Abstract
This review focuses on the (mal)adaptive processes in atrial excitation-contraction coupling occurring in patients with chronic atrial fibrillation. Cellular remodeling includes shortening of the atrial action potential duration and effective refractory period, depressed intracellular Ca2+ transient, and reduced myocyte contractility. Here we summarize the current knowledge of the ionic bases underlying these changes. Understanding the molecular mechanisms of excitation-contraction-coupling remodeling in the fibrillating human atria is important to identify new potential targets for AF therapy.
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia found in the clinical practice, affecting >2 million people in the United States alone.[1] AF is often associated with other cardiovascular disorders, such as coronary artery disease, valve dysfunction, congestive heart failure (CHF), and is characterized by significant morbidity. A key determinant of this morbidity is embolic stroke,[2] with loss of atrial contractility being one of the major causes of thrombus formation. AF is characterized by a rapid and irregular heartbeat caused when the atria quiver (fibrillate) erratically, sometimes faster than 200 times per minute.[2]
Several studies have investigated the molecular and ionic mechanisms involved in the remodeling occurring in the atria of patients with AF, and suggest that structural, electrophysiological, and contractile remodeling are critical factors in the disease progression, i.e., they contribute to the development of a substrate that facilitates the tendency for persistence of AF.[3,4] Structural remodeling involves changes in atrial myocyte and tissue morphology (e.g., cell hypertrophy, fibrosis).[3,5,6] Electrical remodeling includes changes in Ca2+ and K+ currents leading to shortening of the action potential (AP) duration (APD) and loss of APD rate-dependent adaptation.[6] A growing body of experimental evidence points to perturbations in intracellular Ca2+ handling as important players in AF-induced atrial remodeling,[7,8] with intracellular Ca2+ transients (CaTs) being reduced. Myofilament protein changes in AF are also likely to contribute to atrial contractile dysfunction.[9] However, the mechanisms leading to self-perpetuation of the arrhythmia and depressed cardiac contractility are yet poorly understood. Recently, Llach et al. have studied the basis of irregular beat-to-beat response of human atrial myocytes when subjected to elevations of the beating frequency (which often precedes cardiac arrhythmias) and suggested that stability or instability of the response was determined by the sarcoplasmic reticulum (SR) and L-type Ca2+ channel activities.[10]
In this review, we present the current knowledge about the changes occurring in excitation-contraction (E-C) coupling that characterize the remodeled human atrial myocytes from patients with chronic AF (cAF), and the postulated underlying ionic mechanisms.
Phenotypic Consequences of AF on AP, CaT, and Contractility
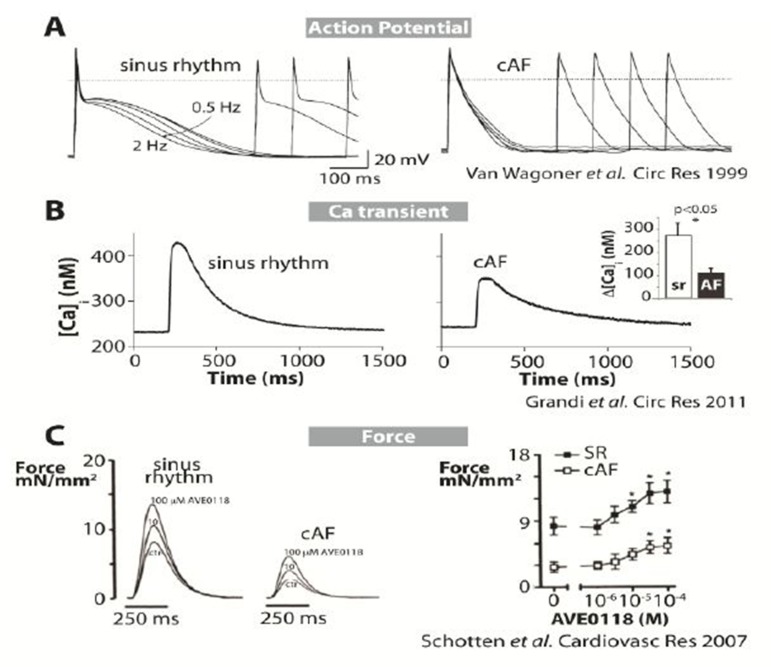
Myocytes from cAF patients are characterized by shorter APs (Fig. 1A) and effective refractory period (ERP), and loss of rate adaptation of both atrial repolarization (Fig. 1A) and refractoriness.[11-15] Typically, the human atrial AP duration at 90% repolarization (APD90) shortens when paced at faster frequencies, but in myocytes isolated from cAF patients this shortening is severely attenuated (Fig. 1A). CaT amplitude is strongly depressed in myocytes from cAF patients compared to those from subjects in sinus rhythm (Fig. 1B),[16] although the SR Ca2+ content is unaltered.[16-19] CaTs decay more slowly in cAF compared to sinus rhythm.[16,18] Elevated diastolic [Ca2+]i has been reported and attributed to enhanced leak of Ca2+ from the SR.[17] Intracellular [Ca2+] measurements with aequorin light signals in atrial tissue from patients in sinus rhythm display a positive dependency of CaT amplitude on the pacing rate.[20] Our recently published mathematical model of the human atrial AP and CaT recapitulated this positive rate-dependence, and importantly showed that this is impaired when simulating cAF conditions.[16]
Figure 1. Altered E-C coupling in human AF. A) APs recorded at different pacing rates in a control human atrial myocyte (left) and in a cell from a cAF patient (right) (11). B) Intracellular Ca2+ transients measured in human atrial cells from sinus rhythm (left) and cAF (right) patients at physiological temperature. C) Twitch force measurements (22) in myocytes from sinus rhythm and cAF patients are shown at various doses of AVE0118 (IKur blocker).
Our simulations indicated that APD rate adaptation in sinus rhythm atrial cells involves accumulation of intracellular Na+ ([Na+]i) at high frequencies, which causes outward shifts in Na+/Ca2+ exchange and Na+/K+ pump currents. The model also predicted that E-C coupling remodeling in cAF would reduce Na+ accumulation, thus causing a blunted APD rate-dependent response.[16]
Baseline force of contraction of atrial trabeculae is also reduced in human cAF by approximately 70% compared to patients in sinus rhythm (Fig. 1C).[21-23]
Ionic Bases of Altered E-C Coupling in AF
The molecular bases of AF-induced alterations in E-C coupling are summarized in Table 1 and discussed in detail in the following paragraphs. E-C coupling remodeling can occur at the level of ion channels/transporters expression, or by modification of ion channel/transporter properties (for example, trafficking or phosphorylation). Furthermore, alterations of myofilament proteins may be involved in AF-induced hypocontractility.
Table 1. Molecular bases of altered E-C Coupling in human AF (changes vs. sinus rhythm) [Modified from [16]].
| Cell Size/Structure | |
|---|---|
| Size | Increased length and width (17) |
| Membrane capacitance | Increased (24) |
| Sarcolemmal Ion Channels | |
| INa | No changes (15, 35) Steady-state inactivation shifted right (15) Slightly reduced current density (36) Late current increased (36) |
| ICaL | Reduced current density by ~50% (5, 11, 12, 18, 40) No changes in voltage dependence of activation and inactivation (11) |
| If | Increased mRNA levels (44) |
| Ito | Reduced density -80% in the RA -45% in the LA (5, 12, 15, 24, 45-47) |
| IKur | Reduced density -55% in the RA -45% in the LA (5, 24, 45, 47, 49) Unchanged (12, 15, 46) |
| IKs | Increased 2-fold (24) |
| IK1 | Upregulated +100% (5, 12, 13, 15, 45) |
| IK,ACh | Increased basal current by receptor-independent, constitutively active component; increased (15) or reduced carbachol-activated current (13, 56, 57) |
| IKATP | Decreased (60) Increased (61) |
| Ca+ and Na+ handling | |
| INCX | Upregulated (17, 18, 21, 34) |
| SERCA | Reduced maximal pump rate (18) and protein expression (34) |
| PLN | Enhanced PKA and CaMKII phosphorylation (34) Unaltered CaMKII-dependent phosphorylation (17) |
| RyR | Increased phosphorylation at PKA and CaMKII sites (17, 18, 67) resulting in increased channel open probability (68) and SR Ca2= leak (17, 111) |
| INKA | Unchanged function (65) |
| Ankyrin-B | Downregulated (70) |
| Protein kinases and phosphatases | |
| CaMKII | Increased expression (32) and phosphorylation (17) |
| PKA | Similar activity in cAF vs. sinus rhythm (34) |
| PP1, PP2A | Higher activity (34) |
| Myofilaments | Reduced maximum rate of tension generation and maximum active tension, reduced passive tension, and increase in myofilament Ca+ sensitivity (9) No changes in maximum force and passive force, reduced rate of tension redevelopment (71) Increased phosphorylation of cMyBP-C (9) Decreased phosphorylation of cMyBP-C (34) No changes in cTnI phosphorylation (9, 34) |
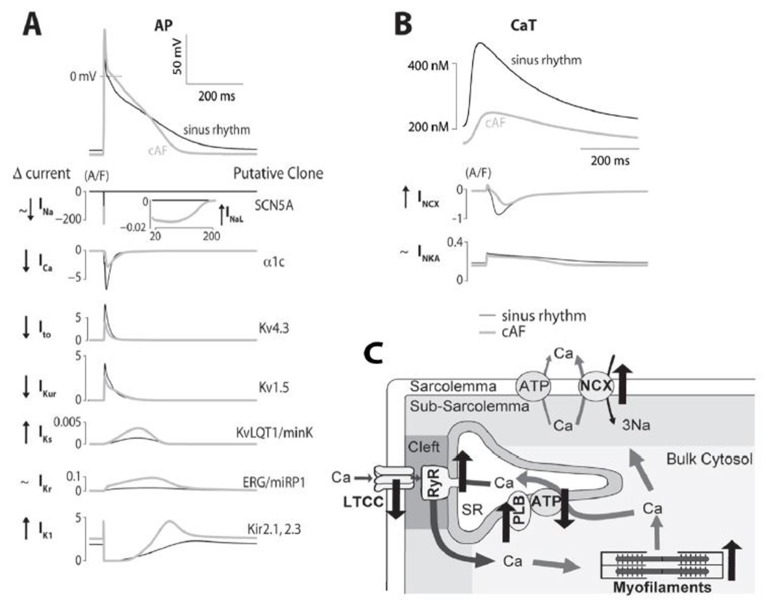
Figure 2 depicts simulated APs and CaT for sinus rhythm and cAF myocytes (from (16)) and the major ionic currents that are active during the cardiac cycle, and provides a graphical representation of the main changes occurring in the electrophysiological and Ca2+ handling processes in human AF.
