly associated with recovery of PV conduction despite acute PV isolation at the time of the procedure.[7,8] In the future, 3D imaging of the pattern and permanence of ablation lesions could be used to guide additional ablation to fill in "gaps" in ablation lines. This review will discuss the current and emerging roles of MRI and CT before and after radiofrequency (RF) catheter ablation of AF
Pre-procedure CT and MRI of Left Atrium and Pulmonary Veins for Guiding Ablation
Understanding the anatomic relationship of the left atrium (LA) and PVs is important for efficiently guiding successful AF ablation. CT angiography (CTA) and MRI angiography (MRA) provide a detailed 3D perspective of the left atrium (LA) and the number, location, size, and geometry of the pulmonary veins. CTA and MRA appear to characterize complex left atrial anatomy better than 2D imaging modalities such as fluoroscopy and echo.[9,10] Since an early MRA study by Kato, et.al. outlined PV anatomic relationships,[11] many CTA and MRA studies have confirmed the common occurrence of pulmonary vein variants. Supernumerary PVs, most often right sided but also potentially from the atrial roof or left side, occur in 10-39% of people in 3D angiogram studies.[11–14] AF triggering, foci can be located within these additional veins requiring their isolation.[15] Early branching from the PV ostium can also be seen and appears more common for the right PVs.[16] Being alerted to the presence of such veins is important because ablating near small or early branching PVs increases the risk of pulmonary vein stenosis.[17,18] A left common PV trunk results in a broad PV junction and is present in 12 to 29% of people in 3D angiogram studies.[11–14] A recent study by Hunter, et.al. reported that common PV trunk anatomy may reduce single procedure success rates.[19] This could suggest a need to focus on better catheter contact during ablation around these veins or point to difficulty confirming true electrical isolation post-ablation due to sub-optimal circular mapping catheter positioning. For such reasons, CTA or MRA is recognized as an appropriate pre-AF ablation study and two-thirds of centers participating in the AF Consensus Task Force routinely perform such imaging.[4,20]
Pre-procedure 3D angiograms are now commonly imported into standard electroanatomic mapping systems (EAM) to provide a real-time sense of catheter position in relation to a patient's specific LA and PV anatomy. The topic of "image integration" with EAM as has been nicely discussed in a number of recent reviews.[21–23] Early experiences suggested that image integration was qualitatively helpful for tailoring ablation to variant PV anatomy and for guiding lesion placement in areas where stable catheter positioning was difficult.[17,24] (Figure 1) Increased confidence in catheter manipulation is supported by randomized studies, which have reported that less fluoroscopy is needed when image integration is used.[25–28] However, the consensus of these studies is that image integration does not reduce AF recurrence following ablation. Though this likely reflects that AF ablation outcomes are limited by factors that are not addressed by use of detailed angiographic "road-maps", some of this lack of efficacy may be related to potential errors when registering the EAM space with pre-procedure 3D angiograms. Inaccurate registration can give a false impression of catheter/tissue contact, which affects the ability to reliably create permanent ablation lesions.[29]
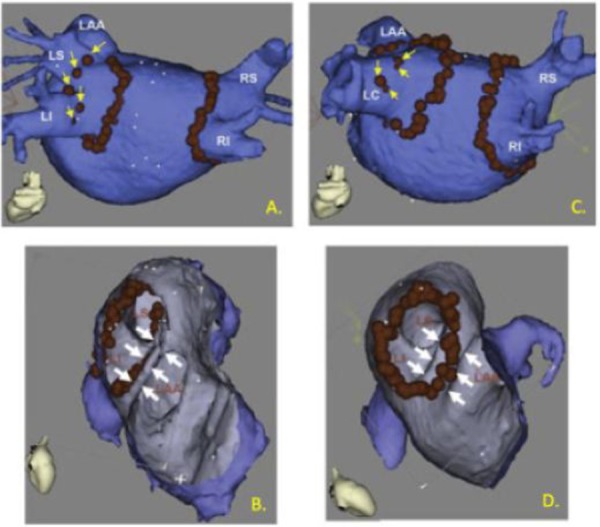
Figure 1. Anatomy tailored ablation by merging 3D angiogram information with electroanatomic mapping catheter guidance. Red dots indicate locations were ablation energy was delivered. A) A procedure where pulmonary vein isolation was achieved by circumferential ablation around the right and left pair of PVs followed by segmental ablation, encroaching closer to the veins, marked by yellow arrows. B) Ablation was guided to the PV side of a narrow left atrial appendage ridge, marked by white arrows. C) A patient with a left common PV and D) ablation could be directed onto a wider left atrial appendage ridge. (Adapted from Dong, et.al. Circulation 2006).
Intra-procedure imaging could reduce registration errors that result from changes in volume status, respiratory phase, cardiac rhythm, and patient position that can occur after pre-procedure imaging. Recently introduced C-arm CT, or rotational angiography, generates a 3D angiogram using the same fluoroscopy system used to perform the AF ablation procedure. This technology appears capable of accurately characterizing LA anatomy compared with conventional CT [30] and with a potentially lower radiation dose.[31] Because rotational angiography is inherently registered to the fluoroscopy system, these 3D angiograms are more readily overlaid on conventional fluoroscopic images.[32] This feature may provide more informed catheter positioning within the LA and PVs independent of EAM guidance. (Figure 2) Intracardiac echocardiography (ICE) is already commonly used intra-procedure imaging modality that has recently been applied to generate 3D anatomy for EAM guidance.[33] ICE depiction of 3D atrial anatomy is less detailed than that obtained by CT or MRI, but has the benefit of being inherently registered to the EAM catheter position when an ICE catheter with an EAM position sensor is used. A recent report described combining these two techniques to get the registration accuracy of ICE while retaining the anatomic detail of rotation angiography.[34] To date, the ability of these intra-procedure imaging modalities to improve AF ablation outcomes has not been demonstrated. However these techniques appear capable of at least improving procedure efficiency compared with pre-procedure image integration.[31–35]
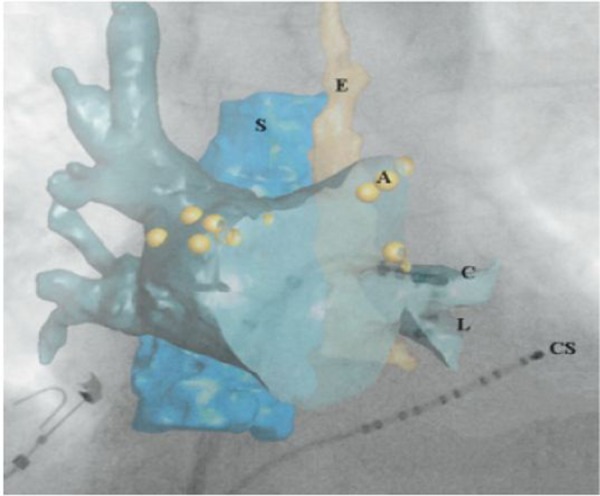
Figure 2. Illustrates how overlay of C-arm CT anatomy on top of conventional fluoroscopy may assist in catheter guidance. 3D structures of interest, in this case the left atrium, spine (S), and esophagus (E), were first segmented from the C-arm CT image and then displayed on top of the fluoroscopic image. Additional registration was not required because the C-arm CT was acquired with the same X-ray system as the live fluoroscopy. The underlying fluoroscopic image clearly depicts the ablation (C), lasso (L), and coronary sinus (CS) catheters, but contains minimal soft tissue detail. However, the location of the ablation catheter and lasso catheter within the left inferior PV is clear when anatomic structures from the C-arm CT are overlaid. In this study, ablation locations (A) could also be displayed with the C-arm CT data. (Adapted from Li, et.al. Heart Rhythm 2009).
