Abstract
Patients with atrial fibrillation (AF) frequently present with symptoms suggestive of myocardial isch- aemia, even in the absence of significant CAD, that seem to be attributable to abnormalities of myocardial perfusion and perfusion reserve. According to the results of recent human and previous experimen- tal studies the increase in coronary artery blood flow during AF is smaller, while the coronary vascular resistance during the arrhythmia does not decrease as much as we would expect, suggesting a mismatch between coronary blood flow and myocardial metabolic demand. AF itself diminishes coronary flow reserve, especially in the subendocardial layer, partly as a result of the increase in the myocardial com- ponent of coronary vascular resistance, and it is possible that irregular ventricular rhythm may play an important role. The mismatch of coronary blood flow and myocardial metabolic demand, especially in view of the severe reduction in coronary flow reserve, may have deleterious consequences that are not limited to patients with CAD.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, particularly in the elderly, that may cause significant symptoms and impair both functional status and the quality of life.1 Patients with AF may be at increased risk of death and deteriorating haemodynamics—due to the increased heart rate, loss of atrioventricular synchrony and progressive dysfunction of both atria and ventricles—and are also vulnerable to stroke and other embolic events caused by atrial thrombi.[1] In addition to age, hypertensive heart disease and coronary heart disease are the most common well-recognised disorders that are also closely linked to AF in developed countries.[1]
However, even in the absence of coronary artery disease (CAD), patients with AF frequently pres- ent with angina-like chest pain and transient isch-aemic-type ST-segment depression. In addition, elevated markers of myocardial damage have been reported during tachyarrhythmias. Impaired myocardial perfusion due to abnormalities in cor- onary blood supply may explain the angina-like symptoms and the electrocardiographic and laboratory findings associated with AF. The aim of this article is to provide an overview of the effects AF may have on the coronary blood flow pattern in humans.
The association between CAD and AF has rarely been investigated; the role of myocardial blood flow and coronary resistance in AF has been studied even less.
Experimental Studies
Early experimental studies in dogs demonstrated that artificially induced AF resulted in alterations in coronary circulation. Saito D and colleagues found that the induction of AF produced a marked decrease in coronary blood flow with a significant increase in coronary vascular resistance, while the termination of the arrhythmia had an opposite effect.[2] Interestingly(MBF), while subendocardial myocardial blood flow during AF was reduced, and was followed by a marked increase after the restoration of sinus rhythm, the subepicardial flow declined only slightly.
The influence of AF on the coronary circulation of 21 anaesthetised open-chest dogs was rather different according to the study of Wichmann J et al.[3] When AF was induced, either by the local applica- tion of acetylcholine to the left atrial appendage or by electrical stimulation, mean aortic pressure fell, heart rate rose significantly and coronary blood flow remained unchanged, while coronary vascular resistance and sinus oxygen saturation decreased. However, control of the heart rate revealed the vasoconstrictor effect of AF. Interestingly, AF-induced coronary vasoconstriction was greater during maximal coronary dilatation than during control. Similarly, early studies that compared patients with mitral stenosis and AF to patients in sinus rhythm and to patients who had undergone successful cardioversion suggested an impaired coronary blood flow.
Myocardial Perfusion Imaging Studies in Patients with AF
Felix T. Range and colleagues used 15H2O-positron emission tomography (PET) scanning to measure resting myocardial blood flow, maximal vasodilated myocardial blood flow after adenosine, and myocardial blood flow during a cold pressor test among 25 individuals with an average age of 58 years who had persistent idiopathic AF.[4] The results were compared with those from 13 age- and risk factor-matched controls in sinus rhythm, and nine young healthy controls with a mean age of 33 years. They found that individuals with idiopathic AF, who had no evidence of CAD, had impaired resting, hyperaemic, and post-cold pressor test myocardial blood flow and increased hyperaemic coronary vascular resistance. Furthermore, cardioversion restored the patients' resting and cold-pres- sor MBF, and improved hyperaemic myocardial blood flow, indicating that AF causes the impaired coronary flow.