Figure 2. Ionic bases of altered E-C coupling in AF. A) Simulated time courses of human atrial APs and contributing ionic currents are shown for sinus rhythm and cAF. Currents are listed on the left (with changes in cAF vs. sinus rhythm), and the genes encoding the channels are shown on the right. B) Simulated human atrial Ca2+ transients for sinus rhythm and cAF are shown with NCX and NKA currents (with changes in cAF vs. sinus rhythm on the left). C) Schematic representation of a human atrial myocyte illustrating the cAF-induced changes in Ca2+ handling proteins.
Atrial Cell Morphology
Cell capacitance of myocytes from cAF patients is greater than that of myocytes from SR patients, suggesting that AF cells are hypertrophied.[24] In fact, cells from AF patients are both longer and wider than those from patients in sinus rhythm.[17] Cell hypertrophy may contribute to cAF-induced global atrial dilation, along with changes of the extracellular matrix (with fibrosis and glycogen accumulation). Atrial dilation may itself have important consequences on cellular remodeling and alteration in protein composition and function of the atrial myocytes, as discussed later in this review.
It has recently been shown that atrial myocytes from human tissue sections exhibit extensive t-tubule networks.[25] The presence of t-tubules in the human atria (not detected in isolated human atrial myocytes),[26] may play an important role in determining the spatio-temporal properties of the intracellular CaT.[25] Notably, one can speculate that t-tubules could be subject to remodeling and contribute to perturbed E-C coupling in cAF, as suggested in sheep[27] and dog.[28] However, further investigations will be required to confirm this.
Protein Kinases and Phosphatases
Intracellular CaT is dynamically regulated via phosphorylation by protein kinase A (PKA) and Ca/calmodulin-dependent protein kinase II (CaMKII) of key Ca2+ handling and regulatory proteins, such as L-type Ca2+ channels, ryanodine receptors (RyRs), and phospholamban (PLN).[29,30] In addition, sarcomere proteins and various sarcolemmal ion channels are targets of both PKA and CaMKII.[29,31] The phosphorylation state of target proteins is also controlled by serine/threonine protein-phosphatases that are differentially regulated in distinct cardiomyocyte microdomains. Thus, altered protein kinase and phosphatase activity may importantly contribute to E-C coupling remodeling in AF. Indeed, CaMKII has been found to be more expressed and more phosphorylated in human cAF.[17,32] Similar PKA activity was found in cAF vs. sinus rhythm in goats,[33] but El-Armouche et al. detected a higher total activity of type 1 and type 2A phosphatases in human cAF, causing inhomogeneous changes in protein phosphorylation in different cellular compartments.[34] This may specifically amplify PKA and CaMKII effects on certain targets without having significant effects on others (e.g., higher phosphatase activity/lower phosphorylation in thick vs. thin myofilaments, cell membrane vs. SR).[34] Thus there is growing interest in the potential role of CaMKII and protein phosphatase inhibitors in preventing arrhythmogenic remodeling in cAF.
Sarcolemmal Ion Channels
INa
The Na+ current (INa) plays a crucial role in cardiac E-C coupling by initiating the AP, and is also a major determinant of the cardiac AP propagation. Bosch et al. reported that INa density and voltage-dependence of activation were not altered in human AF,[15] the steady-state inactivation was shifted to the right,[15] and no changes were detected in mRNA levels of the Na+ channel gene SCN5A.[35] In contrast, Sossalla et al. provided recent evidence that expression of Nav1.5 and peak INa density is decreased (slightly) in the atrial myocardium of patients with cAF.[36]
Although it is unclear whether altered fast INa (Fig. 2A, 2nd row) contributes to the electrical remodeling in human AF, it has recently been shown that the late Na+ current component, INaL (inset), is significantly increased in cAF patients.[36] Sossalla et al.[36] proposed that this increase could be due to the increase in neuronal Na+ channel isoforms (Nav1.1 expression is increased), or mediated by CaMKII, which is increased in AF[17,32] and known to regulate INaL,[37] or caused by oxidative stress.[38,39] However, our simulations suggested that an increased INaL does not contribute significantly to repolarization in cAF, where the overall APD90 was still shorter than that in normal healthy cells.[16] On the other hand, an increase in INaL may cause cellular Na+ and Ca2+ overload and lead to contractile dysfunction and electrical instability (via reverse-mode Na+/Ca2+ exchange).[29]
ICaL
The L-type Ca2+ current (ICaL) critically regulates E-C coupling by triggering SR Ca2+ release, and modulating AP shape and duration, i.e., maintaining the AP plateau.[29] Reduction in ICaL density (-50% vs. sinus rhythm, Fig. 2A, 3rd row) is one of the most consistent electrophysiological features of electrical remodeling in human AF (as seen in (5, 11, 12, 16, 18, 40)).Christ et al.[40] demonstrated that decreased ICaL density in cAF is not accompanied by altered expression of the corresponding α1c and β2a channel subunits (although other studies found different results),[41] and proposed that lower basal ICaL is due to decreased channel phosphorylation in AF, which results from an altered ratio of protein kinase/phosphatase activity in favor of increased phosphatase activity. An analogous explanation was proposed for the blunted effect of CaMKII inhibition on ICaL in human cAF.[17] It has been shown that blocking ICaL with nifedipine in normal human atrial cells results in an AP characteristic typically seen in AF[11] with respect to morphology, duration and impaired rate-dependent adaptation, i.e., reduction in ICaL seems to be a critical component of the remodeled atrial electrical phenotype. However, Workman et al. found that nifedipine did not significantly alter ERP in sinus rhythm myocytes (although APD was shorter), thus supporting the idea that ICaL downregulation may not be sufficient by itself to explain the remodeled atrial electrical phenotype.[12]
ICaT
There is no evidence of a T-type Ca2+ current (ICaT) in human atrial myocytes.[42,43]
If
The hyperpolarization-activated pacemaker current, If, ion channel has been found to be increased in human AF compared to sinus rhythm, at least at the mRNA level,[44] and could contribute to ectopic atrial pacemaker activity. However, functional evidence for If involvement is lacking at present.
