Abstract
Objective
Abdominal aortic aneurysms are a major cause of death in developed countries, and thrombus and calcification of the aneurysm have been linked to increased complications. This study was conducted in order to identify the biochemical marker associated to the presence of intraluminal thrombus or calcification progression of the aneurysm.
Design
Several clinical laboratory parameters were measured in patients with abdominal aortic aneurysms, in particular those already demonstrated to be related to the pathology, such as lipoprotein (a), white blood cell count, fibrinogen and high-sensitivity C-reactive protein. Most of the patients were analysed for the presence of thrombus or aorta calcification using CT angiography.
Results
Unlike previous findings, we found no association between intraluminal thrombus formation and lipoprotein (a), but we evidenced that patients with lower grade of calcification tend to have higher plasma high-sensitivity C-reactive protein values compared with patients with a higher degree of calcification. Instead, no association was found with either white blood cell count or fibrinogen level.
Conclusions
This study suggests that high-sensitivity C-reactive protein is a useful biomarker to assess the evolution of calcification and could be used in triaging patients to identify those who should undergo a rapid imaging, thus allowing prompt initiation of treatment or rule-out suspicious patients from non-essential imaging repetition.
Keywords: Abdominal aortic aneurysm, calcification, biomarker, hsCRP
Introduction
Abdominal aortic aneurysm (AAA) is a >3 cm dilation found in the distal aorta. The ultimate complication of AAA is the rupture of the aneurysm, which is a life-threatening condition with an approximate overall mortality rate of at least 80%.1–5 Efforts to limit the mortality rate depend on early detection and elective AAA repair. Although different authors have demonstrated that the risk of rupture increases exponentially with maximal aortic diameter, often reporting the relationship with biomarkers, aneurysm size does not completely represent the natural history of AAA.6–8
Notably, in every patient with a small and not yet “surgical” AAA, there are no clear predictors of a fast or slow progression of its own AAA, i.e. the best interval between a radiological check and the next step is not defined. Different additional risk factors have already been identified and tested intensively, in particular the presence of intraluminal thrombus, and calcification has been extensively investigated as AAA complication. Most AAAs contain significant quantities of intraluminal thrombus, and generally, the volume is correlated with the severity of the aortic dilatation. It has been suggested that lipoprotein (Lp)(a) plays a role in thrombus formation which may explain its rise particularly in other vascular pathology where mural thrombus formation occurs, and indeed, several investigators have measured Lp(a), to assess its possible role in the pathogenesis or progression of AAA. Various degrees of mural calcification have been found to exist in most AAA9,10 and the risk of rupture seems to be associated with the degree of calcification.9,11 Until recently, vascular calcification was considered a purely degenerative, unregulated process. Since then, a causal role of inflammation in vascular calcification has been proposed12–14 and different studies have analysed the values of analytes such as white blood cell count (WBC), fibrinogen, and C-reactive protein (CRP). The evolution of thrombus and calcification is often progressive and the lack of established prognostic indices makes the repetition of imaging to monitor AAA expansion necessary, with some important limitations such as cost or availability. Indeed, the most used tool for AAA monitoring is echography, which has a very good cost/effectiveness ratio regarding diameters, but shows several limits when it comes to a careful assessment of calcification and thrombus. An accessible and cost-effective measure such as a blood test predicting subsequent AAA progression in thrombus or calcification could be used to rule in and/or rule out patients for more expensive (MRI) and radiation inducing (AngioCT) radiological diagnostic imaging, with benefit for patients and caregivers and with important reduction of cost.
Our goal was to evaluate whether calcification and its progression are correlated with biochemical markers of inflammation and if specific biomarker(s) could be used to monitor thrombus formation.15,16 For the purpose of this study, we selected several bioassays based on their pathophysiologic relationship, on their economical motivation, and on their availability at our research facility.16 In particular, we evaluated classical inflammation markers such as WBC count, fibrinogen, and high-sensitivity CRP (hsCRP), together with Lp(a). The identification of biochemical marker(s) linked to AAA calcification will be used in triaging patients, in order to discriminate those who should undergo rapid imaging to allow a prompt initiation of treatment.