Beyond 3D angiography, imaging atrial wall characteristics could provide additional information for guiding AF ablation. CT has demonstrated significant variations in atrial wall thickness between different regions of the left atrium, ranging from 0.5 to 3mm with significant inter-patient variability.[36] Patient specific variations in regional wall thickness might be useful for directing increasing ablation power in thicker regions, such as the appendage ridge, mitral isthmus, and superior roof, in order to increase the likelihood of transmural lesion formation. Conversely, power could be dedecreased in thinner regions, such as the superior posterior wall, to avoid complications such as perforation. MRI is capable of visualizing atrial tissue changes following ablation. In the future, such ablation lesion imaging could potentially guide additional ablation to fill in "gaps" between lesions. The current state of ablation lesion imaging will be discussed in a later section of this review. Imaging more detailed atrial tissue characteristics such as fibrosis might also be useful for guiding ablation strategy as will be discussed below.
Pre-procedure CT and MRI for Evaluating Arrhythmogenic Atrial Substrate and Ablation Patient Selection
AF requires not only a trigger but also an atrial substrate that is able to sustain the arrhythmia.[37] This understanding and the desire to improve AF ablation outcomes have motivated study of atrial substrate features that impact the success and failure of ablation procedures. Gross atrial volume and shape reflect some of aspects of pro-arrhythmic atrial substrate. For example, progressively larger atrial volume by CTA has been associated with incrementally higher odds of AF recurrence following ablation.[38] A more flattened atrial roof shape may also identify patients who require additional trigger ablation or substrate modification outside of PV isolation.[39] Imaging more detailed tissue characteristics such as atrial fibrosis and pericardial fat are also being investigated as more specific predictors of ablation outcome.
MRI shows promise as a more direct method of assessing the atrial fibrotic changes that parallel the progression of atrial fibrillation to more persistent forms.[40] Delayed gadolinium enhancement MRI (DEMRI) is based on the preferential retention of intravenous gadolinium contrast within myocardium that has disrupted vasculature.[41] Oakes, et.al. first reported the possibility of detecting atrial fibrosis using DEMRI.[42] They found that the extent of pre-ablation LA DEMRI enhancement was 3 to 4 times more predictive of response to drug therapy and AF ablation than LA volume. In this study, AF recurrence after ablation occurred in 75% of patients with extensive enhancement, 43% of those with moderate enhancement, and only 14% of those with mild enhancement. Follow-up studies by this group have supported this finding, leading to proposal of a "fibrosis" scoring system based on total amount of DEMRI atrial enhancement. (Figure 3) These studies reported no post-ablation AF recurrence in patients with very low(< 5%) enhancement but 56% to 96% recurrence in those with more than 35% enhancement.[43,44] This was despite 40% of patients with very low enhancement having persistent AF and a quarter of patients with more than 35% enhancement having paroxysmal AF. These results suggest the limitation of relying on standard paroxysmal and persistant clinical categories for predicting response to AF ablation. To date, more widespread use of atrial DEMRI and confirmation of these findings have been limited by technical challenges. Still, these studies suggest the potential of atrial substrate imaging to refine our understanding of AF in individual patients and to identify groups of patients for whom current ablation strategies have a high likelihood of success or failure.
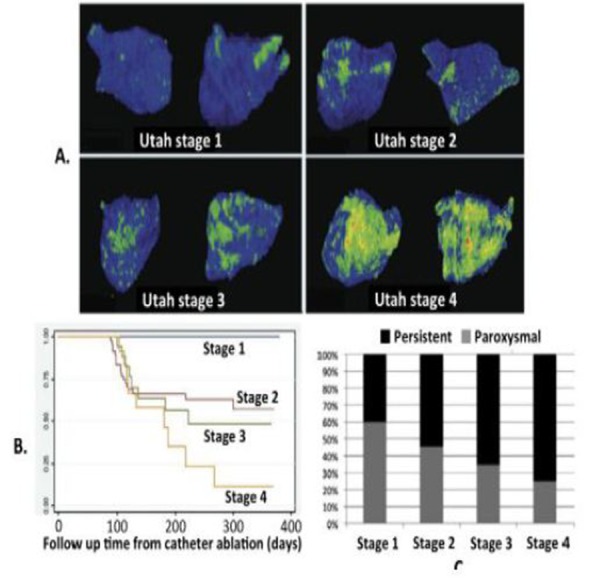
Figure 3. Visualization of left atrial “fibrosis” based on pre-ablation 3D DEMRI. A) Illustrates a scoring system based on quantity of DEMRI enhancement that is proposed to reflect low (Utah Stage 1) to high (Utah Stage 4) amounts of atrial “fibrosis”. Differential coloring was used to better distinguish contrast-enhanced atrium (green to red) from non-enhancing atrium (blue). B) Plots freedom from AF over days after ablation and shows that low, intermediate, and high amounts of enhancement differentiated patients with excellent to poor outcomes despite C) significant number of patients with minimal enhancement having persistent AF and with higher amounts of enhancement having paroxysmal AF. (Adapted from Akoum, et.al. JCEP 2011).
MRI and CT are also capable of localizing and quantifying pericardial fat, which is thought to play a role in local inflammation and could promote AF. Studies by Batal, et.al. and Thanassoulis, et.al. provided evidence that the presence and quantity of pericardial fat measured by CT was associated with increasingly persistent forms of AF despite adjustment for other clinical factors including non-pericardial fat deposits.[45,46] Most recently, using MRI, Wong, et.al. corroborated that pericardial fat volume was associated with the presence and chronicity of AF. They also found that pericardial fat deposits were predictive of AF recurrence after ablation whereas systemic measures of adiposity such as BMI were not.[47] Such studies will likely motivate further evaluation of the role pericardial fat plays in AF.