Indeed, coronary vascular resistance, calculated as the mean arterial pressure divided by the corre-sponding myocardial blood flow, was unchanged at rest in AF patients compared with either control group, but was significantly greater in AF patients under hyperaemic conditions, compared with both matched controls and younger controls.[4] In addition, hyperaemic coronary flow reserve, calculated as the ratio of hyperaemic to baseline myocardial blood flow, was lower in AF patients than controls.
Interestingly, the investigators demonstrated an impairment of both myocardial perfusion and hyperaemic perfusion reserve, in addition to an increase in coronary resistance in individuals suffering from idiopathic persistent AF.[4] These findings could explain the angina-like symptoms seen during AF. However, since the perfusion abnormali- ties were not completely reversible after the resto- ration of sinus rhythm, an additional factor apart from the arrhythmia itself might be involved.
AF resulted in a marked decreased of myocardial perfusion at rest and under hyperaemia, whereas coronary vascular resistance was increased, even in patients with dilated cardiomyopathy (DCM) compared to patients with DCM who were in sinus rhythm.[5] In the study of Felix T. Range and colleagues, 12 men who suffered from DCM and persistent AF were compared with a group of 18 men who had DCM and sinus rhythm and with 22 healthy controls.[5] Although the DCM patients in sinus rhythm showed impaired hyperaemic perfu- sion and perfusion reserve compared with healthy controls, DCM patients with AF showed an addi- tional impairment of both resting and hyperaemic perfusion. Compared with controls, DCM patients with AF showed a further diminution of perfusion reserve accompanied by the highest coronary vascular resistance of all groups. If the investigators had restored sinus rhythm in the DCM patients with AF, we would have been able to postulate whether these changes were associated with, or independent of, the arrhythmia.
The value of myocardial perfusion imaging in the assessment of the risk of CAD in patients with AF is uncertain. Smit et al compared the results of myocardial perfusion single-photon emission computed tomography (SPECT) from 129 patients with a history of AF to the results of 124 age- and sex-matched controls.[6] A positive SPECT result was similar in patients with AF and controls. Nev- ertheless, in AF patients a positive SPECT outcome was less often related to significant CAD in those patients who subsequently underwent coronary angiography. These results emphasise the limitations of myocardial perfusion SPECT with regard to the detection of underlying CAD in patients with a history of AF. Thus, myocardial perfusion SPECT does not seem to be an accurate method for the diagnosis or exclusion of significant CAD in patients with AF. These findings confirm the results of previous studies, indicating decreased coronary blood flow and myocardial ischaemia in the absence of significant CAD in patients with AF.
Atrial Fibrillation and Coronary Circulation in Humans
Although there are several studies that have inves- tigated the effect of AF on coronary flow in animals, as well as non-invasive myocardial perfusion im- aging studies in AF patients, there are only scanty data concerning the effects of AF in humans. The data we have are mainly derived from invasive coronary angiography studies, given the fact that the methods used until now for the assessment of coronary flow were difficult or impossible to apply to human subjects.[2–6]
Recently, we used Doppler flowmetry to investigate the effects of experimentally induced AF on both the coronary blood flow and flow reserve in humans.[7] To assess the contribution of an accelerated heart rate to these outcomes, we compared the effects of AF with those of right atrial pacing at a similar heart rate. Additionally, in order to assess the importance of the atrial contraction itself, we evaluated the changes in coronary flow when AF was induced in a group of patients who had dual- chamber pacemakers for complete atrioventricular block (atrioventricular sequence vs. AF with the same, regular, paced ventricular rate).
In 16 patients with normal coronary vessels, baseline and hyperaemic blood pressure and Doppler phasic coronary flow velocity were measured, during sinus rhythm, experimentally induced AF, and right atrial pacing with a similar ventricular response to that during AF. Coronary flow velocity integral per minute increased significantly during both right atrial pacing and AF compared to sinus rhythm. The increase, however, was greater during right atrial pacing than during AF. This difference persisted even after correction for the product of heart rate and blood pressure. In the 12 paced patients the induction of AF (atrial fibrillation with a regular RR interval) caused no significant changes in coronary flow velocity variables.
