Abstract
Lung herniation after minimally invasive thoracoscopic pulmonary vein ablation has never been described before so far. We report for the first time, of its incidence in a 62-year-old patient, 6 weeks after a minimally invasive cardiac surgery (MICS) for atrial fibrillation ablation. We suggest that even after MICS, a high resolution computerized tomography scan should be performed in patients presenting with breathing difficulty and chest pain to rule out this condition too, other than pulmonary vein stenosis.
Keywords: Thoracoscopic Surgical Ablation, Atrial Fibrillation, Minimally Invasive, Complication, Atraumatic Lung Hernia, LAA Clip
Introduction
Pulmonary vein (PV) isolation is considered key to atrial fibrillation (AF) ablation.[1] Due to limitations of medical therapy in achieving sinus rhythm, there has been recent upsurge in surgical and catheter-based technologies to achieve this goal.[2-4] Recently, a new concept of bringing the advantages together of a cardiac surgeon and a electrophysiologist in order to overcome the drawbacks and suboptimal results of both catheter ablation and surgical AF treatment is successful with promising results[5,6] Atraumatic lung hernia is a rare entity secondary to minimally invasive cardiac surgery (MICS)[7,8] To the best of our knowledge, it has never been described secondary to any lateral approach thoracoscopic MICS. With increasing number of surgical arrhythmia procedures in future, cardiac surgeons and physicians must keep a watchful eye for such complication.
We report herein a case of lung herniation after MICS for AF ablation.
Case report
A 62-year-old woman with a 3-year history of symptomatic paroxysmal AF was refractory to anti arrhythmic drugs including amiodarone, inspite of undergoing endocardial PV isolation by radiofrequency (RF) catheter a year ago. Echocardiography showed a structurally and functionally normal heart with mildly dilated left atrium.
Hybrid Procedure
`The details of the procedure have been described elsewhere.[9] To summarize, after inducing general anaesthesia, a 12 mm videoport in 5th intercostal space and 2 working ports of 5 mm and 12 mm were inserted along anterior axillary line in 3rd and 6th or 7th intercostal spaces respectively. The pericardium was opened posterior to phrenic nerve and it was extended upto the diaphragm and superior vena cava on opposite sides. A bipolar RF clamp (Atricure, West Chester, OH, USA) was placed at the antrum of the PVs. Four to 6 applications of about 15 seconds with a median output of 10 to 15 Watt were performed. Both roofline and an inferior line were made epicardially using a bipolar RF pen device (Coolrail, Atricure). At the end of procedure, incision for a left working port (12 mm) was extended to 3 cms to facilitate positioning the epicardial left atrial appendage (LAA) clip (AtriClip, Atricure Inc.). Using the Gillinov-Cosgrove™ Selection Guide (Atricure Inc.) a 35 mm LAA clip was selected. Avoiding the pulmonary and circumflex arteries, the clip was placed at the base of the appendage. The clip can be repositioned before deployment, if the clip’s position is not satisfactory. After the clip was in optimal location, it was closed and released from the deployment tool manually. The Clip did not migrate intraoperatively.
After endocardial placement of His and coronary sinus catheters, a transseptal puncture was done using long 8-F sheath (SL0, St. Jude Medical) into the left atrium guided by fluoroscopy and trans-esophageal echocardiography. PVs and linear lesions were checked for electrical isolation. AF inducibility was attempted using endocardial burst pacing and adenosine administration. No endocardial touchup was needed due to intact surgical ablation lines and no evidence of dormant PV reconduction. The pericardium was approximated with a stitch, and a chest tube was placed in both pleural cavities. There was no drain left in the pericardial space. The insertion sites for working ports were closed using surgical skin staplers. The exclusion of appendage was successful as assessed by postoperative trans-esophageal echocardiography.
Post Procedure Events
Post- procedure recovery was uneventful. Six weeks later, she presented at our hospital’s clinic with complaints of pain in left thorax region associated with dyspnea (NYHA class 2). Pain used to increase on inspiration, was dull aching and non-radiating type. General physical examination was otherwise normal. Transthoracic echocardiography showed no pericardial effusion, no valvular (aortic or mitral) dysfunction and normal left ventricular function.
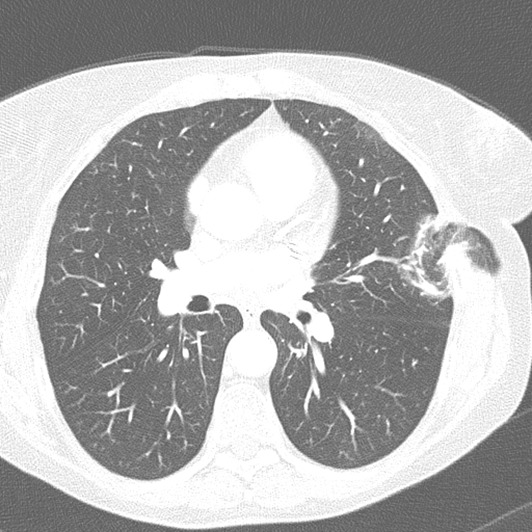
Her chest radiograph was within normal limits and subsequently advised medical management with painkillers. After 3 days, she returned with similar complaints. Due to history of MICS and strong clinical suspicion to rule out any complication, high resolution computerized tomographic (HRCT) scan was ordered. The scan showed (figure 1 and 2) a lung herniation in left chest wall through two intercostal levels that was caused by ports placement (and extra widening) in the chest wall. Finally, she underwent surgical release of the entrapped lung tissue through hernial sac, freed from adhesions, and reduced back into the thoracic cavity. Now after 12 weeks follow up, she is asymptomatic.