Post-procedure Imaging for Assessing Ablation Lesions
Using imaging to establish creation of permanent ablation lesions at the time of the procedure could address an important limitation of current ablation procedures. Recovery of PV conduction is typical in patients who undergo repeat AF ablation despite documentation of electrical pulmonary vein isolation (PVI) after previous procedures.[7,8,48,49] This is likely due to resolution of transient factors such as edema that acutely affect conduction but can resolve in the days to weeks following ablation.[50]
Among imaging modalities, MRI has the most established history for assessing lesion characteristics following cardiac ablation.[51–53] In 2007 Peters, et.al. introduced the concept of high-resolution atrial ablation lesion imaging following PVI using DEMRI.[54] McGann, et.al. expanded upon this technique and used a standardized workflow for 3D ablation lesion quantification and visualization.[55] They found that despite a uniform and extensive ablation protocol, there was significant patient-to-patient variation in post-ablation LA enhancement. They also found that a greater amount of post-ablation enhancement was associated with less AF recurrence after ablation. Looking more closely at the relationship between attempted ablation and actual lesion formation, Taclas et.al. reported that 20% of lesions they marked with EAM did not have evidence of associated DEMRI atrial enhancement.[56] (Figure 4) They also related more "gaps" in DEMRI enhancement to greater AF recurrence after ablation. Conversely, Badger, et.al. found that achieving circumferential DEMRI enhancement around all pulmonary veins was difficult and only seen in 7% of patients after initial ablation.[57] However, all patients who achieved circumferential enhancement of all veins were free from AF at one year. They also noted that in the 18 patients undergoing repeat ablation, recovery of PV conduction was only seen in veins with non-circumferential ablation. Akoum, et.al. recently refined these findings by combining pre-procedure DEMRI fibrosis assessment and post-procedure DEMRI lesion assessment.44 After PVI plus extensive substrate ablation, they found that achieving circumferential DEMRI enhancement around the PVs predicted procedural success only in patients with relatively less DEMRI atrial fibrosis. However, in patients with more atrial fibrosis only overall post-ablation atrial enhancement, including the posterior wall and septum, was predictive of success. This study suggests that imaging detailed atrial tissue characteristics may be useful for identifying patients who are likely to require an ablation strategy beyond PVI.
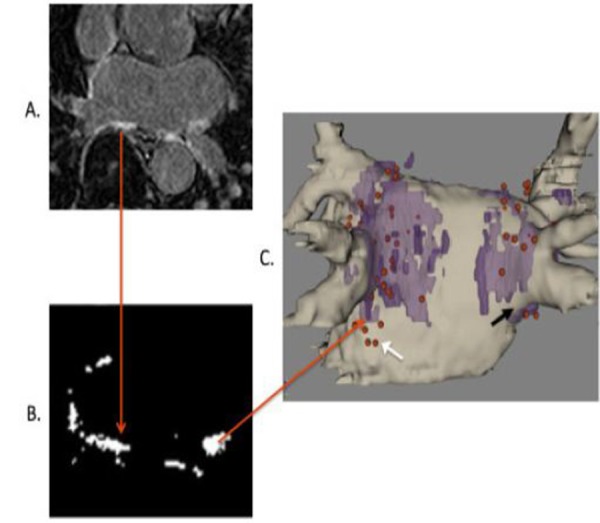
Figure 4. Atrial ablation lesion visualization using 3D DEMRI. A) A 2D DEMRI slice through the left atrium. The red arrow end marks an area of post-ablation enhancement. B) Segmenting enhanced regions isolates ablated areas from the rest of the image. The red arrow tip marks one area of ablation. C) Segmented “lesions” from the whole 3D image (colored purple) can be overlaid on the 3D angiogram (colored tan) to clarify the distribution of left atrial ablation lesions. Red dots indicate areas marked by the EAM mapping system where ablation energy was delivered. The black arrow illustrates a region of incomplete ablation below the right inferior PV. The white arrow illustrates an area of ineffective ablation where RF energy was delivered but no resulting ablation lesion was seen. (Adapted from Taclas, et.al. Heart Rhythm 2010).
One limitation of the studies above is that lesion imaging was performed weeks after ablation. Further investigation is required to identify permanent lesion characteristics at the time of the procedure when additional ablation could be targeted to lesion "gaps". Badger, et.al reported that the amount of DEMRI enhancement seen within 24 hours of ablation was significantly greater than the enhancement seen at 3 and 6 months after ablation.[58] This reflects that early post-ablation, gadolinium appears to be retained in areas of transient atrial injury that do not correlate well with eventual atrial scarring. A recent follow-up study by McGann et.al suggested that, non-enhancing "dark" regions on DEMRI might better reflect regions that will eventually form durable ablation lesions.[59]
Even if permanent ablation lesion characteristics can be visualized early post-ablation, the difficulty of transporting patients between the EP lab and MRI scanner limits the practicality of using DEMRI lesion imaging for guiding ablation. This limitation is one motivation for research into performing ablation procedures within the MRI scanner using real-time MRI guidance.[51,60–62] Lesion visualization using other modalities that may be more amenable to intra-procedure integration is also under investigation. Recently, animal studies using C-arm CT have demonstrated the feasibility of RF ablation lesion imaging in the ventricle.[63] However, reductions of radiation and contrast dose are needed for clinical use and the ability to visualize atrial lesions remains to be demonstrated. ICE methods for cardiac ablation lesion imaging have also been studied.[64,65] Though ICE currently has limited clinical utility for reliably imaging ablation lesions, new methods are in early stages of investigation.[64,66,67]
CT and MRI for Avoiding and Evaluating Complications
In a recent worldwide survey, the risk of significant complications following AF ablation was reported to be around 4.5%.[6] These complications include stroke, cardiac tamponade, pulmonary vein stenosis, and esophageal and phrenic nerve injury. Appreciation of the left atrial structure and relationship to surrounding anatomy is important to minimize mechanical and ablation related complications. Anatomic considerations of relevance to transseptal puncture include the relationship of the foramen ovale to the aorta, left atrial appendage, and posterior, superior, and lateral atrial walls. The risk of catheter-mediated perforation can be mitigated by additional localization of the PV junctions and knowledge of regional variations in atrial thickness. Ablation injury of adjacent structures can be minimized by understanding the anatomic relationships of these structures such as the course of the right phrenic nerve between the right superior PV and superior vena cava/right atrium, the proximity of the left circumflex coronary artery to the mitral isthmus and coronary sinus, and the proximity of the bronchi to the atrial roof. CT and MRI are useful for depicting these anatomic relationships and as previously discussed can be combined with EAM catheter tracking systems to assist procedure guidance. As reviewed next, CT and MRI have also been specifically evaluated for the prevention of stroke and post-ablation evaluation of pulmonary vein stenosis, esophageal injury, and reduction of atrial function.
Stroke can be a debilitating complication of AF ablation and left atrial thrombus is an absolute contraindication for the procedure. Though TEE remains the standard for excluding left atrial appendage thrombus, an ability to combine thrombus evaluation with pre-procedure CT or MRI could avoid need for an additional study. The largest study on this subject included 402 patients and suggested that absence of an appendage filling defect on CT angiogram may be sufficient to exclude thrombus in patients with a low CHADS2 score.[68,69] Though MRI is excellent for detecting ventricular thrombus, it has been less studied for evaluating atrial thrombus. There is some support that a negative non-contrast MRI may exclude atrial appendage thrombus.[70] However, conflicting data from contrast MRI studies suggest that further investigation is needed.[71] Beyond exclusion of existing atrial thrombus, imaging could have a broader role in defining an individual's stroke risk. A recent report found that patients with prior stroke had significantly more atrial "fibrosis" by DEMRI than those without prior stroke and suggested that LA fibrosis was associated with stroke independent of CHADS2 risk factors.[72] If confirmed to be prospective predictors of stroke risk, such imaging measures of atrial remodeling may be useful for guiding the need for long-term anticoagulation.