Methods
Patients and specimens
We selected a consecutive sample of 100 Caucasian patients admitted to the Vascular Surgery Unit of Brescia University “Spedali Civili” hospital in Brescia, Northern Italy, between 2013 and 2015, for AAA resection, meeting the following criteria: we selected patients for whom a CT angiography was performed within one month before surgery. Patients who had a CRP>10 mg/L, ruptured AAA, recent infections, fever, recent trauma, patients with inflammatory aneurysm based on CT findings, with symptomatic or ruptured aneurysm, inflammatory or infectious aneurysm, or anastomotic pseudoaneurysm and malignant disease were not considered to be included in the study. The risk factors, including age (continuous), gender (male vs. female), smoking (current vs. never or former), were collected. Patients were defined diagnosed with hypertension, if the systolic blood pressure was >140 mmHg or their diastolic blood pressure >85 mmHg for more than two measurements, or if they were treated with antihypertensive drugs. A family history of cardiovascular disease was recorded if any cardiovascular disease was present within second-degree relatives. Patients were classified as affected by diabetes mellitus if the glycated haemoglobin values were higher than 6.5% or if they were prescribed with antidiabetic drugs.
The study excluded patients who had ruptured AAA, recent infections, fever, recent trauma, and CRP >10 mg/L that could represent an active inflammatory process. Demographic data and medical history of each patient were collected. The study conformed to the ethical guidelines of the “World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th World Medical Association General Assembly, Helsinki, Finland, June 1964, and revised in Tokyo in 2004. Institutional ethic committees approved the study, and all patients provided a written informed consent (approval reference number: 1353). Participants did not receive any form of financial compensation.
Imaging assessment of aneurysm calcifications and thrombus
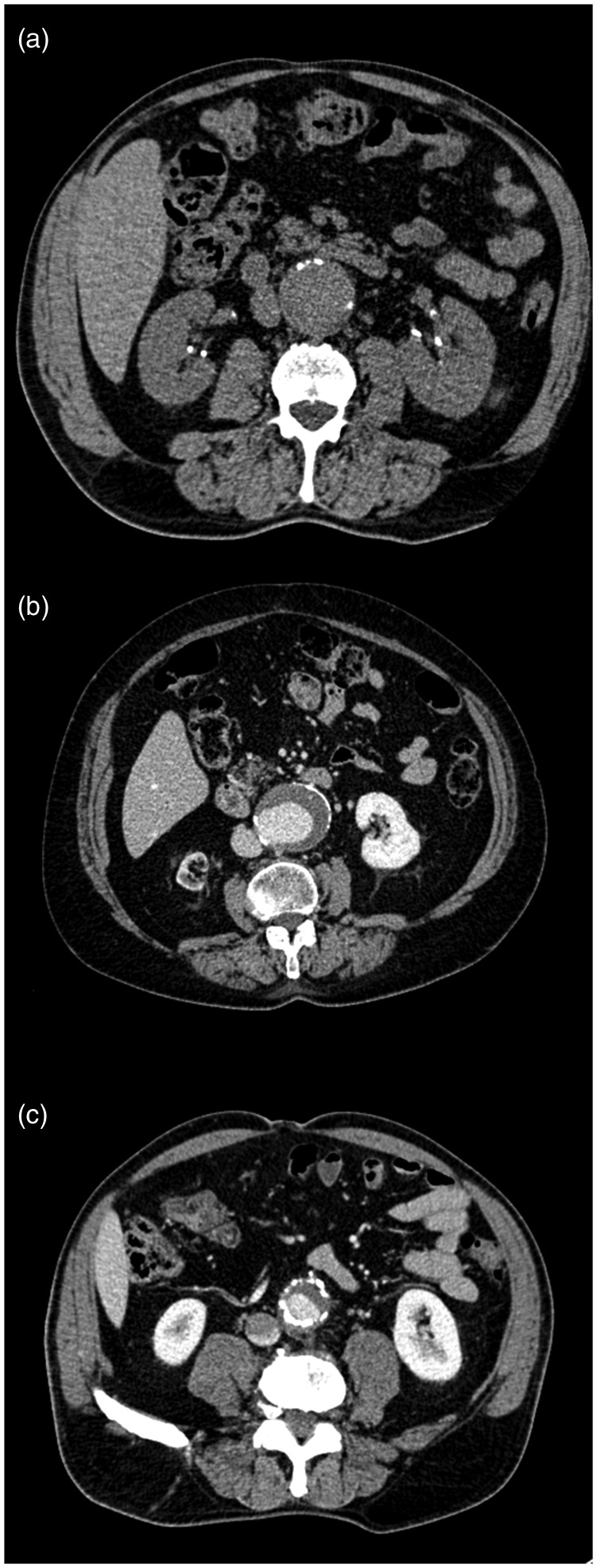
By a single radiologist, calcifications were evaluated on axial multiplanar reconstructions using 10 mm thick maximum intensity projection on three different levels: upper, middle and lower portion of the aneurysm. The absence of calcifications was scored = 0; calcifications covering less than one third of aortic circumference were scored = 1; between one and two-thirds a score = 2, and more than two-thirds a score = 3. The score at the upper and lower aneurysm level was multiplied by a factor of 0.5, in order to reflect the changes in aneurysm circumference due to aneurysm shape. A global score = 0 was not observed in any of the analysed patients. Scores ≤2 indicated a grade I, scores 3 and 4 corresponded to grade II, and scores ≥5 to grade III calcifications (Figure 1). Patients with grade II (n = 30) and III (n = 13) calcifications were grouped together in the subsequent statistical analysis in order to obtain two well-balanced groups (grade I vs. grade II+III). Calcification assessment was repeated by a resident in Vascular Surgery, optimal inter-rater agreement with the radiologist regarding final calcification grade was found (k > 0.8). Aneurysm mural thrombus has been visually assessed by dicotomic variables (presence/absence; concentric/eccentric) and measured (maximum thickness in mm on a plane orthogonal to aortic major axis).