Ito and IKur
The Ca2+-independent transient outward K+ current (Ito) and the ultra-rapid delayed rectifier K+ current (IKur) dominate the early AP repolarization phase and confer the atrial AP a characteristic triangular shape. Human cAF is associated with strong reduction of Ito (Fig. 2A, 4th row) density[5,12,15,24,45-47] and downregulation of its channel α subunit Kv4.3.[35,48] IKur(Fig. 2A, 5th row) was reduced in cAF[24,45,47,49] paralleled by diminished expression of Kv1.5.[35,45,[48] However, others have reported no changes in IKur density.[12,15,46] Inconsistent results about IKur function have been commented on previously by Christ et al. and attributed to different strategies for identification of IKur (e.g., pharmacological or with Ito-inactivating prepulse), and to a fraction of IKur that is not accounted for by Kv1.5.[49] The reduction in Ito and IKur explains the slight prolongation in earlier phases of the AP (Fig. 2A, 1st row).[16,50]
It has been shown that CaMKII (increased in cAF) positively regulates Ito in human atrial myocytes in acute conditions, as the application of the CaMKII inhibitor KN-93 caused loss of channel function.[32] The authors speculated that, by reducing the extent of inactivation of Ito, upregulation of CaMKII during atrial fibrillation reduces Ca2+ influx and therefore minimizes Ca+ overload. On the other hand, CaMKII overexpression in cAF may impact channel expression, thus contributing to Ito downregulation, as recently shown in CaMKII-overexpressing transgenic mice.[51]
Experimental evidence suggests that block of IKur enhances force of contraction of isolated human atrial trabeculae both in patients in sinus rhythm and AF.[22,23,52] We have recently predicted that block of IKur results in prolongation and elevation of the AP plateau, which augments the CaT amplitude that would elicit a positive inotropic effect.[16] Taken together, these studies suggest that IKur might be a potentially useful atrial-specific target to potentially counteract hypocontractility associated with cAF. A slight AP prolongation associated to IKur blockade may also be beneficial.
Caballero et al. have recently looked at differences in current density and AF-induced alterations in the right vs. left human atrium. They found heterogeneity in the repolarizing currents between the atria in sinus rhythm, and demonstrated that cAF reduced the Ito amplitude and density more markedly in the left than in the right atrium, thus creating a right-to-left gradient, whereas IKur was more markedly reduced in the right than in the left atrium, thus dissipating the left-to-right gradient detected in sinus rhythm.[24] However, the data concerning intra-atrial heterogeneities in repolarizing currents in human atrial myocytes are still limited, and it is unclear whether and how these changes may contribute to the perpetuation of arrhythmia.[16]
IKs and IKr
The delayed rectifier K+ currents have proven much harder to record and study in isolated human atrial cells.[53] Nevertheless, their contribution is likely to be small in cells that lack an appreciable plateau phase (e.g., see current densities in Fig. 2A, 6th and 7th rows).[54] The block of the rapidly activating delayed rectifier K+ current, IKr, has been shown to prolong human atrial APD in the late phase of repolarization by a small amount,[23] and to date no experimental evidence has suggested its involvement in AF-induced electrical remodeling.
Recently, Caballero et al. provided the first demonstration that cAF significantly increased the amplitude of the slow delayed rectifier K+ current, IKs, in both atria.[24] They suggested that IKs increase could contribute to cAF-induced shortening of APD and to further promote fibrillatory conduction, especially with current accumulation at high frequencies.
IK1 and IK,ACh
The inwardly rectifying K+ current (IK1) primarily controls the resting potential of the cardiac cell, and its much lower density in atrial than in ventricular myocytes[55] confers the atrial AP a more depolarized resting potential.[16] In cAF, increases in both current density[5,12,13,45,56] and mRNA levels[5,13] have been reported (Fig. 2A, 8th row). Increased IK1 causes a more negative resting membrane potential in cAF vs. sinus rhythm human atrial myocytes.[13,16,56]
Patients with chronic AF exhibit agonist-independent constitutive IK,ACh activity that contributes to the enhanced basal inward rectifier current and may result from abnormal channel phosphorylation by PKC.[13,56,57] Constitutively active IK,ACh is considered to support the maintenance of AF, together with increased IK1, by stabilizing reentrant activity sustained by rotors (faster activation, less meander).[58]
Recently, Voigt et al. found significant left-to-right gradients in IK1 and constitutively active IK,ACh in patients with paroxysmal AF, which were dissipated in cAF, raising the idea that this may contribute to left-to-right dominant frequency gradients that are often more evident in paroxysmal AF vs. cAF.[56]
IKATP
The ATP-sensitive K+ (IKATP) channels generate an inward rectifying current that activates with a decrease in intracellular ATP concentration.[59] Gene expression and electrophysiological studies in patients with atrial fibrillation demonstrated reduced mRNA levels of Kir6.248 and current activation,[60] but increased current was also reported.[61] Interestingly, a KATP channel mutation has been shown to confer risk for adrenergic atrial fibrillation originating from the vein of Marshall,[62] and it has been proposed that KATP channel deficit could play a broader role in the pathogenesis of electrical instability.[63] It is also conceivable that metabolic and mechanosensitive gating of KATP channels could be altered with structural heart disease and atrial dilation, thus providing a substrate for the more common acquired form of atrial fibrillation.[63]
Ca2+ and Na+ Handling
INCX
The Na+/Ca2+ exchanger current (INCX) is the main Ca2+ extrusion and Na+ influx pathway in cardiac myocytes. It extrudes 1 Ca+ in exchange for 3 Na+, thus generating an inward current that influences cardiac repolarization and arrhythmogenesis.[29] Increased expression[18,21,34] and abnormal function of INCX protein[16,18] are implicated in human AF pathophysiology. An increase in INCX may be an adaptive response to cellular Ca2+ loading and contribute to diminish the Ca2+ overload induced by rapid atrial pacing (along with ICa downregulation). Indeed, the decay rate of caffeine-evoked CaT (attributable to Ca2+ removal by NCX) is shown to be faster in human cAF vs. sinus rhythm myocytes.[16-18] Note that simulated INCX during an AP is smaller in AF than in sinus rhythm (Fig. 2B, 2nd row), due to the reduced CaT (Fig. 2B, 1st row). Na+ overload-induced Ca2+ influx via reverse-mode NCX has been implicated in Ca2+ overload and related arrhythmogenesis, whereas increase Ca2+ extrusion via forward-mode has been linked to delayed-afterdepolarizations.[29,64] Indeed, Na+ and Ca2+ loading are more favored at increased atrial rates (with AF). However, more studies are needed to assess whether delayed afterdepolarizations (DADs) are important in initiating arrhythmias in AF, and the underlying role of NCX in mediating them, since an increased IK1in cAF will tend to oppose the occurrence of such DADs. These studies will help determine if blocking NCX represents a novel therapeutic strategy in suppressing arrhythmia triggers in cAF.
INKA
The Na+/K+ pump (NKA) is the main route of Na+ efflux in cardiac cells thus regulating intracellular [Na+]. By extruding 3 Na+ in exchange for 2 K+ it generates an outward current that is known to influence resting membrane popotential and repolarization.[29] Workman et al. found no difference in NKA pump current in myocytes from cAF patients compared to sinus rhythm, and concluded that INKA is not involved in AF-induced electrophysiological remodeling in patients.[65] Our simulations show different NKA current underlying the AP (Fig. 2B, 3rd row) because of altered Na+ loading in cAF. Intracellular [Na+] changes may contribute to the human cAF phenotype, as we postulated in our modeling study[16] but have not yet measured.
Ryanodine Receptors
RyRs directly control SR Ca2+ release in cardiac muscles, activating contraction during E-C coupling.[29] Spontaneous Ca2+-release events (Ca2+ sparks) and Ca2+ waves through leaky RyR channels have been reported in myocytes from cAF patients[17,18,66,67] despite unaltered SR Ca2+ content. One potential contributor to RyR hyperactivity may be oxidative stress, which is known to play a critical role in AF pathophysiology[38] and increase RyR open probability. Neef et al. suggested that the CaMKII-dependent increase in SR Ca2+ leak caused by RyR hyperphosphorylation in AF is a potential arrhythmogenic mechanism,[17] because elimination of Ca2+ via inward INCX could lead to cell depolarization and cause DADs. Voigt et al. measured directly single RyRs isolated from cAF patients and demonstrated a higher channel open probability in cAF that responded to CaMKII inhibition.[68] Thus CaMKII inhibition may reduce the propensity for atrial arrhythmias.
SR Ca2+ ATP-ase and PLN
The SR Ca2+ ATP-ase (SERCA) is responsible for pumping Ca2+ back into the SR after Ca2+ release.[29] The endogenous inhibitor PLN regulates SERCA and releases its inhibition when phosphorylated by either PKA or CaMKII.[29,30] A decrease in SERCA activity, associated with smaller SERCA protein expression,[18,34] is evident in human cAF and explains the slower CaT decay compared to sinus rhythm.[16,18,34] On the other hand, reduced inhibition of SERCA by hyperphosphorylated PLN[34] in cAF could help to maintain a normal SR Ca2+ load despite increased RyR activity.