Pulmonary vein stenosis is a well-recognized complication of AF ablation that results from ablation within or near the PVs. CT and MRI are the preferred modalities for assessing this complication and grade stenosis as mild (< 50%), moderate (50-70%), and severe (> 70%).[4] The risk of PV stenosis decreases with ablation further outside the PVs, but was reported as high as 42% after focal ablation of triggers within the PVs.[73] Dill, et.al. reported a lower risk around 18% following segmental PV isolation, but that the risk of stenosis increases when ablation is delivered to increasingly circumferential regions of the PV ostia.[74] With contemporary antral PV isolation, one group reported around a 3% risk of moderate stenosis and 1% risk of severe stenosis.[75] Still, some advocate screening for PV stenosis three months following ablation because many patients with severe stenosis remain asymptomatic.[75] This is relevant because stenosis can progress over time and the window for intervention may be lost if total PV occlusion occurs.[76] Though most groups do not routinely perform PV stenosis screening, it has been recommended that centers beginning to perform AF ablation or those beginning to use new ablation modalities consider screening for quality control purposes.[4]
Atrial-esophageal fistula is a rare but often fatal complication of AF ablation that can result from ablation along the posterior LA. CT and MRI are the standard diagnostic modalities for assessing this condition.[77] Because esophageal injury has potentially devastating consequences, there has been recent interest in early detection that could prompt earlier intervention. Badger, et.al. proposed that DEMRI might be able to identify esophageal injury after ablation.[78] However a follow-up study suggested that esophageal enhancement at 24 hours did not correlate with EGD evidence of esophageal injury and that MRI appeared overly sensitive.[79] The primary strategy for preventing this serious complication is avoidance. Pre-procedure CT and MRI have documented the nearly ubiquitous contact of the esophagus with the posterior LA, significant variability in esophageal course relative to the left and right PVs, and that the esophagus is often compressed and contact can span most of the posterior LA wall.[80] However, because the esophagus can shift many centimeters from the time of pre-procedural imaging,[81] more "real-time" measures of esophageal position such as esophageal temperature probe monitoring, ICE, or potentially C-arm CT are recommended.[4,30]
The shift to ablating larger regions of the atrium, particularly in cases of persistent atrial fibrillation, raises concern for adversely impacting atrial structure and function. Using post-ablation DEMRI to quantify LA "scarring" after ablation, Wylie, et.al. found a linear correlation between increasing amount of ablation and decreasing atrial ejection fraction (EF).[82] Though a recent meta-analysis reported overall improvement in atrial function following successful ablation[83], a subsequent CT angiography study by Masuda, et.al. refined this finding.[84] This study found that while post-ablation LA volume and EF improved in patients with larger baseline LA volume and poorer baseline EF, atrial function worsened in those with more preserved baseline atrial function. Gibson et.al. also recently described a "stiff left atrial syndrome" characterized by atrial diastolic dysfunction and worsening pulmonary hypertension in the absence of PV stenosis.[85] This syndrome was noted after AF ablation in 1.4% of over a thousand patients. Severe pre-ablation atrial scarring, along with diabetes and sleep apnea, were risk factors for this condition. Though this study used invasive mapping to identify pre-ablation atrial scar, MRI might be useful for non-invasive, pre-procedure characterization of atrial scarring as discussed above.
Additional CT and MRI Considerations
Though in current use CT and MRI are often interchangeable, these modalities have some relative advantages and disadvantages. CT has the advantage of short imaging times and high spatial resolution. Current CT systems are capable of generating 3D angiograms with 0.5x0.5x0.5mm resolution within around 10 heart beats for 64 detector systems and within one heart beat for 320 detector systems.[86] In contrast, a 20 to 30 second breath hold is typically required for 3D MRA with a resolution around 1.5x1.5x1.5mm. More significantly, much longer imaging times of several minutes are currently required for the "fibrosis" and ablation lesion DEMRI studies discussed above. Reliably mitigating the effects of patient and physiologic motion over these longer imaging times remains a challenge. Improving the speed and motion sensitivity of MRI is an area of active investigation that has seen a number of promising recent developments.[87–89]
An advantage of MRI over CT is that it does not require ionizing radiation. Minimizing radiation exposure is a consideration in the AF ablation population because prolonged fluoroscopy and repeat ablation procedures can result in significant cumulative radiation dose. MRI also has the advantage of more flexible soft tissue contrast compared with CT. This has lead to the more rapid advances in MRI of atrial substrate and ablation lesions that have been discussed in this review.
The use of both CT and MRI is limited in patients with significant renal dysfunction. The incidence and severity of renal dysfunction with iodine-based CT contrast agents markedly increases in patients with GFR < 30 ml/min.[90] Gadolinium MRI contrast has been associated with a very rare but serious condition, nephrogenic systemic fibrosis (NSF). The occurrence of NSF has been limited to patients with GFR < 30 ml/min and typically dialysis dependent.[91] Non-contrast MRA has been proposed for left atrial and PV imaging in such patients.[88]
Conclusions
Pre-procedure CT and MRI are widely accepted for ablation planning to delineate the complex and variable anatomy of the left atrium, PVs, and surrounding structures. The information from pre-procedure 3D angiography is commonly incorporated into electroanatomic mapping systems to guide ablation lesion placement and can reduce need for fluoroscopic radiation exposure. Post-procedure imaging using these modalities has also proven useful for evaluating complications such as PV stenosis, esophageal injury, and reduced atrial function. In the future, incorporating MRI or CT information about tissue characteristics such as regional fibrosis, pericardial fat, and local wall thickness may provide additional information for selecting patients for different ablation strategies or for guiding ablation. A major limitation of current ablation procedures is an inability to consistently create permanent, transmural ablation lesions. Ablation lesion imaging is a current focus of research toward the goal of detecting incomplete regions of ablation in order to guide additional lesion placement. Together with advances in real-time imaging modalities such as ICE and MRI, alternate ablation delivery systems, and better understanding of AF pathophysiology, ablation promises to evolve into a more uniformly successful treatment for atrial fibrillation.
Disclosures
No disclosures relevant to this article were made by the author.