Figure 1.
Representative example of different score of calcifications. The absence of calcifications was scored = 0; calcifications covering less than one third of aortic circumference were scored = I (a); between one and two thirds a score = II (b), and more than two thirds a score = III (c).
Blood collection and laboratory measurements
Venous blood samples were obtained from fasting overnight patients via an anticubital vein puncture without venous stasis before AAA resection. Commercially available assays were used according to manufacturer’s instruction: serum Lp(a) was measured on a nephelometer (Behring analyser II) with the latex lipoprotein reagent where the lowest detection level of the method was less than 0.3 g/L. For WBC count, specimens were collected in peripheral blood sampling microtainer tube containing K2EDTA and analyzed using an automated blood analyzer (Coulter LH 750) within 4 h from collection. The laboratory tests for fibrinogen were performed on blood collected with sodium citrate on ACL TOP (Instrumentation Laboratory, Milan, Italy), according to the manufacturer's specifications and using HemosIL reagent system17 (Instrumentation Laboratory). To determine hsCRP plasma levels, blood was collected in lithium-heparin tube and concentrations were measured using an immunoassay technique (Siemens Healthcare Diagnostics, Den Hague, the Netherlands) with a lower detection limit of 0.2 mg/L carried out in Dimension® Vista™ 1500 analyzer (Siemens Diagnostics) according to the manufacturer's instructions. If the values were high (>10 mg/L), indicating an acute inflammatory process, the measurement was discarded or repeated. The instruments were calibrated against appropriate proprietary reference standard material and verified by using the registered quality controls.
Statistical analysis
Laboratory results were reported as mean, standard deviation, or percentages and analyzed using univariate analysis according to the presence of thrombus and calcification. Differences were tested using Student’s t-test for continuous variables and the logistic regression for dichotomous and unordered categorical data. p Values < 0.05 were considered statistically significant.
Results
Among the 100 patients who were enrolled for the study, 15 were rejected because of an hsCRP higher than 10 mg/L, 40 were diagnosed for the presence of intraluminal thrombus and 83 patients were assigned with a grade of AAA calcification. Indeed, 40 patients showed a calcification grade I, while the remaining 43 possessed calcification grades II and III.