Figure 1. Three-dimensional reconstruction of high-resolution computerized tomography scans of the lung hernia.
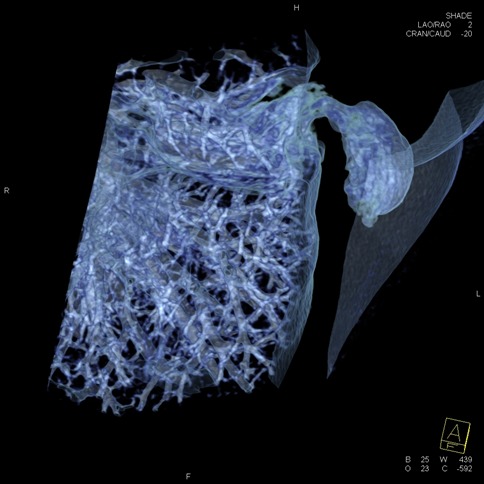
Figure 2. High-resolution computerized tomography scans (axial view) of lung hernia.
Discussion
MICS for AF ablation is a novel tool. It has evolved over time, to reduce the complications and cosmetic disadvantages of conventional cardiac surgery incision leading to a shorter hospital stay.[10] Herniation of pulmonary tissue was described earliest in 1499 by Roland.[11,12] Later in 1946, Maurer and Blades established the definition of lung herniation as “a protrusion of the pleural-covered lung beyond its normal boundaries through an abnormal opening in the thoracic enclosure".[13] They are also classified according to their etiology as spontaneous, traumatic or post-thoracotomy and according to their location as cervical, thoracic, abdominal, or thoraco-abdominal.[14]
To decrease the future risk of thromboembolism, a LAA exclusion or excision surgery is recommended in patients undergoing surgical treatment for AF or mitral valve.[15] LAA clip occlusion epicardially may not only provide stroke prevention but also reduce the recurrence of AF by causing acute electrical isolation of the LAA.[16] The Clip used in this procedure is pre-loaded on a disposable Clip applier and used for open occlusion of the heart’s LAA. No device-related perioperative complication has been observed and follow up with CT imaging has shown stable clip placement.[17,18]
Most of the previous cases of lung hernia happened with minimal-access thoracotomy in anterior wall of thorax, however particular for our patient has been their lateral and posterior location. We concluded main culprit being the extra operative trauma due to widening of the mini invasions of 12 mms (initially) to 3 cms to accomodate an epicardial LAA clip. If epicardial Clip was not indicated for a patient, the size of working port lesion would not been widened. The other possible reasons are inadequate closure of lesions (for insertion of videoports and interports), inherent weakness of intercostal muscles and intensive cauterization of the intercostal muscles. However, we did not observe any unexpected bleeding from sites of working ports insertion during the procedure. The patient’s activity during the period of chest-wall healing should be limited regardless of the size of the skin incision.[12]
Important Findings
The classical triad of patient’s history and the clinical symptoms and physical examination brings the physician closer to the diagnosis. However, it may sometimes not bring close enough as in this case. CT scan helped to fill the necessary void by delineating the size and location of the defect and dimensions of the hernia and leading to correct diagnosis.[19] The patient may present with a well-demarcated bulge increasing in size by expiration and vice-versa and more evident during coughing or Valsalva maneuver. Hernia may not pose a serious threat unless associated with incarceration and strangulation of the pulmonary tissue. The diagnosis of lung hernia should be confirmed after ruling out bronchopleural fistula, subcutaneous emphysema, lipoma and a gas-forming infection which require different treatment strategy.[12,20]
Conclusion
Lung hernia may occur after MICS for AF ablation, as a rare complication. Thus, it seems important to recommend CT scan in similarly symptomatic patients during their routine follow up.
Disclosures
Mark La Meir and Laurent Pison are also consultant to Atricure.