Ankyrin-B
Ankyrin-B (encoded by ANK2) is an adaptor protein expressed in excitable cells that targets ion channels (e.g., Na+ and Ca2+channels), transporters (e.g., NKA and NCX), and signaling molecules to specific membrane domains. In the heart, ankyrin-B loss-of-function mutations in humans lead to Long QT syndrome, AF, sinus node dysfunction and stress-induced ventricular arrhythmias.[69] Recently, reduced ankyrin-B expression has been demonstrated in atrial samples of patients with paroxysmal AF, and supported an association between ankyrin-B and AF.[70] A new potential molecular mechanism underlying ankyrin-associated AF has been proposed involving disrupted Cav1.3 (atrial L-type Ca2+ channels) membrane targeting in atrial myocytes.[70] It will be interesting to further explore the role of ankyrin in cAF.
Myofilaments
Altered Ca2+ handling (namely, downregulation of the L-type Ca2+ channels and increased Ca2+ extrusion via NCX) could account for the depressed contractility in remodeled atria, but a reduction of the maximum force generating capacity of the myofilaments and its Ca2+-sensitivity may also be involved. Indeed, recent studies have highlighted the potential role of sarcomeric proteins in the cAF induced hypocontractility,[9,34,71] although results are somewhat controversial. Compared to sinus rhythm myofibrils, cAF myofibrils exhibited reduced maximum rate of tension generation and maximum active tension, reduced passive tension, and increased in myofilament Ca2+ sensitivity.[9] An earlier study did not show significant changes in maximum force and passive force, but did report reduced rate of tension redevelopment in cAF.[71] One major difference between the two studies is that the former used left atrial samples whereas the latter used right atrial samples.
Altered phosphorylation state of various myofilament proteins was found in cAF vs. sinus rhythm. Phosphorylation of the primary sarcomere target of PKA, cTnI, was not altered in cAF atria.[9,34] The expression of the slow β-myosin heavy chain isoform (cMyBP-C)[9,34,71] was upregulated in cAF, and its phosphorylation levels were found significantly increased[9] or decreased.[34] It has been suggested that discrepancies between these results may be explained by a decrease in cMyBP-C phosphorylation in cAF reflecting atrial dilatation rather than being a component of cAF.[9] Another potential reason is the use of samples from the left atrium in the former study and from the right atrium in the latter.
Further studies are needed to resolve these inconsistencies. Furthermore, cell shortening data that are currently missing in human atrial myocytes may help in linking these molecular changes to functional alterations.
It is becoming increasingly clear that studies of remodeling of human atrium by chronic AF are frequently and unavoidably influenced in part by multiple confounding clinical variables such as patient age, sex, disease history, and drug treatments. Furthermore, the changes in ion currents and APs should be considered to be associated with, rather than necessarily caused by, the chronic AF. Nevertheless, the concordance between these human chronic AF data and AF/atrial tachypacing-induced changes in animal models (see Table 1 in([72]))supports the view that chronic AF causes atrial electrophysiological remodeling in humans.
Consequences of Ventricular Dysfunction and Atrial Dilation on Human Atrial AP
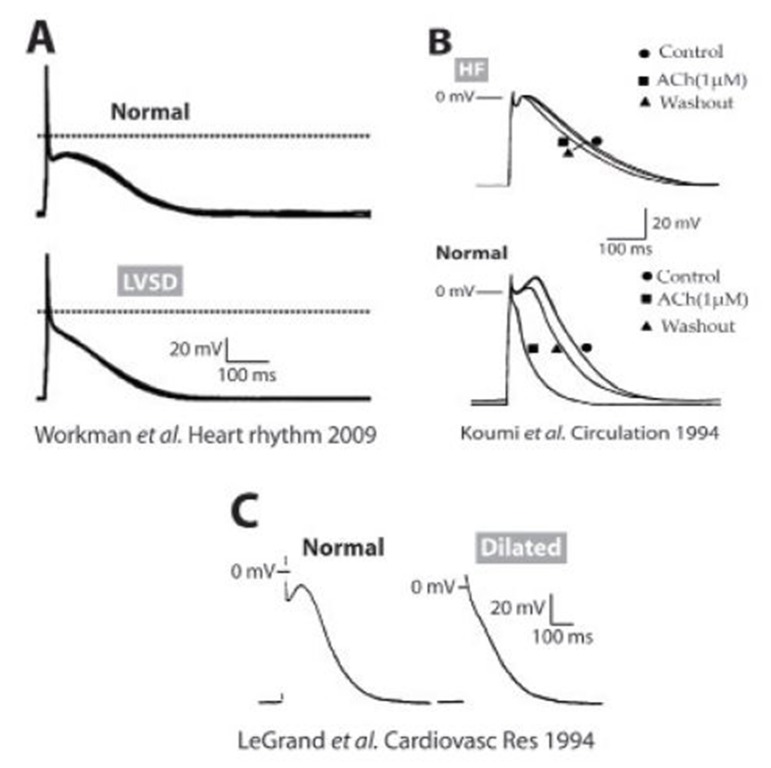
Structural, electrophysiological, mechanical, metabolic and neurohumoral remodeling associated with cardiac disorders, such as coronary artery disease, CHF, and left ventricular systolic dysfunction (LVSD) may increase AF risk.[73] In atrial cells isolated from patients with CHF or LVSD, AP duration was either unchanged (Fig. 3A)[74,75] or increased (Fig. 3B).[76] In patients in sinus rhythm with reduced left ventricular ejection fraction (< 45%) APD90 was shorter than in patients with higher ejection fraction,[75] and there was a significant correlation between cellular ERP shortening and decreasing left ventricular ejection fraction.[75] Furthermore, multivariate analysis adjusting for 10 relevant clinical covariates confirmed that LVSD was independently associated with atrial cellular ERP-shortening, which may, therefore, be expected to contribute to a predisposition to AF in these patients. The features of ionic remodeling in CHF or LVSD in human atrium are not fully understood. ICaL was either decreased in patients with coronary artery disease, aortic valve disease, or mitral valve disease[77] or unchanged in LVSD or CHF patients.[75,78] Schreieck et al. found increased Ito in human atrial myocytes of patients with reduced left ventricular function, with no change in its voltage dependence or decay, but with enhanced reactivation.[74] However, this Ito increase may have been confounded by the lower proportion of patients treated with β-blockers in the reduced LV function group, since such treatment is associated with decreased Ito in human atrium.[79] In contrast, Workman et al. found that LVSD was associated with decreased Ito, a positive shift in its activation voltage, and no change in its decay kinetics.[75] Koumi et al. reported low resting membrane potential in atrial myocytes from CHF patients, possibly due to reduced density of IK1 and IK,ACh.[76] Workman et al. reported unchanged IK1 in LVSD,[75] although Ba2+-sensitive IK1 or IK,ACh were not measured. Unchanged atrial IKur has also been reported in human LVSD.[74,75]
Figure 3. Altered atrial APs in human ventricular dysfunction. A) Atrial APs from patients with moderate or severe LVSD (bottom) and from patients without LVSD (top) (75). B) Representative AP and response to ACh in isolated atrial myocytes from HF (top) and donor (bottom) hearts at a stimulation frequency of 1 Hz (76). C) Representative APs from a non-dilated (normal, left) and a dilated (right) atrium recorded at a pacing frequency of 1 Hz (80).
Cardiac dilatation is known to develop frequently during the course of cardiac failure.[80] In trabeculae and myocytes taken from dilated atria the AP was shorter and the plateau was markedly depressed (Fig. 3C) compared to trabeculae and myocytes from non-dilated atria.[80] However, it must be noted that the ventricular dysfunction was not quantified in these patients. AP changes were explained with more severely depressed ICaL compared to the reduction in total outward current.[80]
Overall, the ionic bases of altered atrial function in patients with ventricular dysfunction, and how it predisposes to more frequent AF episodes culminating in cAF, remains poorly understood.