References
- 1.Haïssaguerre M, Shah D C, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102 (20):2463–5. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 3.Robbins I M, Colvin E V, Doyle T P, Kemp W E, Loyd J E, McMahon W S, Kay G N. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998 Oct 27;98 (17):1769–75. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 4.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991 Aug;22 (8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 6.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang Feifan, Antz Matthias, Ernst Sabine, Hachiya Hitoshi, Mavrakis Hercules, Deger Florian T, Schaumann Anselm, Chun Julian, Falk Peter, Hennig Detlef, Liu Xingpeng, Bänsch Dietmar, Kuck Karl-Heinz. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005 Jan 18;111 (2):127–35. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 8.Verma Atul, Kilicaslan Fethi, Pisano Ennio, Marrouche Nassir F, Fanelli Raffaele, Brachmann Johannes, Geunther Jens, Potenza Domenico, Martin David O, Cummings Jennifer, Burkhardt J David, Saliba Walid, Schweikert Robert A, Natale Andrea. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005 Aug 02;112 (5):627–35. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 9.Wood Mark A, Wittkamp Michael, Henry Daniel, Martin Robert, Nixon J V, Shepard Richard K, Ellenbogen Kenneth A. A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am. J. Cardiol. 2004 Jan 01;93 (1):49–53. doi: 10.1016/j.amjcard.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Hamdan Ashraf, Charalampos Kriatselis, Roettgen Rainer, Wellnhofer Ernst, Gebker Rolf, Paetsch Ingo, Jahnke Cosima, Schnackenburg Bernhard, Tang Min, Gerds-Li Hong, Fleck Eckart. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am. J. Cardiol. 2009 Dec 01;104 (11):1540–6. doi: 10.1016/j.amjcard.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Kato Ritsushi, Lickfett Lars, Meininger Glenn, Dickfeld Timm, Wu Richard, Juang George, Angkeow Piamsook, LaCorte Jennifer, Bluemke David, Berger Ronald, Halperin Henry R, Calkins Hugh. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003 Apr 22;107 (15):2004–10. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- 12.Lickfett Lars, Kato Ritsushi, Tandri Harikrishna, Jayam Vinod, Vasamreddy Chandrasekhar R, Dickfeld Timm, Lewalter Thorsten, Luderitz Berndt, Berger Ronald, Halperin Henry, Calkins Hugh. Characterization of a new pulmonary vein variant using magnetic resonance angiography: incidence, imaging, and interventional implications of the "right top pulmonary vein". J. Cardiovasc. Electrophysiol. 2004 May;15 (5):538–43. doi: 10.1046/j.1540-8167.2004.03499.x. [DOI] [PubMed] [Google Scholar]
- 13.Mansour Moussa, Holmvang Godtfred, Sosnovik David, Migrino Raymond, Abbara Suhny, Ruskin Jeremy, Keane David. Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004 Apr;15 (4):387–93. doi: 10.1046/j.1540-8167.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman David, Lacomis Joan, Wigginton William G. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J. Am. Coll. Cardiol. 2003 Apr 16;41 (8):1349–57. doi: 10.1016/s0735-1097(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsao H M, Wu M H, Yu W C, Tai C T, Lin Y K, Hsieh M H, Ding Y A, Chang M S, Chen S A. Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2001 Dec;12 (12):1353–7. doi: 10.1046/j.1540-8167.2001.01353.x. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Lugones Alejandro, Schvartzman Paulo R, Schweikert Robert, Tchou Patrick J, Saliba Walid, Marrouche Nassir F, Castle Lon W, White Richard D, Natale Andrea. Three-dimensional reconstruction of pulmonary veins in patients with atrial fibrillation and controls: morphological characteristics of different veins. Pacing Clin Electrophysiol. 2003 Jan;26 (1 Pt 1):8–15. doi: 10.1046/j.1460-9592.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 17.Mansour Moussa, Refaat Marwan, Heist Edwin Kevin, Mela Theofanie, Cury Ricardo, Holmvang Godtfred, Ruskin Jeremy N. Three-dimensional anatomy of the left atrium by magnetic resonance angiography: implications for catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Jul;17 (7):719–23. doi: 10.1111/j.1540-8167.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 18.Scharf Christoph, Sneider Michael, Case Ian, Chugh Aman, Lai Steve W K, Pelosi Frank, Knight Bradley P, Kazerooni Ella, Morady Fred, Oral Hakan. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J. Cardiovasc. Electrophysiol. 2003 Feb;14 (2):150–5. doi: 10.1046/j.1540-8167.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 19.Hunter Ross J, Ginks Matthew, Ang Richard, Diab Ihab, Goromonzi Farai C, Page Stephen, Baker Victoria, Richmond Laura, Tayebjee Muzahir, Sporton Simon, Earley Mark J, Schilling Richard J. Impact of variant pulmonary vein anatomy and image integration on long-term outcome after catheter ablation for atrial fibrillation. Europace. 2010 Dec;12 (12):1691–7. doi: 10.1093/europace/euq322. [DOI] [PubMed] [Google Scholar]
- 20.Hendel Robert C, Patel Manesh R, Kramer Christopher M, Poon Michael, Hendel Robert C, Carr James C, Gerstad Nancy A, Gillam Linda D, Hodgson John McB, Kim Raymond J, Kramer Christopher M, Lesser John R, Martin Edward T, Messer Joseph V, Redberg Rita F, Rubin Geoffrey D, Rumsfeld John S, Taylor Allen J, Weigold Wm Guy, Woodard Pamela K, Brindis Ralph G, Hendel Robert C, Douglas Pamela S, Peterson Eric D, Wolk Michael J, Allen Joseph M, Patel Manesh R. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J. Am. Coll. Cardiol. 2006 Oct 03;48 (7):1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.H. Oral, F. Morady. “Integrative Approaches to Imaging: Fluoroscopy, CT, MRI, Echocardiography, and Three-Dimensional Electroanatomical and Noncontact Mapping,”. in Contemporary Cardiology: Atrial Fibrillation, From Bench to Bedside, A. Natale and J. Jalife, Eds., 1 ed Totowa, NJ: Humana Press. 2008;0:349–362. [Google Scholar]
- 22.Ponti Roberto De, Marazzi Raffaella, Lumia Domenico, Picciolo Giuseppe, Biddau Roberto, Fugazzola Carlo, Salerno-Uriarte Jorge A. Role of three-dimensional imaging integration in atrial fibrillation ablation. World J Cardiol. 2010 Aug 26;2 (8):215–22. doi: 10.4330/wjc.v2.i8.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govil Ashul, Calkins Hugh, Spragg David D. Fusion of imaging technologies: how, when, and for whom? J Interv Card Electrophysiol. 2011 Dec;32 (3):195–203. doi: 10.1007/s10840-011-9616-7. [DOI] [PubMed] [Google Scholar]
- 24.Dong Jun, Dickfeld Timm, Dalal Darshan, Cheema Aamir, Vasamreddy Chandrasekhar R, Henrikson Charles A, Marine Joseph E, Halperin Henry R, Berger Ronald D, Lima Joao A C, Bluemke David A, Calkins Hugh. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 May;17 (5):459–66. doi: 10.1111/j.1540-8167.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 25.Kistler Peter M, Rajappan Kim, Harris Stuart, Earley Mark J, Richmond Laura, Sporton Simon C, Schilling Richard J. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. Eur. Heart J. 2008 Dec;29 (24):3029–36. doi: 10.1093/eurheartj/ehn453. [DOI] [PubMed] [Google Scholar]