References
- 1.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Galperín J, Elizari M V, Chiale P A, Molina R T, Ledesma R, Scapín A O, Vázquez Blanco M. Efficacy of amiodarone for the termination of chronic atrial fibrillation and maintenance of normal sinus rhythm: a prospective, multicenter, randomized, controlled, double blind trial. J. Cardiovasc. Pharmacol. Ther. 2001 Oct;6 (4):341–50. doi: 10.1177/107424840100600403. [DOI] [PubMed] [Google Scholar]
- 3.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 4.Cox J L, Schuessler R B, Cain M E, Corr P B, Stone C M, D'Agostino H J, Harada A, Chang B C, Smith P K, Boineau J P. Surgery for atrial fibrillation. Semin. Thorac. Cardiovasc. Surg. 1989 Jul;1 (1):67–73. [PubMed] [Google Scholar]
- 5.Stamou Sotiris C, Khabbaz Kamal R, Mahmood Feroze, Zimetbaum Peter, Hagberg Robert C. A multidisciplinary approach to the minimally invasive pulmonary vein isolation for treatment of atrial fibrillation. Ann. Thorac. Surg. 2010 Feb;89 (2):648–50. doi: 10.1016/j.athoracsur.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 6.Pison Laurent, La Meir Mark, van Opstal Jurren, Blaauw Yuri, Maessen Jos, Crijns Harry J. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2012 Jul 03;60 (1):54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 7.Ross R T, Burnett C M. Atraumatic lung hernia. Ann. Thorac. Surg. 1999 May;67 (5):1496–7. doi: 10.1016/s0003-4975(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 8.Athanassiadi Kalliopi, Bagaev Erik, Simon Andre, Haverich Axel. Lung herniation: a rare complication in minimally invasive cardiothoracic surgery. Eur J Cardiothorac Surg. 2008 May;33 (5):774–6. doi: 10.1016/j.ejcts.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 9.La Meir Mark, Gelsomino Sandro, Lucà Fabiana, Lorusso Roberto, Gensini Gian Franco, Pison Laurant, Wellens Francis, Maessen Jos. Minimally invasive thoracoscopic hybrid treatment of lone atrial fibrillation: early results of monopolar versus bipolar radiofrequency source. Interact Cardiovasc Thorac Surg. 2012 Apr;14 (4):445–50. doi: 10.1093/icvts/ivr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng P C, Chua A N, Swanson M S, Koutlas T C, Chitwood W R, Elbeery J R. Anterior thoracotomy wound complications in minimally invasive direct coronary artery bypass. Ann. Thorac. Surg. 2000 May;69 (5):1338–40. doi: 10.1016/s0003-4975(00)01222-4. [DOI] [PubMed] [Google Scholar]
- 11.Roland. De pulmonibus sanaripot. In: Chavliac G, editor. Cyrurgia. Liber III (cap) 0;0:0–0. [Google Scholar]
- 12.Gouda Hossam, Multz Alan S, Khan Arfa, Rossoff Leonard J, Green L Michael, Graver Daniel. Lung hernia as a sequela to limited-access mitral valve surgery. Tex Heart Inst J. 2002;29 (3):203–5. [PMC free article] [PubMed] [Google Scholar]
- 13.MAURER E, BLADES B. Hernia of the lung. J Thorac Surg. 1946 Apr;15 ():77–98. [PubMed] [Google Scholar]
- 14.Sulaiman Abdulrazzaq, Cottin Vincent, De Souza Neto Edmundo Pereira, Orsini Alessandro, Cordier Jean-Francois, Gamondes Jean-Paul, Tronc Francois. Cough-induced intercostal lung herniation requiring surgery: Report of a case. Surg. Today. 2006;36 (11):978–80. doi: 10.1007/s00595-006-3284-8. [DOI] [PubMed] [Google Scholar]
- 15.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 16.Starck Christoph T, Steffel Jan, Emmert Maximilian Y, Plass Andre, Mahapatra Srijoy, Falk Volkmar, Salzberg Sacha P. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012 Sep;15 (3):416–8. doi: 10.1093/icvts/ivs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salzberg Sacha P, Gillinov Alan Marc, Anyanwu Anelechi, Castillo Javier, Filsoufi Farzan, Adams David H. Surgical left atrial appendage occlusion: evaluation of a novel device with magnetic resonance imaging. Eur J Cardiothorac Surg. 2008 Oct;34 (4):766–70. doi: 10.1016/j.ejcts.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Salzberg Sacha P, Plass Andre, Emmert Maximillian Y, Desbiolles Lotus, Alkadhi Hatem, Grünenfelder Jurg, Genoni Michele. Left atrial appendage clip occlusion: early clinical results. J. Thorac. Cardiovasc. Surg. 2010 May;139 (5):1269–74. doi: 10.1016/j.jtcvs.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla M, Leitman B S, Forcade C, Stern E, Naidich D P, McCauley D I. Lung hernia: radiographic features. AJR Am J Roentgenol. 1990 Jan;154 (1):51–3. doi: 10.2214/ajr.154.1.2104725. [DOI] [PubMed] [Google Scholar]
- 20.Eisa Naseem, Alraiyes Abdul Hamid, Shaheen Khaldoon, Alraies M Chadi. Lung hernia. BMJ Case Rep. 2013 Apr 29;2013 () doi: 10.1136/bcr-2013-009391. [DOI] [PMC free article] [PubMed] [Google Scholar]