Autonomic Changes in Chronic AF and Related Myocardial Diseases
The autonomic nervous system, and particularly the relative activities of the sympathetic (adrenergic) and parasympathetic (cholinergic) branches, have a major influence on the occurrence of AF. Furthermore, chronic AF, and certain predisposing cardiac pathologies, remodel atrial electrophysiological responses to catecholamines and acetylcholine and thus influence the electrophysiological mechanisms of AF. β-adrenergic stimulation increases human atrial ICaL,[11,41,42,81] IKur[79,82] and If,[83,84] has no effect on IK1,[79] IKA,Ch[85] or Ito,[79] and has markedly different effects on connexin conductance or expression, depending upon their main molecular correlate; i.e. Cx,[40] Cx,[43] Cx45.[86] The increased ICaL and IKur, with lack of effect on other repolarizing currents, results in no net effect of β-stimulation on atrial APD,[90] as predicted by our model,[16] consistent with 5 of 6 reports in human atrial cells or tissues.[87] However, the increased ICaL markedly elevates the AP plateau[81] and, coupled with increased [Ca2+]i from PLN phosphorylation by adrenergic stimulation,[88] favors non-reentrant activity such as afterdepolarizations.[87]
Human atrial studies of α-adrenergic stimulation are sparse: phenylephrine inhibited IK1,[85,89] IK,ACh[85] and IKur[82] also potentially promoting afterdepolarizations. Chronic AF consistently potentiates the effect of β-adrenergic stimulation to increase human atrial ICaL.[11,40,43,90] While this could, in theory, increase the propensity for afterdepolarizations in the presence of catecholamines, chronic AF also markedly decreases basal ICaL[6] and attenuates the effects of α-stimulation on IK1 and IK,ACh.[85] Chronic AF may also cause increased atrial adrenergic innervation, “neural remodeling”, in patients.[91] The effects of chronic AF on [Ca2+-]iresponses to adrenergic stimulation have yet to be studied in human atrium. Data on effects of myocardial diseases that predispose to AF, on human atrial adrenergic responses, are equivocal: the ability of β-stimulation to increase ICaL was attenuated,[92,93] unchanged[75] or potentiated[77] in association with HF or LVSD. An attenuated ICaL-increase was also reported in cells obtained from dilated atria from explanted hearts.[78] Attenuated β-responses may involve reduced β-receptor density or function.[87] Post-operative AF was not predicted by any change in the pre-operative atrial ICaL response to β-stimulation.[94] Cholinergic elevation and increased levels of acetylcholine activate IK,ACh and also antagonize effects of catecholamines on ICaL, both shortening APD and ERP, thus promoting reentry.[95] Also, combined adrenergic/cholinergic-stimulation may produce “late phase EADs”,[96] possibly by concurrently shortening APD and increasing [Ca2+].[97] Chronic AF induces a constitutively active IK,ACh in human atrium,[57] likely resulting from a PKC isoform switch.[98] However, the acetylcholine-mediated increase in atrial IK,ACh[13,57] and shortening in atrial APD13 were each attenuated in chronic AF, and the cholinergic receptors GIRK[1] and GIRK[4] were generally downregulated.[5] The attenuation of atrial cholinergic responses by chronic AF may be restricted to the right atrium.[56] The ability of acetylcholine to increase IK,ACh and/or shorten atrial APD may be attenuated by HF, as shown in dogs.[99] However, corroborative data from human patients with HF or LVSD are sparse and confounded by the presence of chronic AF.[76] While much progress has been made, the complex and interacting influences of chronic AF and its predisposing myocardial pathologies on the involvement of the autonomic system in AF are yet to be resolved.
Oxidative Stress and Inflammation-related Changes in Human AF
Patients undergoing cardiac surgery often experience post-operative AF. It has been shown that these patients did not exhibit the electrophysiological remodeling seen in patients with cAF as far as Ca2+ and K+ currents and AP characteristics are concerned,[94] whereas altered atrial Ca2+ handling in post-operative AF patients has not yet been studied. However, Van Wagoner et al. showed that patients with the highest ICaL density pre-surgery, were associated with post-operative AF, thus indirectly suggesting a role for Ca2+overload, mediated via oxidative/inflammatory stress, as a possible trigger.[11] This is because increased levels of inflammatory markers are often recorded after cardiac surgery, and recent evidence suggests oxidative stress may play an important role in the pathogenesis and perpetuation of post-operative AF.[100]
Several studies have shown increased myocardial oxidative stress associated with AF.[38,101] In addition, inflammatory markers such as interleukin-6 and C-reactive protein have been found elevated in AF patients.[101,102] Evidence suggests that in several pathophysiological conditions inflammation and oxidative stress are highly interrelated, whereby inflammation augments oxidative stress and viceversa, and may be involved in AF pathogenesis. Importantly, oxidant and inflammatory mechanisms may contribute to the described structural, electrophysiological, and contractile remodeling that favors maintenance of AF,[103] and thus could be considered as targets for AF-treatment, as discussed in more comprehensive reviews on the topic.[101,104] Inflammatory processes may contribute to atrial injury resulting in myocyte hypertrophy and fibrosis. Furthermore, several Ca2+ channels and transporters are the subject of redox modulation.[105] For example, oxidative stress may play an important role in ICa changes, as it has been shown that S-nitrosylation of the L-type Ca2+ channel α subunit is increased in AF, and exogenously applied glutathione partially restores the AF-related ICa reduction.[106] We also discussed above the potential contribution of oxidation in AF-associated RyR hyperactivity. Several K+ channels (e.g., Ito[107] and IKATP) are also sensitive to redox state. Kv1.5 currents are inhibited by oxidation by S-nitrosylation,[108] which may contribute to IKur suppression in AF. Redox-dependent modulation of Na+ channel activity has also been reported.[109] Additionally, oxidative stress may affect myofilament protein function[38] and influence the activity of protein kinases (e.g., CaMKII[110]) and phosphatases that alter E-C coupling via phosphorylation of target proteins and are also redox sensitive. Suppressing E-C coupling remodeling with anti-inflammatory and antioxidant drugs (such as glucocorticoids and statins) has proven clinically useful in some cases in preventing AF recurrence.[104] In sum, we are still limited in our understanding regarding how oxidative/inflammatory stress influences E-C coupling, particularly in the context of chronic AF, and further studies are warranted.
Conclusions
Chronic AF is associated with altered expression and activity of numerous sarcolemmal ion channels, transporters, Ca2+ handling and myofilament proteins. Understanding the ionic mechanisms underlying E-C coupling remodeling in fibrillating human atria, and distinguishing compensatory responses from maladaptive mechanisms, may allow for identification of new therapeutic targets to improve electrical and contractile function in cAF patients.
Acknowledgements
Supported by NHLBI Grants P01HL039707, P01HL087226, and the Leducq Foundation (to Dr. José Jalife), NHLBI Grants P01-HL080101, R37-HL30077-29 and the Leducq Foundation (to Dr. Donald Bers). We would like to thank Drs. José Jalife, Donald Bers, and Dobromir Dobrev for useful feedback and discussions.
References
- 1.Damani Samir B, Topol Eric J. Molecular genetics of atrial fibrillation. Genome Med. 2009 May 22;1 (5) doi: 10.1186/gm54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin Emelia J, Chen Peng-Sheng, Bild Diane E, Mascette Alice M, Albert Christine M, Alonso Alvaro, Calkins Hugh, Connolly Stuart J, Curtis Anne B, Darbar Dawood, Ellinor Patrick T, Go Alan S, Goldschlager Nora F, Heckbert Susan R, Jalife José, Kerr Charles R, Levy Daniel, Lloyd-Jones Donald M, Massie Barry M, Nattel Stanley, Olgin Jeffrey E, Packer Douglas L, Po Sunny S, Tsang Teresa S M, Van Wagoner David R, Waldo Albert L, Wyse D George. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009 Feb 03;119 (4):606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nattel Stanley, Burstein Brett, Dobrev Dobromir. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008 Apr;1 (1):62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 4.Wakili Reza, Voigt Niels, Kääb Stefan, Dobrev Dobromir, Nattel Stanley. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Invest. 2011 Aug;121 (8):2955–68. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrev Dobromir, Ravens Ursula. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res. Cardiol. 2003 May;98 (3):137–48. doi: 10.1007/s00395-003-0409-8. [DOI] [PubMed] [Google Scholar]