- 26.Tang Kai, Ma Jian, Zhang Shu, Zhang Jing-ying, Wei Yi-dong, Chen Yan-qing, Yu Xue-jing, Xu Ya-wei. A randomized prospective comparison of CartoMerge and CartoXP to guide circumferential pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Chin. Med. J. 2008 Mar 20;121 (6):508–12. [PubMed] [Google Scholar]
- 27.Della Bella Paolo, Fassini Gaetano, Cireddu Manuela, Riva Stefania, Carbucicchio Corrado, Giraldi Francesco, Maccabelli Giuseppe, Trevisi Nicola, Moltrasio Massimo, Pepi Mauro, Galli Claudia A, Andreini Daniele, Ballerini Giovanni, Pontone Gianluca. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J. Cardiovasc. Electrophysiol. 2009 Mar;20 (3):258–65. doi: 10.1111/j.1540-8167.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- 28.Caponi Domenico, Corleto Antonella, Scaglione Marco, Blandino Alessandro, Biasco Luigi, Cristoforetti Yvonne, Cerrato Natascia, Toso Elisabetta, Morello Mara, Gaita Fiorenzo. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: a randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010 Aug;12 (8):1098–104. doi: 10.1093/europace/euq107. [DOI] [PubMed] [Google Scholar]
- 29.Wittkampf Fred H M, Nakagawa Hiroshi. RF catheter ablation: Lessons on lesions. Pacing Clin Electrophysiol. 2006 Nov;29 (11):1285–97. doi: 10.1111/j.1540-8159.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 30.Nölker Georg, Gutleben Klaus Jürgen, Marschang Harald, Ritscher Guido, Asbach Stefan, Marrouche Nassir, Brachmann Johannes, Sinha Anil Martin. Three-dimensional left atrial and esophagus reconstruction using cardiac C-arm computed tomography with image integration into fluoroscopic views for ablation of atrial fibrillation: accuracy of a novel modality in comparison with multislice computed tomography. Heart Rhythm. 2008 Dec;5 (12):1651–7. doi: 10.1016/j.hrthm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Kriatselis Charalampos, Nedios Sotirios, Akrivakis Spyridon, Tang Min, Roser Mattias, Gerds-Li Jin-Hong, Fleck Eckart, Orlov Michael. Intraprocedural imaging of left atrium and pulmonary veins: a comparison study between rotational angiography and cardiac computed tomography. Pacing Clin Electrophysiol. 2011 Mar;34 (3):315–22. doi: 10.1111/j.1540-8159.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 32.Li Jonathan H, Haim Moti, Movassaghi Babak, Mendel Jeffrey B, Chaudhry G Muqtada, Haffajee Charles I, Orlov Michael V. Segmentation and registration of three-dimensional rotational angiogram on live fluoroscopy to guide atrial fibrillation ablation: a new online imaging tool. Heart Rhythm. 2009 Feb;6 (2):231–7. doi: 10.1016/j.hrthm.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Banchs Javier E, Patel Parag, Naccarelli Gerald V, Gonzalez Mario D. Intracardiac echocardiography in complex cardiac catheter ablation procedures. J Interv Card Electrophysiol. 2010 Sep;28 (3):167–84. doi: 10.1007/s10840-010-9474-8. [DOI] [PubMed] [Google Scholar]
- 34.Nölker Georg, Gutleben Klaus-Jürgen, Asbach Stefan, Vogt Jürgen, Heintze Johannes, Brachmann Johannes, Horstkotte Dieter, Sinha Anil M. Intracardiac echocardiography for registration of rotational angiography-based left atrial reconstructions: a novel approach integrating two intraprocedural three-dimensional imaging techniques in atrial fibrillation ablation. Europace. 2011 Apr;13 (4):492–8. doi: 10.1093/europace/eur003. [DOI] [PubMed] [Google Scholar]
- 35.Pratola Claudio, Baldo Elisa, Artale Paolo, Marcantoni Lina, Toselli Tiziano, Percoco Gianfranco, Sassone Biagio, Ferrari Roberto. Different image integration modalities to guide AF ablation: impact on procedural and fluoroscopy times. Pacing Clin Electrophysiol. 2011 Apr;34 (4):422–30. doi: 10.1111/j.1540-8159.2010.02989.x. [DOI] [PubMed] [Google Scholar]
- 36.Beinart Roy, Abbara Suhny, Blum Andrew, Ferencik Maros, Heist Kevin, Ruskin Jeremy, Mansour Moussa. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2011 Nov;22 (11):1232–6. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki Yu-ki, Nishida Kunihiro, Kato Takeshi, Nattel Stanley. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011 Nov 15;124 (20):2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 38.Hof Irene, Chilukuri Karuna, Arbab-Zadeh Armin, Scherr Daniel, Dalal Darshan, Nazarian Saman, Henrikson Charles, Spragg David, Berger Ronald, Marine Joseph, Calkins Hugh. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J. Cardiovasc. Electrophysiol. 2009 Sep;20 (9):1005–10. doi: 10.1111/j.1540-8167.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 39.Burstein Brett, Nattel Stanley. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 40.Burstein Brett, Nattel Stanley. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 41.Ordovas Karen G, Higgins Charles B. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology. 2011 Nov;261 (2):358–74. doi: 10.1148/radiol.11091882. [DOI] [PubMed] [Google Scholar]
- 42.Oakes Robert S, Badger Troy J, Kholmovski Eugene G, Akoum Nazem, Burgon Nathan S, Fish Eric N, Blauer Joshua J E, Rao Swati N, DiBella Edward V R, Segerson Nathan M, Daccarett Marcos, Windfelder Jessiciah, McGann Christopher J, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009 Apr 07;119 (13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahnkopf Christian, Badger Troy J, Burgon Nathan S, Daccarett Marcos, Haslam Thomas S, Badger Christopher T, McGann Christopher J, Akoum Nazem, Kholmovski Eugene, Macleod Rob S, Marrouche Nassir F. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm. 2010 Oct;7 (10):1475–81. doi: 10.1016/j.hrthm.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akoum Nazem, Daccarett Marcos, McGann Chris, Segerson Nathan, Vergara Gaston, Kuppahally Suman, Badger Troy, Burgon Nathan, Haslam Thomas, Kholmovski Eugene, Macleod Rob, Marrouche Nassir. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 2011 Jan;22 (1):16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batal Omar, Schoenhagen Paul, Shao Mingyuan, Ayyad Ala Eddin, Van Wagoner David R, Halliburton Sandra S, Tchou Patrick J, Chung Mina K. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):230–6. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanassoulis George, Massaro Joseph M, O'Donnell Christopher J, Hoffmann Udo, Levy Daniel, Ellinor Patrick T, Wang Thomas J, Schnabel Renate B, Vasan Ramachandran S, Fox Caroline S, Benjamin Emelia J. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010 Aug;3 (4):345–50. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong Christopher X, Abed Hany S, Molaee Payman, Nelson Adam J, Brooks Anthony G, Sharma Gautam, Leong Darryl P, Lau Dennis H, Middeldorp Melissa E, Roberts-Thomson Kurt C, Wittert Gary A, Abhayaratna Walter P, Worthley Stephen G, Sanders Prashanthan. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J. Am. Coll. Cardiol. 2011 Apr 26;57 (17):1745–51. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 48.Weerasooriya Rukshen, Khairy Paul, Litalien Jean, Macle Laurent, Hocini Meleze, Sacher Frederic, Lellouche Nicolas, Knecht Sebastien, Wright Matthew, Nault Isabelle, Miyazaki Shinsuke, Scavee Christophe, Clementy Jacques, Haissaguerre Michel, Jais Pierre. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J. Am. Coll. Cardiol. 2011 Jan 11;57 (2):160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 49.Hussein Ayman A, Saliba Walid I, Martin David O, Bhargava Mandeep, Sherman Minerva, Magnelli-Reyes Christina, Chamsi-Pasha Mohammed, John Seby, Williams-Adrews Michelle, Baranowski Bryan, Dresing Thomas, Callahan Thomas, Kanj Mohamed, Tchou Patrick, Lindsay Bruce D, Natale Andrea, Wazni Oussama. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):271–8. doi: 10.1161/CIRCEP.111.962100. [DOI] [PubMed] [Google Scholar]