In this study population, the induction of AF resulted in an increase in coronary blood flow, although to a lesser degree compared to the effects of right atrial pacing with the same cycle length.[7] This difference could be explained by the fact that the increase in myocardial oxygen demand, as indi- cated by the rate-pressure product, is smaller during AF than during right atrial pacing at the same heart rate. However, other factors were probably involved in preventing the coronary blood flow from increasing as much during acute AF as it does during right atrial pacing, since the difference in the percentage increase in coronary flow between AF and right atrial pacing remained even after correction for the rate-pressure product.
In addition, as in the experimental studies, AF resulted in a reduction in coronary flow reserve that could not be attributed solely to the increase in heart rate, while there was also a decrease in coronary vascular resistance. The latter finding suggests that the coronary vessels dilated, at least initially, in response to the accelerated heart rate and to the increase in metabolic demand that oc- curs in AF.
However, the reduction in coronary vascular re- sistance was smaller during acute AF than during right atrial pacing at a similar heart rate, whereas the coronary vascular resistance was greater during AF than during either sinus rhythm or right atrial pacing at maximal dilation induced by ad- enosine, when metabolic regulation is blunted.
Therefore, during AF in humans, as in animals, there is some degree of coronary vasoconstriction in addition to vasodilation. Moreover, we demon- strated a beat-to-beat variability in coronary flow pattern during AF and a greater influence of the ar- rhythmia on the diastolic component of coronary blood flow.[7]
Pathophysiological Considerations
According to the results of recent human and previous experimental studies, the increase in coronary artery blood flow during AF is smaller, while the coronary vascular resistance during the arrhythmia does not decrease as much as we should expect, suggesting a mismatch between coronary blood flow and myocardial metabolic demand that occurs in AF but not during atrial pacing at the same average ventricular rate.[2–7] Thus, AF it- self diminishes coronary flow reserve, especially in the subendocardial layer, partly as a result of an increase in the myocardial component of coronary vascular resistance. It is also possible that an irregular ventricular rhythm may play an important part in the rise in extravascular support.
Experimental and clinical pathophysiological investigations provide convincing evidence that during AF there is activation of the sympathetic nervous system.[8–9] Both the increased sympathetic activity and circulating vasoactive peptides may possibly affect the coronary circulation by impeding the increase in coronary flow and exaggerating the reduction in coronary flow reserve during AF.[8–10] In addition to these effects, the acceleration in heart rate and the irregularity of ventricular cycle lengths have independent, negative haemo- dynamic consequences.
Interestingly, our results indicate that the loss of atrial contraction alone, as seen following the induction of AF in patients with complete atrioven- tricular block under pacing (induction of atrial fibrillation with regular paced ventricular rhythm and a heart rate similar to that of the earlier atrio- ventricular sequential pacing rhythm), has only a small effect on coronary flow and tends to increase the coronary flow reserve.[7] Therefore, it is more the irregular rhythm that is responsible for the coronary vasoconstriction that acts in opposition to dilation during AF, thus impeding coro- nary flow and reducing coronary flow reserve.[7]
The underlying mechanism through which an ir- regular ventricular rhythm results in these 'nega- tive' effects is still unclear. Although an irregular sequence of RR intervals could have deleterious haemodynamic consequences, it seems remote, since, according to our results, the changes in coronary flow caused by atrial fibrillation were not cor- related with changes in blood pressure. Rather, the fact that the loss of atrial contraction does not affect the coronary vascular resistance suggests that the irregular rhythm is solely responsible for the coronary vasoconstriction that acts in opposition to dilation during acute atrial fibrillation, thus impeding coronary flow and reducing coronary flow reserve. Moreover, the possibility that the rhythm irregularity also acts directly to inhibit coronary flow can not be excluded.