- 6.Workman Antony J, Kane Kathleen A, Rankin Andrew C. Cellular bases for human atrial fibrillation. Heart Rhythm. 2008 Jun;5 (6 Suppl):S1–6. doi: 10.1016/j.hrthm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrev Dobromir, Nattel Stanley. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J. Cardiovasc. Pharmacol. 2008 Oct;52 (4):293–9. doi: 10.1097/FJC.0b013e318171924d. [DOI] [PubMed] [Google Scholar]
- 8.Dobrev Dobromir, Voigt Niels, Wehrens Xander H T. The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc. Res. 2011 Mar 01;89 (4):734–43. doi: 10.1093/cvr/cvq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belus Alexandra, Piroddi Nicoletta, Ferrantini Cecilia, Tesi Chiara, Cazorla Olivier, Toniolo Luana, Drost Maurice, Mearini Giulia, Carrier Lucie, Rossi Alessandra, Mugelli Alessandro, Cerbai Elisabetta, van der Velden Jolanda, Poggesi Corrado. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ. Res. 2010 Jul 09;107 (1):144–52. doi: 10.1161/CIRCRESAHA.110.220699. [DOI] [PubMed] [Google Scholar]
- 10.Llach Anna, Molina Cristina E, Fernandes Jacqueline, Padró Josep, Cinca Juan, Hove-Madsen Leif. Sarcoplasmic reticulum and L-type Ca²⁺ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J. Physiol. (Lond.) 2011 Jul 01;589 (Pt 13):3247–62. doi: 10.1113/jphysiol.2010.197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Wagoner D R, Pond A L, Lamorgese M, Rossie S S, McCarthy P M, Nerbonne J M. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ. Res. 1999 Sep 03;85 (5):428–36. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 12.Workman A J, Kane K A, Rankin A C. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc. Res. 2001 Nov;52 (2):226–35. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 13.Dobrev D, Graf E, Wettwer E, Himmel H M, Hála O, Doerfel C, Christ T, Schüler S, Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001 Nov 20;104 (21):2551–7. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 14.Boutjdir M, Le Heuzey J Y, Lavergne T, Chauvaud S, Guize L, Carpentier A, Peronneau P. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol. 1986 Nov;9 (6 Pt 2):1095–100. doi: 10.1111/j.1540-8159.1986.tb06676.x. [DOI] [PubMed] [Google Scholar]
- 15.Bosch R F, Zeng X, Grammer J B, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 1999 Oct;44 (1):121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 16.Grandi Eleonora, Pandit Sandeep V, Voigt Niels, Workman Antony J, Dobrev Dobromir, Jalife José, Bers Donald M. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ. Res. 2011 Oct 14;109 (9):1055–66. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neef Stefan, Dybkova Nataliya, Sossalla Samuel, Ort Katharina R, Fluschnik Nina, Neumann Kay, Seipelt Ralf, Schöndube Friedrich A, Hasenfuss Gerd, Maier Lars S. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ. Res. 2010 Apr 02;106 (6):1134–44. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 18.Voigt N, Trafford AW, Ravens U, Dobrev D. Abstract 2630: Cellular and Molecular Determinants of Altered Atrial Ca2+ Signaling in Patients With Chronic Atrial Fibrillation. Circulation. 2009;120:0–0. [Google Scholar]
- 19.Liang Xin, Xie Hong, Zhu Pei-Hong, Hu Jun, Zhao Qiang, Wang Chun-Sheng, Yang Cheng. Ryanodine receptor-mediated Ca2+ events in atrial myocytes of patients with atrial fibrillation. Cardiology. 2008;111 (2):102–10. doi: 10.1159/000119697. [DOI] [PubMed] [Google Scholar]
- 20.Maier L S, Barckhausen P, Weisser J, Aleksic I, Baryalei M, Pieske B. Ca(2+) handling in isolated human atrial myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000 Sep;279 (3):H952–8. doi: 10.1152/ajpheart.2000.279.3.H952. [DOI] [PubMed] [Google Scholar]
- 21.Schotten Ulrich, Greiser Maura, Benke Dirk, Buerkel Kai, Ehrenteidt Britta, Stellbrink Christoph, Vazquez-Jimenez Jaime F, Schoendube Friedrich, Hanrath Peter, Allessie Maurits. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc. Res. 2002 Jan;53 (1):192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 22.Schotten Ulrich, de Haan Sunniva, Verheule Sander, Harks Erik G A, Frechen Dirk, Bodewig Eva, Greiser Maura, Ram Rashmi, Maessen Jos, Kelm Malte, Allessie Maurits, Van Wagoner David R. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc. Res. 2007 Jan 01;73 (1):37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Wettwer Erich, Hála Ottó, Christ Torsten, Heubach Jürgen F, Dobrev Dobromir, Knaut Michael, Varró András, Ravens Ursula. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004 Oct 19;110 (16):2299–306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 24.Caballero Ricardo, de la Fuente Marta González, Gómez Ricardo, Barana Adriana, Amorós Irene, Dolz-Gaitón Pablo, Osuna Lourdes, Almendral Jesús, Atienza Felipe, Fernández-Avilés Francisco, Pita Ana, Rodríguez-Roda Jorge, Pinto Angel, Tamargo Juan, Delpón Eva. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J. Am. Coll. Cardiol. 2010 May 25;55 (21):2346–54. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Richards M A, Clarke J D, Saravanan P, Voigt N, Dobrev D, Eisner D A, Trafford A W, Dibb K M. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am. J. Physiol. Heart Circ. Physiol. 2011 Nov;301 (5):H1996–2005. doi: 10.1152/ajpheart.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrev D, Teos Leyla Y, Lederer W J. Unique atrial myocyte Ca2+ signaling. J. Mol. Cell. Cardiol. 2009 Apr;46 (4):448–51. doi: 10.1016/j.yjmcc.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenaerts Ilse, Bito Virginie, Heinzel Frank R, Driesen Ronald B, Holemans Patricia, D'hooge Jan, Heidbüchel Hein, Sipido Karin R, Willems Rik. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ. Res. 2009 Oct 23;105 (9):876–85. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- 28.Wakili Reza, Yeh Yung-Hsin, Yan Qi Xiao, Greiser Maura, Chartier Denis, Nishida Kunihiro, Maguy Ange, Villeneuve Louis-Robert, Boknik Peter, Voigt Niels, Krysiak Judith, Kääb Stefan, Ravens Ursula, Linke Wolfgang A, Stienen Gerrit J M, Shi Yanfen, Tardif Jean-Claude, Schotten Ulrich, Dobrev Dobromir, Nattel Stanley. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol. 2010 Oct;3 (5):530–41. doi: 10.1161/CIRCEP.109.933036. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers. 2001;0:0–0. [Google Scholar]
- 30.Maier Lars S, Bers Donald M. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 2007 Mar 01;73 (4):631–40. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Bers Donald M, Grandi Eleonora. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009 Sep;54 (3):180–7. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tessier S, Karczewski P, Krause E G, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier J J, Hatem S N. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ. Res. 1999 Oct 29;85 (9):810–9. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 33.Greiser Maura, Neuberger Hans-Ruprecht, Harks Erik, El-Armouche Ali, Boknik Peter, de Haan Sunniva, Verheyen Fons, Verheule Sander, Schmitz Wilhelm, Ravens Ursula, Nattel Stanley, Allessie Maurits A, Dobrev Dobromir, Schotten Ulrich. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J. Mol. Cell. Cardiol. 2009 Mar;46 (3):385–94. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 34.El-Armouche Ali, Boknik Peter, Eschenhagen Thomas, Carrier Lucie, Knaut Michael, Ravens Ursula, Dobrev Dobromir. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006 Aug 15;114 (7):670–80. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 35.Brundel B J, Van Gelder I C, Henning R H, Tieleman R G, Tuinenburg A E, Wietses M, Grandjean J G, Van Gilst W H, Crijns H J. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001 Feb 06;103 (5):684–90. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 36.Sossalla Samuel, Kallmeyer Birte, Wagner Stefan, Mazur Marek, Maurer Ulrike, Toischer Karl, Schmitto Jan D, Seipelt Ralf, Schöndube Friedrich A, Hasenfuss Gerd, Belardinelli Luiz, Maier Lars S. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J. Am. Coll. Cardiol. 2010 May 25;55 (21):2330–42. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 37.Wagner Stefan, Dybkova Nataliya, Rasenack Eva C L, Jacobshagen Claudius, Fabritz Larissa, Kirchhof Paulus, Maier Sebastian K G, Zhang Tong, Hasenfuss Gerd, Brown Joan Heller, Bers Donald M, Maier Lars S. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006 Dec;116 (12):3127–38. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mihm M J, Yu F, Carnes C A, Reiser P J, McCarthy P M, Van Wagoner D R, Bauer J A. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001 Jul 10;104 (2):174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 39.Wagner Stefan, Ruff Hanna M, Weber Sarah L, Bellmann Sarah, Sowa Thomas, Schulte Timo, Anderson Mark E, Grandi Eleonora, Bers Donald M, Backs Johannes, Belardinelli Luiz, Maier Lars S. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ. Res. 2011 Mar 04;108 (5):555–65. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christ T, Boknik P, Wöhrl S, Wettwer E, Graf E M, Bosch R F, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004 Oct 26;110 (17):2651–7. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 41.Brundel B J, van Gelder I C, Henning R H, Tuinenburg A E, Deelman L E, Tieleman R G, Grandjean J G, van Gilst W H, Crijns H J. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc. Res. 1999 May;42 (2):443–54. doi: 10.1016/s0008-6363(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 42.Li G R, Nattel S. Properties of human atrial ICa at physiological temperatures and relevance to action potential. Am. J. Physiol. 1997 Jan;272 (1 Pt 2):H227–35. doi: 10.1152/ajpheart.1997.272.1.H227. [DOI] [PubMed] [Google Scholar]