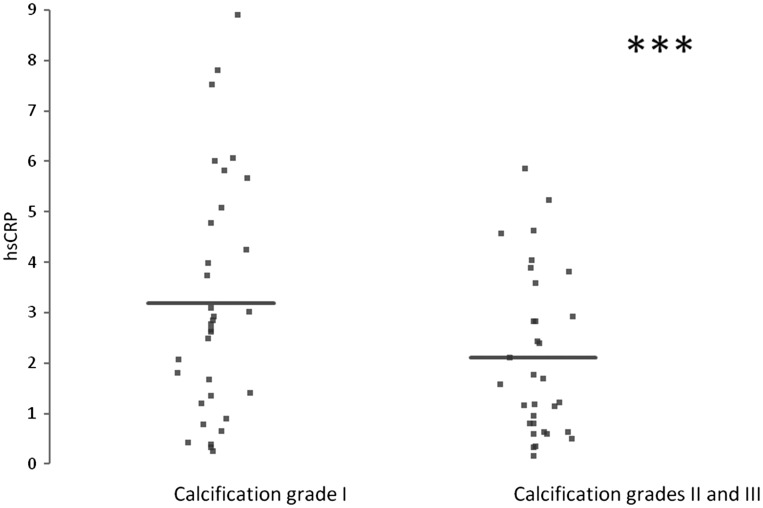
Patients differed by age, sex, hypertension, obesity, glucose tolerance, renal failure, family history of cardiovascular diseases, and chronic obstructive pulmonary disease. No differences were observed in AAAs localization among the enrolled patients. The median serum Lp(a) level in patients with diagnosed AAA was 0.25 g/L; the median WBC count was 7300 cells/µl; the fibrinogen median value was 338 mg/L, and hsCRP level was 2.25 mg/L. These results together with statistical values are reported in Table 1. Thus, Lp(a) serum levels of AAA patients are undistinguishable from normal values and, in addition, the presence of Lp(a) does not correlate with the presence of thrombus. No significant difference was observed in both WBC count and fibrinogen level between the patients with calcification I and the group that comprises grades II and III, probably suggesting the presence of inflammation in both cases. In agreement with literature, data hsCRP levels were elevated in almost all AAA patients but interestingly enough the patients with a lower calcification (group I) tend to have increased plasma hsCRP levels compared with patients carrying a higher grade of calcification; in particular, the median values of hsCRP of patients with calcification I were 3.1 mg/L, while the median values of hsCRP of patients with calcification II were 2.6 mg/L. The results are shown in Figure 2 and from the statistical analysis, after adjustment for age, gender, and clinical diagnosis, a significant inverse correlation between hsCRP levels and grade of calcification is observed (p value of 0.01).
Table 1.
Median values of circulating biomarker in AAA patients.
| Biomarker | Mean values | References values | Units |
|---|---|---|---|
| Lp(a) | 0.25 | <0.3 | g/L |
| WBC | 7.30 | 4.00–10.80 | ×103/µL |
| Fibrinogen | 338 | 170–410 | mg/L |
| hsCRP | 2.25 | <0.30 | mg/L |
Figure 2.
hsCRP levels in different grades of AAA calcification (95% CI: −0.122 to −0.005). *** indicates a p value <0.05.
As shown in Table 2, we explore the potential association between classical risk factors and hsCRP and found an association between hsCRP and hypertension. We also stratify these parameters by grade of aneurysm calcification with no significant association.
Table 2.
Correlation of clinical parameter with hsCRP and calcification grade.
| Clinical parameter | Correlation with hsCRP value | Correlation with calcification grade |
|---|---|---|
| Smoking | p = 0.4 | p = 0.5 |
| Hypertension | p = 0.034 | p = 0.6 |
| Diabetes | p = 0.6 | p = 0.4 |
| Family history of cardiovascular disease | p = 0.7 | p = 0.7 |
Discussion
We report the identification of a biomarker, hsCRP, that could be associated with the evolution of AAA calcification.