- 50.Ranjan Ravi, Kato Ritsushi, Zviman Menekhem M, Dickfeld Timm M, Roguin Ariel, Berger Ronald D, Tomaselli Gordon F, Halperin Henry R. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):279–86. doi: 10.1161/CIRCEP.110.960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lardo A C, McVeigh E R, Jumrussirikul P, Berger R D, Calkins H, Lima J, Halperin H R. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000 Aug 08;102 (6):698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- 52.Dickfeld Timm, Kato Ritsushi, Zviman Menekhem, Lai Shenghan, Meininger Glenn, Lardo Albert C, Roguin Ariel, Blumke David, Berger Ronald, Calkins Hugh, Halperin Henry. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2006 Jan 17;47 (2):370–8. doi: 10.1016/j.jacc.2005.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickfeld Timm, Kato Ritsushi, Zviman Menekem, Nazarian Saman, Dong Jun, Ashikaga Hiroshi, Lardo Albert C, Berger Ronald D, Calkins Hugh, Halperin Henry. Characterization of acute and subacute radiofrequency ablation lesions with nonenhanced magnetic resonance imaging. Heart Rhythm. 2007 Feb;4 (2):208–14. doi: 10.1016/j.hrthm.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters Dana C, Wylie John V, Hauser Thomas H, Kissinger Kraig V, Botnar René M, Essebag Vidal, Josephson Mark E, Manning Warren J. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007 Jun;243 (3):690–5. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 55.McGann Christopher J, Kholmovski Eugene G, Oakes Robert S, Blauer Joshua J E, Daccarett Marcos, Segerson Nathan, Airey Kelly J, Akoum Nazem, Fish Eric, Badger Troy J, DiBella Edward V R, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2008 Oct 07;52 (15):1263–71. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 56.Taclas Jason E, Nezafat Reza, Wylie John V, Josephson Mark E, Hsing Jeff, Manning Warren J, Peters Dana C. Relationship between intended sites of RF ablation and post-procedural scar in AF patients, using late gadolinium enhancement cardiovascular magnetic resonance. Heart Rhythm. 2010 Apr;7 (4):489–96. doi: 10.1016/j.hrthm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badger Troy J, Daccarett Marcos, Akoum Nazem W, Adjei-Poku Yaw A, Burgon Nathan S, Haslam Thomas S, Kalvaitis Saul, Kuppahally Suman, Vergara Gaston, McMullen Lori, Anderson Paul A, Kholmovski Eugene, MacLeod Rob S, Marrouche Nassir F. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):249–59. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badger Troy J, Oakes Robert S, Daccarett Marcos, Burgon Nathan S, Akoum Nazem, Fish Eric N, Blauer Joshua J E, Rao Swati N, Adjei-Poku Yaw, Kholmovski Eugene G, Vijayakumar Sathya, Di Bella Edward V R, MacLeod Rob S, Marrouche Nassir F. Temporal left atrial lesion formation after ablation of atrial fibrillation. Heart Rhythm. 2009 Feb;6 (2):161–8. doi: 10.1016/j.hrthm.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 59.McGann Christopher, Kholmovski Eugene, Blauer Joshua, Vijayakumar Sathya, Haslam Thomas, Cates Joshua, DiBella Edward, Burgon Nathan, Wilson Brent, Alexander Alton, Prastawa Marcel, Daccarett Marcos, Vergara Gaston, Akoum Nazem, Parker Dennis, MacLeod Rob, Marrouche Nassir. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J. Am. Coll. Cardiol. 2011 Jul 05;58 (2):177–85. doi: 10.1016/j.jacc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vergara Gaston R, Vijayakumar Sathya, Kholmovski Eugene G, Blauer Joshua J E, Guttman Mike A, Gloschat Christopher, Payne Gene, Vij Kamal, Akoum Nazem W, Daccarett Marcos, McGann Christopher J, Macleod Rob S, Marrouche Nassir F. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm. 2011 Feb;8 (2):295–303. doi: 10.1016/j.hrthm.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nordbeck Peter, Hiller Karl-Heinz, Fidler Florian, Warmuth Marcus, Burkard Natalie, Nahrendorf Matthias, Jakob Peter M, Quick Harald H, Ertl Georg, Bauer Wolfgang R, Ritter Oliver. Feasibility of contrast-enhanced and nonenhanced MRI for intraprocedural and postprocedural lesion visualization in interventional electrophysiology: animal studies and early delineation of isthmus ablation lesions in patients with typical atrial flutter. Circ Cardiovasc Imaging. 2011 May;4 (3):282–94. doi: 10.1161/CIRCIMAGING.110.957670. [DOI] [PubMed] [Google Scholar]
- 62.Kolandaivelu Aravindan, Zviman Menekhem M, Castro Valeria, Lardo Albert C, Berger Ronald D, Halperin Henry R. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using MRI thermography. Circ Arrhythm Electrophysiol. 2010 Oct;3 (5):521–9. doi: 10.1161/CIRCEP.110.942433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard Erin E, Al-Ahmad Amin, Rosenberg Jarrett, Luong Richard, Moore Teri, Lauritsch Günter, Boese Jan, Fahrig Rebecca. Contrast-enhanced C-arm CT evaluation of radiofrequency ablation lesions in the left ventricle. JACC Cardiovasc Imaging. 2011 Mar;4 (3):259–68. doi: 10.1016/j.jcmg.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren J F, Callans D J, Schwartzman D, Michele J J, Marchlinski F E. Changes in local wall thickness correlate with pathologic lesion size following radiofrequency catheter ablation: an intracardiac echocardiographic imaging study. Echocardiography. 2001 Aug;18 (6):503–7. doi: 10.1046/j.1540-8175.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 65.Szili-Torok Tamas, Kimman Geert-Jan, Scholten Marcoen, Thornton Andrew, Ten Cate Folkert, Roelandt Jos, Jordaens Luc. Ablation lesions in Koch's triangle assessed by three-dimensional myocardial contrast echocardiography. Cardiovasc Ultrasound. 2004 Dec 09;2 () doi: 10.1186/1476-7120-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khoury Dirar S, Rao Liyun, Ding Chuxiong, Sun Huabin, Youker Keith A, Panescu Dorin, Nagueh Sherif F. Localizing and quantifying ablation lesions in the left ventricle by myocardial contrast echocardiography. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1078–87. doi: 10.1046/j.1540-8167.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu Dalong, Ebbini Emad S. Real-time 2-D temperature imaging using ultrasound. IEEE Trans Biomed Eng. 2010 Jan;57 (1):12–6. doi: 10.1109/TBME.2009.2035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez Matthew W, Kirsch Jacobo, Williamson Eric E, Syed Imran S, Feng DaLi, Ommen Steve, Packer Douglas L, Brady Peter A. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009 Jan;2 (1):69–76. doi: 10.1016/j.jcmg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Garcia Mario J. Detection of left atrial appendage thrombus by cardiac computed tomography: a word of caution. JACC Cardiovasc Imaging. 2009 Jan;2 (1):77–9. doi: 10.1016/j.jcmg.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Ohyama Hideo, Hosomi Naohisa, Takahashi Tsutomu, Mizushige Katsufumi, Osaka Kunihiko, Kohno Masakazu, Koziol James A. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke. 2003 Oct;34 (10):2436–9. doi: 10.1161/01.STR.0000090350.73614.0F. [DOI] [PubMed] [Google Scholar]