- 43.Skasa M, Jüngling E, Picht E, Schöndube F, Lückhoff A. L-type calcium currents in atrial myocytes from patients with persistent and non-persistent atrial fibrillation. Basic Res. Cardiol. 2001 Apr;96 (2):151–9. doi: 10.1007/s003950170065. [DOI] [PubMed] [Google Scholar]
- 44.Lai L P, Su M J, Lin J L, Tsai C H, Lin F Y, Chen Y S, Hwang J J, Huang S K, Tseng Y Z, Lien W P. Measurement of funny current (I(f)) channel mRNA in human atrial tissue: correlation with left atrial filling pressure and atrial fibrillation. J. Cardiovasc. Electrophysiol. 1999 Jul;10 (7):947–53. doi: 10.1111/j.1540-8167.1999.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Wagoner D R, Pond A L, McCarthy P M, Trimmer J S, Nerbonne J M. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 1997 Jun;80 (6):772–81. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 46.Grammer J B, Bosch R F, Kühlkamp V, Seipel L. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2000 Jun;11 (6):626–33. doi: 10.1111/j.1540-8167.2000.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 47.Brandt M C, Priebe L, Böhle T, Südkamp M, Beuckelmann D J. The ultrarapid and the transient outward K(+) current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation. J. Mol. Cell. Cardiol. 2000 Oct;32 (10):1885–96. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- 48.Brundel B J, Van Gelder I C, Henning R H, Tuinenburg A E, Wietses M, Grandjean J G, Wilde A A, Van Gilst W H, Crijns H J. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J. Am. Coll. Cardiol. 2001 Mar 01;37 (3):926–32. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 49.Christ T, Wettwer E, Voigt N, Hála O, Radicke S, Matschke K, Várro A, Dobrev D, Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br. J. Pharmacol. 2008 Aug;154 (8):1619–30. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Wagoner D R, Nerbonne J M. Molecular basis of electrical remodeling in atrial fibrillation. J. Mol. Cell. Cardiol. 2000 Jun;32 (6):1101–17. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 51.Wagner Stefan, Hacker Elena, Grandi Eleonora, Weber Sarah L, Dybkova Nataliya, Sossalla Samuel, Sowa Thomas, Fabritz Larissa, Kirchhof Paulus, Bers Donald M, Maier Lars S. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009 Jun;2 (3):285–94. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata E F, Drury T, Refsum H, Aldrete V, Giles W. Contributions of a transient outward current to repolarization in human atrium. Am. J. Physiol. 1989 Dec;257 (6 Pt 2):H1773–81. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- 53.Firek L, Giles W R. Outward currents underlying repolarization in human atrial myocytes. Cardiovasc. Res. 1995 Jul;30 (1):31–8. [PubMed] [Google Scholar]
- 54.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ. Res. 1993 Aug;73 (2):276–85. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- 55.Schram Gernot, Pourrier Marc, Melnyk Peter, Nattel Stanley. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ. Res. 2002 May 17;90 (9):939–50. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 56.Voigt Niels, Trausch Anne, Knaut Michael, Matschke Klaus, Varró András, Van Wagoner David R, Nattel Stanley, Ravens Ursula, Dobrev Dobromir. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Oct;3 (5):472–80. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005 Dec 13;112 (24):3697–706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 58.Pandit Sandeep V, Berenfeld Omer, Anumonwo Justus M B, Zaritski Roman M, Kneller James, Nattel Stanley, Jalife José. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys. J. 2005 Jun;88 (6):3806–21. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Haixia, Flagg Thomas P, Nichols Colin G. Cardiac sarcolemmal K(ATP) channels: Latest twists in a questing tale! J. Mol. Cell. Cardiol. 2010 Jan;48 (1):71–5. doi: 10.1016/j.yjmcc.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balana Bartosz, Dobrev Dobromir, Wettwer Erich, Christ Torsten, Knaut Michael, Ravens Ursula. Decreased ATP-sensitive K(+) current density during chronic human atrial fibrillation. J. Mol. Cell. Cardiol. 2003 Dec;35 (12):1399–405. doi: 10.1016/s0022-2828(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 61.Wu Gang, Huang Cong-xin, Tang Yan-hong, Jiang Hong, Wan Jun, Chen Hui, Xie Qiang, Huang Zheng-rong. Changes of IK, ATP current density and allosteric modulation during chronic atrial fibrillation. Chin. Med. J. 2005 Jul 20;118 (14):1161–6. [PubMed] [Google Scholar]
- 62.Olson Timothy M, Alekseev Alexey E, Moreau Christophe, Liu Xiaoke K, Zingman Leonid V, Miki Takashi, Seino Susumu, Asirvatham Samuel J, Jahangir Arshad, Terzic Andre. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007 Feb;4 (2):110–6. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olson Timothy M, Terzic Andre. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010 Jul;460 (2):295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Priori S G, Corr P B. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am. J. Physiol. 1990 Jun;258 (6 Pt 2):H1796–805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 65.Workman Antony J, Kane Kathleen A, Rankin Andrew C. Characterisation of the Na, K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc. Res. 2003 Sep 01;59 (3):593–602. doi: 10.1016/s0008-6363(03)00466-8. [DOI] [PubMed] [Google Scholar]
- 66.Chelu Mihail G, Sarma Satyam, Sood Subeena, Wang Sufen, van Oort Ralph J, Skapura Darlene G, Li Na, Santonastasi Marco, Müller Frank Ulrich, Schmitz Wilhelm, Schotten Ulrich, Anderson Mark E, Valderrábano Miguel, Dobrev Dobromir, Wehrens Xander H T. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J. Clin. Invest. 2009 Jul;119 (7):1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vest John A, Wehrens Xander H T, Reiken Steven R, Lehnart Stephan E, Dobrev Dobromir, Chandra Parag, Danilo Peter, Ravens Ursula, Rosen Michael R, Marks Andrew R. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005 Apr 26;111 (16):2025–32. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 68.Voigt Niels, Li Na, Wang Qiongling, Wang Wei, Trafford Andrew W, Abu-Taha Issam, Sun Qiang, Wieland Thomas, Ravens Ursula, Nattel Stanley, Wehrens Xander H T, Dobrev Dobromir. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012 May 01;125 (17):2059–70. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohler Peter J, Schott Jean-Jacques, Gramolini Anthony O, Dilly Keith W, Guatimosim Silvia, duBell William H, Song Long-Sheng, Haurogné Karine, Kyndt Florence, Ali Mervat E, Rogers Terry B, Lederer W J, Escande Denis, Le Marec Herve, Bennett Vann. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003 Feb 06;421 (6923):634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 70.Cunha Shane R, Hund Thomas J, Hashemi Seyed, Voigt Niels, Li Na, Wright Patrick, Koval Olha, Li Jingdong, Gudmundsson Hjalti, Gumina Richard J, Karck Matthias, Schott Jean-Jacques, Probst Vincent, Le Marec Herve, Anderson Mark E, Dobrev Dobromir, Wehrens Xander H T, Mohler Peter J. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation. 2011 Sep 13;124 (11):1212–22. doi: 10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eiras S, Narolska N A, van Loon R B, Boontje N M, Zaremba R, Jimenez C R, Visser F C, Stooker W, van der Velden J, Stienen G J M. Alterations in contractile protein composition and function in human atrial dilatation and atrial fibrillation. J. Mol. Cell. Cardiol. 2006 Sep;41 (3):467–77. doi: 10.1016/j.yjmcc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 72.Workman A J, Smith G L, Rankin A C. Mechanisms of termination and prevention of atrial fibrillation by drug therapy. Pharmacol. Ther. 2011 Aug;131 (2):221–41. doi: 10.1016/j.pharmthera.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neuberger Hans-Ruprecht, Mewis Christian, van Veldhuisen Dirk J, Schotten Ulrich, van Gelder Isabelle C, Allessie Maurits A, Böhm Michael. Management of atrial fibrillation in patients with heart failure. Eur. Heart J. 2007 Nov;28 (21):2568–77. doi: 10.1093/eurheartj/ehm341. [DOI] [PubMed] [Google Scholar]
- 74.Schreieck J, Wang Y, Overbeck M, Schömig A, Schmitt C. Altered transient outward current in human atrial myocytes of patients with reduced left ventricular function. J. Cardiovasc. Electrophysiol. 2000 Feb;11 (2):180–92. doi: 10.1111/j.1540-8167.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 75.Workman Antony J, Pau Davide, Redpath Calum J, Marshall Gillian E, Russell Julie A, Norrie John, Kane Kathleen A, Rankin Andrew C. Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF. Heart Rhythm. 2009 Apr;6 (4):445–51. doi: 10.1016/j.hrthm.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koumi S, Arentzen C E, Backer C L, Wasserstrom J A. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994 Nov;90 (5):2213–24. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- 77.Dinanian Sylvie, Boixel Christophe, Juin Christophe, Hulot Jean-Sébastien, Coulombe Alain, Rücker-Martin Catherine, Bonnet Nicolas, Le Grand Bruno, Slama Michel, Mercadier Jean-Jacques, Hatem Stéphane N. Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation. Eur. Heart J. 2008 May;29 (9):1190–7. doi: 10.1093/eurheartj/ehn140. [DOI] [PubMed] [Google Scholar]