The management of patients with AAA might significantly benefit from the measurement of circulating biomarkers that facilitate an early diagnosis and that could have a direct correlation with a possible fast growth of a known lesion. In particular, thrombus formation and calcification evolution are often progressive and lack established prognostic indices; this has, as a consequence, a repetition of the imaging of AAA complications with important limitation such as cost, availability, or waiting time. Our study was designed to identify circulating markers that could substantially help to identify appropriate patients for different monitoring protocols and intervention. For the purpose of this study, we selected biomarkers based on their pathophysiologic relationship, economical reasons and availability at our research facility. This study, has some limitations such as the number of participants – although it was greater than the other studies of AAA disease, the number is still quite limited; in particular, we were obliged to group calcification II with III since group III included 13 patients only. Furthermore, aneurysm calcifications have been analyzed in a standardized but qualitative way. Further studies should include a quantitative semi-automated calcium scoring method in order to improve repeatability. It could also be argued that the study is missing negative controls, but most of the patients subjected to abdominal echography and who were negative to AAA, had an inflammatory disease diagnosis, which automatically excluded them from the possibility of becoming a negative control. Moreover, we did not include plasma derived from routine laboratory examination, since we could not exclude asymptomatic AAA. Further studies with negative control will need to be carried out.
The association of AAA with increasing concentration of peculiar biomarkers has been an interesting subject for different reasons, considering the currently accepted deficiencies in the management of this condition. Most commonly, investigators have measured blood concentrations of analytes to assess its possible role in the pathogenesis or progression of AAA,8,18,19 and recently Folsom et al.16 found that multiple positive biomarkers of inflammation identify a subgroup of patients at high risk of AAA. Most of the studies performed showed a direct association between AAA diameter and biomarker increase and proposed some of the laboratory tests, in particular inflammatory markers, for the prediction of subsequent AAA progression.20–22 However, additional complication needs to be explored. The implication of thrombus in the evolution of AAA complications has already been demonstrated and a number of studies, with the exception of some, have shown that circulating Lp(a) concentration may be higher in patients with AAA compared to healthy people, thus indicating that Lp(a) could be involved in thrombus formation. In our analysis, we assayed Lp(a) using the gold standard nephelometric technique; patients with AAA do not show higher levels of circulating Lp(a). We could speculate that the different results obtained in previous studies could be indeed related to inaccurate techniques used to evaluate Lp(a). The statistical analysis of our data revealed the absence of the association between Lp(a) values and the presence of thrombus. As a consequence, we believe that Lp(a) does not represent a useful biomarker for monitoring AAA intraluminal thrombus formation.
Though computational analysis of calcification of the coronary vessels has been validated rigorously, no such tool has been developed for larger vessels such as the abdominal aorta.23,24 Beside imaging, currently, no accurate methods exist in order to diagnose calcified AAAs, and clinical examination is still doubtful. For the purpose of this study, we analysed the blood levels for WBC, fibrinogen and hsCRP, but the correlation between different grades of calcification and WBC count or fibrinogen levels does not occur.
Even if WBC plays an important role in the inflammatory response involved in the initiation and progression of AAA and different authors demonstrated higher WBC count in patients with symptomatic or ruptured AAA,25 this investigation showed no significant changes of this parameter among different grades of calcification.
The association between plasma fibrinogen and AAA has been studied extensively with conflicting results.16 Our data suggest that no association or correlation exists between fibrinogen level and progression of calcification. It has been supposed that fibrinogen may act through several mechanisms including platelet aggregation and endothelial function.22 Nevertheless, since in calcified AAA tissue, the endothelium is absent and totally replaced by the calcification, the lack of significant results maybe explained by the absence of a fibrinogen deposition mechanism. Therefore, although the evaluated markers are plausible biomarkers for AAA, WBC and fibrinogen are not good candidates for monitoring the presence or the progression of calcification, and other potential biomarkers should also be investigated. Activated inflammatory response in patients with AAA is revealed by elevated hsCRP in almost all the enrolled patients. Indeed, even though patients with hsCRP > 10 mg/L were excluded, the mean serum hsCRP level was above the range for supposedly healthy individuals,7,8 while the values of CRP were normal as shown by different authors who have demonstrated that asymptomatic patients tend to have normal values of CRP compared to patients with symptomatic AAA or with ruptured AAA who were excluded from our study.6 We explore the potential association between the classical risk factors and hsCRP, and, as shown by other authors, we found an association between hypertension and hsCRP values (p < 0.05) thus supporting the theory that increased blood pressure is associated with (vascular) inflammation.26 Very interestingly, we show a significant correlation between hsCRP and vascular calcifications (p = 0.01). Intriguingly, hsCRP levels were higher in patients with a lower grade of calcification. This unexpected result is probably due to the fact that during the calcification process, the originally inflamed tissue is gradually replaced by calcified tissue, thus reducing the level of inflammation and, ultimately, decreasing hsCRP values. Indeed, it has been shown that endothelial cells of different origins are able to produce different molecules that can mediate endothelial function or are involved in systemic physiological or pathological process27–29; in particular, CRP can be produced in aneurysmal tissue28,29 and the loss of living tissue could be justified due to a lower level of hsCRP in patients with higher level of calcification compared to patients with a lower grade of calcification. Quantitative analysis of CRP mRNA and protein in normal and in AAA tissue should give a wider insight into the up-regulation of CRP production during aneurysm formation. Furthermore, due to the limited number of cases and the absence of negative control, the findings from the statistic model need to be repeated in future studies.