- 71.Mohrs Oliver K, Nowak Bernd, Petersen Steffen E, Welsner Matthias, Rubel Christine, Magedanz Annett, Kauczor Hans-Ulrich, Voigtlaender Thomas. Thrombus detection in the left atrial appendage using contrast-enhanced MRI: a pilot study. AJR Am J Roentgenol. 2006 Jan;186 (1):198–205. doi: 10.2214/AJR.04.1504. [DOI] [PubMed] [Google Scholar]
- 72.Daccarett Marcos, Badger Troy J, Akoum Nazem, Burgon Nathan S, Mahnkopf Christian, Vergara Gaston, Kholmovski Eugene, McGann Christopher J, Parker Dennis, Brachmann Johannes, Macleod Rob S, Marrouche Nassir F. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2011 Feb 15;57 (7):831–8. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen S A, Hsieh M H, Tai C T, Tsai C F, Prakash V S, Yu W C, Hsu T L, Ding Y A, Chang M S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999 Nov 02;100 (18):1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 74.Dill Thorsten, Neumann Thomas, Ekinci Okan, Breidenbach Christiane, John Anna, Erdogan Ali, Bachmann Georg, Hamm Christian W, Pitschner Heinz-F. Pulmonary vein diameter reduction after radiofrequency catheter ablation for paroxysmal atrial fibrillation evaluated by contrast-enhanced three-dimensional magnetic resonance imaging. Circulation. 2003 Feb 18;107 (6):845–50. doi: 10.1161/01.cir.0000048146.81336.1d. [DOI] [PubMed] [Google Scholar]
- 75.Baranowski Bryan, Saliba Walid. Our approach to management of patients with pulmonary vein stenosis following AF ablation. J. Cardiovasc. Electrophysiol. 2011 Mar;22 (3):364–7. doi: 10.1111/j.1540-8167.2010.01981.x. [DOI] [PubMed] [Google Scholar]
- 76.Holmes David R, Monahan Kristi H, Packer Douglas. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv. 2009 Apr;2 (4):267–76. doi: 10.1016/j.jcin.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Scanavacca Mauricio, Hachul Denise, Sosa Eduardo. Atrioesophageal fistula--a dangerous complication of catheter ablation for atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007 Nov;4 (11):578–9. doi: 10.1038/ncpcardio1010. [DOI] [PubMed] [Google Scholar]
- 78.Badger Troy J, Adjei-Poku Yaw A, Burgon Nathan S, Kalvaitis Saul, Shaaban Akram, Sommers Daniel N, Blauer Joshua J E, Fish Eric N, Akoum Nazem, Haslem Thomas S, Kholmovski Eugene G, MacLeod Rob S, Adler Douglas G, Marrouche Nassir F. Initial experience of assessing esophageal tissue injury and recovery using delayed-enhancement MRI after atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):620–5. doi: 10.1161/CIRCEP.109.871939. [DOI] [PubMed] [Google Scholar]
- 79.Gorman Darcie R, Peterson Kathryn A, Fang John, Olpin Jeffrey, Sommers Daniel O, McFadden Molly, Morshedzadeh Jack H, Akoum Nazem, Daccarett Marcos, Marrouche Nassir, Adler Douglas G. Cross-sectional imaging obtained immediately following radiofrequency atrial fibrillation ablation does not predict endoscopic evidence of esophageal injury. Dig. Dis. Sci. 2011 Dec;56 (12):3453–8. doi: 10.1007/s10620-011-1829-1. [DOI] [PubMed] [Google Scholar]
- 80.Bahnson Tristram D. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2009 Feb;32 (2):248–60. doi: 10.1111/j.1540-8159.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 81.Good Eric, Oral Hakan, Lemola Kristina, Han Jihn, Tamirisa Kamala, Igic Petar, Elmouchi Darryl, Tschopp David, Reich Scott, Chugh Aman, Bogun Frank, Pelosi Frank, Morady Fred. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2005 Dec 06;46 (11):2107–10. doi: 10.1016/j.jacc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 82.Wylie John V, Peters Dana C, Essebag Vidal, Manning Warren J, Josephson Mark E, Hauser Thomas H. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008 May;5 (5):656–62. doi: 10.1016/j.hrthm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Jeevanantham Vinodh, Ntim William, Navaneethan Sankar D, Shah Sidharth, Johnson Alex C, Hall Burr, Shah Abrar, Hundley W Gregory, Daubert James P, Fitzgerald David. Meta-analysis of the effect of radiofrequency catheter ablation on left atrial size, volumes and function in patients with atrial fibrillation. Am. J. Cardiol. 2010 May 01;105 (9):1317–26. doi: 10.1016/j.amjcard.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 84.Masuda Masaharu, Inoue Koichi, Iwakura Katsuomi, Okamura Atsunori, Koyama Yasushi, Kimura Ryusuke, Toyoshima Yuko, Ito Norihisa, Komuro Issei, Fujii Kenshi. The impact of atrial fibrillation ablation on left atrial function: association with baseline left atrial function. Pacing Clin Electrophysiol. 2012 Mar;35 (3):327–34. doi: 10.1111/j.1540-8159.2011.03284.x. [DOI] [PubMed] [Google Scholar]
- 85.Gibson Douglas N, Di Biase Luigi, Mohanty Prasant, Patel Jigar D, Bai Rong, Sanchez Javier, Burkhardt J David, Heywood J Thomas, Johnson Allen D, Rubenson David S, Horton Rodney, Gallinghouse G Joseph, Beheiry Salwa, Curtis Guy P, Cohen David N, Lee Mark Y, Smith Michael R, Gopinath Devi, Lewis William R, Natale Andrea. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011 Sep;8 (9):1364–71. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 86.Schuleri Karl H, George Richard T, Lardo Albert C. Applications of cardiac multidetector CT beyond coronary angiography. Nat Rev Cardiol. 2009 Nov;6 (11):699–710. doi: 10.1038/nrcardio.2009.172. [DOI] [PubMed] [Google Scholar]
- 87.Niendorf Thoralf, Sodickson Daniel K. Highly accelerated cardiovascular MR imaging using many channel technology: concepts and clinical applications. Eur Radiol. 2008 Jan;18 (1):87–102. doi: 10.1007/s00330-007-0692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akçakaya Mehmet, Hu Peng, Chuang Michael L, Hauser Thomas H, Ngo Long H, Manning Warren J, Tarokh Vahid, Nezafat Reza. Accelerated noncontrast-enhanced pulmonary vein MRA with distributed compressed sensing. J Magn Reson Imaging. 2011 May;33 (5):1248–55. doi: 10.1002/jmri.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adluru Ganesh, Chen Liyong, Kim Seong-Eun, Burgon Nathan, Kholmovski Eugene G, Marrouche Nassir F, Dibella Edward V R. Three-dimensional late gadolinium enhancement imaging of the left atrium with a hybrid radial acquisition and compressed sensing. J Magn Reson Imaging. 2011 Dec;34 (6):1465–71. doi: 10.1002/jmri.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morcos S K, Thomsen H S, Webb J A. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9 (8):1602–13. doi: 10.1007/s003300050894. [DOI] [PubMed] [Google Scholar]
- 91.Marckmann Peter, Skov Lone. Nephrogenic systemic fibrosis: clinical picture and treatment. Radiol. Clin. North Am. 2009 Sep;47 (5):833–40, vi. doi: 10.1016/j.rcl.2009.05.004. [DOI] [PubMed] [Google Scholar]