- 78.Cheng T H, Lee F Y, Wei J, Lin C I. Comparison of calcium-current in isolated atrial myocytes from failing and nonfailing human hearts. Mol. Cell. Biochem. 1996 Apr 12;157 (1-2):157–62. doi: 10.1007/BF00227894. [DOI] [PubMed] [Google Scholar]
- 79.Marshall Gillian E, Russell Julie A, Tellez James O, Jhund Pardeep S, Currie Susan, Dempster John, Boyett Mark R, Kane Kathleen A, Rankin Andrew C, Workman Antony J. Remodelling of human atrial K+ currents but not ion channel expression by chronic β-blockade. Pflugers Arch. 2012 Apr;463 (4):537–48. doi: 10.1007/s00424-011-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Grand B L, Hatem S, Deroubaix E, Couétil J P, Coraboeuf E. Depressed transient outward and calcium currents in dilated human atria. Cardiovasc. Res. 1994 Apr;28 (4):548–56. doi: 10.1093/cvr/28.4.548. [DOI] [PubMed] [Google Scholar]
- 81.Redpath Calum J, Rankin Andrew C, Kane Kathleen A, Workman Antony J. Anti-adrenergic effects of endothelin on human atrial action potentials are potentially anti-arrhythmic. J. Mol. Cell. Cardiol. 2006 May;40 (5):717–24. doi: 10.1016/j.yjmcc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Li G R, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ. Res. 1996 May;78 (5):903–15. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- 83.Hoppe U C, Beuckelmann D J. Characterization of the hyperpolarization-activated inward current in isolated human atrial myocytes. Cardiovasc. Res. 1998 Jun;38 (3):788–801. doi: 10.1016/s0008-6363(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 84.Lonardo Giuseppe, Cerbai Elisabetta, Casini Simona, Giunti Gabriele, Bonacchi Massimo, Battaglia Francesco, Fiorani Brenno, Stefano Pier Luigi, Sani Guido, Mugelli Alessandro. Pharmacological modulation of the hyperpolarization-activated current (I f) in human atrial myocytes: focus on G protein-coupled receptors. J. Mol. Cell. Cardiol. 2005 Mar;38 (3):453–60. doi: 10.1016/j.yjmcc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Voigt N, Bollman B, Wettwer E, Matschke K, Ravens U, Dobrev D. Abstract: Alpha-adrenergic regulation of IK1 and ACh-gated IK,ACh is impaired in patients with atrial fibrillation. Naunyn-Schmiedeberg’s Arch Pharmacol. 2009;379:52–0. [Google Scholar]
- 86.Salameh Aida, Dhein Stefan. Adrenergic control of cardiac gap junction function and expression. Naunyn Schmiedebergs Arch. Pharmacol. 2011 Apr;383 (4):331–46. doi: 10.1007/s00210-011-0603-4. [DOI] [PubMed] [Google Scholar]
- 87.Workman Antony J. Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 2010 Mar;381 (3):235–49. doi: 10.1007/s00210-009-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bers Donald M. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415 (6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 89.Su M.-J., Chi J.-F., Chu S.-H.. Adrenergic Modulation of Potassium Currents in Isolated Human Atrial Myocytes. J. Biomed. Sci. 1994 Jun;1 (3):193–200. doi: 10.1007/BF02253349. [DOI] [PubMed] [Google Scholar]
- 90.Greiser Maura, Halaszovich Christian R, Frechen Dirk, Boknik Peter, Ravens Ursula, Dobrev Dobromir, Lückhoff Andreas, Schotten Ulrich. Pharmacological evidence for altered src kinase regulation of I (Ca,L) in patients with chronic atrial fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 2007 Aug;375 (6):383–92. doi: 10.1007/s00210-007-0174-6. [DOI] [PubMed] [Google Scholar]
- 91.Gould Paul A, Yii Michael, McLean Catriona, Finch Samara, Marshall Tanneale, Lambert Gavin W, Kaye David M. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006 Aug;29 (8):821–9. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 92.Piot C, LeMaire S A, Albat B, Seguin J, Nargeot J, Richard S. High frequency-induced upregulation of human cardiac calcium currents. Circulation. 1996 Jan 01;93 (1):120–8. doi: 10.1161/01.cir.93.1.120. [DOI] [PubMed] [Google Scholar]
- 93.Ouadid H, Albat B, Nargeot J. Calcium currents in diseased human cardiac cells. J. Cardiovasc. Pharmacol. 1995 Feb;25 (2):282–91. doi: 10.1097/00005344-199502000-00014. [DOI] [PubMed] [Google Scholar]
- 94.Workman Antony J, Pau Davide, Redpath Calum J, Marshall Gillian E, Russell Julie A, Kane Kathleen A, Norrie John, Rankin Andrew C. Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by pre-operative atrial cellular electrophysiology. J. Cardiovasc. Electrophysiol. 2006 Nov;17 (11):1230–8. doi: 10.1111/j.1540-8167.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schotten Ulrich, Verheule Sander, Kirchhof Paulus, Goette Andreas. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol. Rev. 2011 Jan;91 (1):265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 96.Burashnikov Alexander, Antzelevitch Charles. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006 Mar;29 (3):290–5. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patterson Eugene, Lazzara Ralph, Szabo Bela, Liu Hong, Tang David, Li Yu-Hua, Scherlag Benjamin J, Po Sunny S. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J. Am. Coll. Cardiol. 2006 Mar 21;47 (6):1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 98.Makary Samy, Voigt Niels, Maguy Ange, Wakili Reza, Nishida Kunihiro, Harada Masahide, Dobrev Dobromir, Nattel Stanley. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ. Res. 2011 Oct 14;109 (9):1031–43. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- 99.Shi Hong, Wang Huizhen, Li Danshi, Nattel Stanley, Wang Zhiguo. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding K+ channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell. Physiol. Biochem. 2004;14 (1-2):31–40. doi: 10.1159/000076924. [DOI] [PubMed] [Google Scholar]
- 100.Kim Young M, Kattach Hassan, Ratnatunga Chandi, Pillai Ravi, Channon Keith M, Casadei Barbara. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008 Jan 01;51 (1):68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 101.Van Wagoner David R. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J. Cardiovasc. Pharmacol. 2008 Oct;52 (4):306–13. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 102.Aviles Ronnier J, Martin David O, Apperson-Hansen Carolyn, Houghtaling Penny L, Rautaharju Pentti, Kronmal Richard A, Tracy Russell P, Van Wagoner David R, Psaty Bruce M, Lauer Michael S, Chung Mina K. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003 Dec 16;108 (24):3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 103.Neuman Robert B, Bloom Heather L, Shukrullah Irfan, Darrow Lyndsey A, Kleinbaum David, Jones Dean P, Dudley Samuel C. Oxidative stress markers are associated with persistent atrial fibrillation. Clin. Chem. 2007 Sep;53 (9):1652–7. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Issac Tim T, Dokainish Hisham, Lakkis Nasser M. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J. Am. Coll. Cardiol. 2007 Nov 20;50 (21):2021–8. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 105.Zima Aleksey V, Blatter Lothar A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006 Jul 15;71 (2):310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 106.Carnes Cynthia A, Janssen Paul M L, Ruehr Mary L, Nakayama Hitomi, Nakayama Tomohiro, Haase Hannelore, Bauer John Anthony, Chung Mina K, Fearon Ian M, Gillinov A Marc, Hamlin Robert L, Van Wagoner David R. Atrial glutathione content, calcium current, and contractility. J. Biol. Chem. 2007 Sep 21;282 (38):28063–73. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 107.Li X, Li S, Xu Z, Lou M F, Anding P, Liu D, Roy S K, Rozanski G J. Redox control of K+ channel remodeling in rat ventricle. J. Mol. Cell. Cardiol. 2006 Mar;40 (3):339–49. doi: 10.1016/j.yjmcc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 108.Núñez Lucía, Vaquero Miguel, Gómez Ricardo, Caballero Ricardo, Mateos-Cáceres Petra, Macaya Carlos, Iriepa Isabel, Gálvez Enrique, López-Farré Antonio, Tamargo Juan, Delpón Eva. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc. Res. 2006 Oct 01;72 (1):80–9. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 109.Fearon Ian M, Brown Stephen T. Acute and chronic hypoxic regulation of recombinant hNa(v)1.5 alpha subunits. Biochem. Biophys. Res. Commun. 2004 Nov 26;324 (4):1289–95. doi: 10.1016/j.bbrc.2004.09.188. [DOI] [PubMed] [Google Scholar]
- 110.Howe Christopher J, Lahair Michelle M, McCubrey James A, Franklin Richard A. Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem. 2004 Oct 22;279 (43):44573–81. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 111.Hove-Madsen Leif, Llach Anna, Bayes-Genís Antoni, Roura Santiago, Rodriguez Font Enrique, Arís Alejandro, Cinca Juan. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004 Sep 14;110 (11):1358–63. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]