The calcification degree of the AAA wall may hold a strong predictive value for the natural history of AAA, and treatment could be offered earlier with less resultant morbidity and mortality30 that need the repetition of imaging to monitor AAA expansion with some important limitations such as cost, availability and time. Even if we believe that the use of hsCRP is not the central point for monitoring evolution, we believe that it could help to select the patients who could be offered diagnostic and therapeutic treatment earlier.
Conclusion
To our knowledge, we report the first identification of a biomarker that could be associated with the evolution of AAA calcification. We suggest that hsCRP evaluation could be used in triaging patients to identify those who should undergo more frequent radiological checks, thus allowing for a prompt initiation of treatment, or rule out suspicious patients from non-essential repeat imaging.
Dedication
To the memory of Prof. Adolfo Turano – “Accedit quod patrem plus etiam quam non modo tu sed quam ipse scit amo” (“I love my father more than, not only you, but even he knows”) (MT Cicerone).
Acknowledgements
We thank the personnel of the surgery wards of Spedali Civili of Brescia for their assistance in sampling the specimens and Kris Hagan for his contribution in the preparation of this manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a local research grant (#6051) from the University of Brescia, Italy.
Ethical approval
This study was approved by the provincial ethical commettee of Brescia with the reference number 1353.
Guarantor
E.G. has official responsibility for the overall integrity of the manuscript and ensures that all statements are true to her knowledge.
Contributorship
E.G. and S.B. contribute to the conception, design, analysis, interpretation of data and wrote the manuscript; A.G., M.R., M.P., and C.Z. helped with acquisition and analysis of data and revised the manuscript, L.C. and P.D.E. helped with interpretation of data and revised the manuscript.
References
- 1.Earnshaw JJ. Comments regarding “Agreement between computed tomography and ultrasound on abdominal aortic aneurysm and implications on clinical decision”. Eur J Vasc Endovasc Surg 2011; 42: 615–616. [DOI] [PubMed] [Google Scholar]
- 2.Svensjö S, Björck M, Gürtelschmid M, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011; 124: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Ghoorah K, Kunadian V. Abdominal aortic aneurysms and risk factors for adverse events: a review. Cardiol Rev 2016; 24: 88–93. [DOI] [PubMed] [Google Scholar]
- 4.Choke E, Lee K, McCarthy M, et al. Risk models for mortality following elective open and endovascular abdominal aortic aneurysm repair: a single institution experience. Eur J Vasc Endovasc Surg 2012; 44: 549–554. [DOI] [PubMed] [Google Scholar]
- 5.Lo RC, Bensley RP, Hamdan AD, et al. Vascular Study Group of New England. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013; 57: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domanovits H, Shillinger M, Mullner M, et al. Acute phase reactants in patients with AAA. Atherosclerosis 2002; 163: 297–302. [DOI] [PubMed] [Google Scholar]
- 7.De Haro J, Acin F, Bleda S, et al. Prediction of asymptomatic abdominal aortic aneurysm expansion by means of rate of variation of C-reactive protein plasma levels. J Vasc Surg 2012; 56: 45–52. [DOI] [PubMed] [Google Scholar]
- 8.Hellenthal FA, Pulinx B, Welten RJ, et al. Circulating biomarkers and abdominal aortic aneurysm size. J Surg Res 2012; 176: 672–678. [DOI] [PubMed] [Google Scholar]
- 9.Buijs RV, Willems TP, Tio RA, et al. Calcification as a risk factor for rupture of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2013; 46: 542–548. [DOI] [PubMed] [Google Scholar]
- 10.Torres WE, Maurer DE, Steinberg HV, et al. CT of aortic aneurysms: the distinction between mural and thrombus calcification. AJR Am J Roentgenol 1988; 150: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary SA, Mulvihill JJ, Barrett HE, et al. Determining the influence of calcification on the failure properties of abdominal aortic aneurysm (AAA) tissue. J Mech Behav Biomed Mater 2015; 42: 154–167. [DOI] [PubMed] [Google Scholar]
- 12.Nordon IM, Hinchliffe RJ, Loftus IM, et al. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011; 8: 92–102. (Review). [DOI] [PubMed] [Google Scholar]
- 13.Brady AR, Thompson SG, Fowkes FG, et al. UK Small Aneurysm Trial Participants. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004; 110: 16–21. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M, Nishikimi N, Sakurai T, et al. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int Angiol 2000; 19: 276–279. [PubMed] [Google Scholar]
- 15.New SE, Aikawa E. Cardiovascular calcification: an inflammatory disease. Circ J 2011; 75: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 16.Folsom AR, Yao L, Alonso A, et al. Circulating biomarkers and abdominal aortic aneurysm incidence: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2015; 132: 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appert-Flory A, Fischer F, Jambou D, et al. Evaluation and performance characteristics of the automated coagulation analyzer ACL TOP. Thromb Res 2007; 120: 733–743. [DOI] [PubMed] [Google Scholar]
- 18.Vega de Céniga M, Esteban M, Quintana JM, et al. Search for serum biomarkers associated with abdominal aortic aneurysm growth – a pilot study. Eur J Vasc Endovasc Surg 2009; 37: 297–299. [DOI] [PubMed] [Google Scholar]
- 19.Stather PW, Sidloff DA, Dattani N, et al. Meta-analysis and meta-regression analysis of biomarkers for abdominal aortic aneurysm. Br J Surg 2014; 101: 1358–1372. (Review). [DOI] [PubMed] [Google Scholar]
- 20.Takagi H, Watanabe T, Mizuno Y, et al. Circulating interleukin-6 levels are associated with abdominal aortic aneurysm presence: a meta-analysis and meta-regression of case-control studies. Ann Vasc Surg 2014; 28: 1913–1922. [DOI] [PubMed] [Google Scholar]
- 21.Siennicka A1, Drozdzynska M, Chelstowski K, et al. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg 2008; 47: 928–935. [DOI] [PubMed] [Google Scholar]
- 22.Flondell-Sité D, Lindblad B, Kölbel T, et al. Markers of proteolysis, fibrinolysis, and coagulation in relation to size and growth rate of abdominal aortic aneurysms. Vasc Endovasc Surg 2010; 44: 262–268. [DOI] [PubMed] [Google Scholar]
- 23.Yoon YE, Choi JH, Kim JH, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging 2012; 5: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 24.Voros S, Maurovich-Horvat P, Marvasty IB, et al. Precision phenotyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: rationale and design of the “Genetic Loci and the Burden of Atherosclerotic Lesions” study. J Cardiovasc Comput Tomogr 2014; 8: 442–451. [DOI] [PubMed] [Google Scholar]
- 25.Tambyraja AL, Dawson R, Valenti D, et al. Systemic inflammation and repair of abdominal aortic aneurysm. World J Surg 2007; 31: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 26.Pruijim M, Vollenwider P, Mooser V, et al. Inflammatory markers and blood pressure: sex differences and the effect of fat mass in the CoLaus Study. J Hum Hypertens 2013; 27: 169–175. [DOI] [PubMed] [Google Scholar]
- 27.Spinella F, Caprara V, Garrafa E, et al. Endothelin axis induces metalloproteinase activation and invasiviness in human LEC. Can J Physiol Pharmacol 2010; 88: 782–787. [DOI] [PubMed] [Google Scholar]
- 28.Vainas T, Lubbers T, Stassen FR, et al. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation 2003; 107: 1103–1105. [DOI] [PubMed] [Google Scholar]
- 29.Lindholt JS. Aneurysmal wall calcification predicts natural history of small abdominal aortic aneurysms. Atherosclerosis 2008; 2: 673–678. [DOI] [PubMed] [Google Scholar]