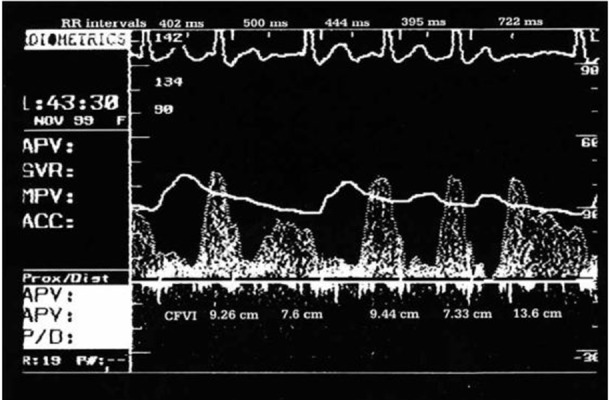
Additionally, the beat-to-beat variability in coro- nary flow during AF was mainly correlated with the current RR interval in our study, (figure 1).[7]In all patients there was beat-to-beat variability for both RR interval and coronary flow velocity inte- grals. Significant correlations of varying strength (r from 0.44 to 0.94) between current RR and coro- nary flow velocity integrals were observed in all patients, (figure 1). Preceding RR interval was a significant independent covariate in only two of the patients, while pre-preceding RR interval did not contribute significantly to the overall correlation in any of the patients. However, it is possible that the effect of short RR intervals could be com- pletely reversed by the increase in coronary flow that was associated with longer RR intervals, thus, the coronary flow per minute may be less than during atrial tachycardia at the same heart rate, (figure 2). Moreover, systoles that follow short RR intervals might generate insufficient pressure to open the aortic valve, resulting in a sufficient inhibition of coronary inflow and a reduction in coronary flow, regardless of the subsequent RR interval. Obviously, such systoles could have a significant negative effect on the coronary flow per minute.
Figure 1. Left anterior descending coronary artery flow veloc- ity pattern from a subject during atrial fibrillation. The beat-to- beat variability in the coronary flow velocity integral (CFVI) during atrial fibrillation is clearly correlated with the current RR interval.
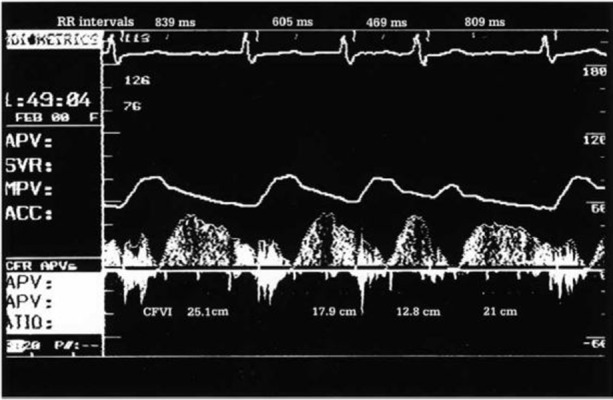
Figure 2. Left anterior descending coronary artery flow velocity pattern from a subject during atrial fibrillation. The second beat generated insufficient pressure to open the aortic valve. The subsequent RR interval has a clearly smaller coronary flow velocity integral (CFVI) than do the first and third RR intervals, even though its duration is longer.
Clinical Implications – Conclusions
Patients with AF frequently present with symptoms suggestive of myocardial ischaemia, even in the absence of significant CAD, that seem to be attributable to abnormalities of myocardial perfu- sion and perfusion reserve. The deleterious effects on haemodynamics and on coronary blood flow could be the result of the acceleration in heart rate, and the irregularity of ventricular cycle lengths. The mismatch of coronary blood flow and myo- cardial metabolic demand, especially in view of the severe reduction in coronary flow reserve, may have deleterious consequences—not limited to pa- tients with CAD—causing or worsening myocar- dial ischaemia to a greater degree than would an elevated heart rate alone.
Even in patients who have no significant coronary artery stenoses, AF could produce subendocardial ischaemia, especially if coronary flow and coronary flow reserve are already compromised, and could thus have a negative impact on ventricular function. The fact that AF mainly influences diastolic coronary flow is a further exacerbating factor, given that perfusion of the subendocardium occurs during diastole.
Since studies of myocardial blood flow in humans with AF are sparse, further ones are required in order to elucidate the underlying pathophysi- ological mechanisms and to clarify the clinical implications of AF-induced alterations in coronary blood flow.
Disclosures
No disclosures relevant to this article were made by the authors.
References
- 1.Chugh S S, Blackshear J L, Shen W K, Hammill S C, Gersh B J. Epidemiology and natural history of atrial fibrillation: clinical implications. J. Am. Coll. Cardiol. 2001 Feb;37 (2):371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 2.Saito D, Haraoka S, Ueda M, Fujimoto T, Yoshida H, Ogino Y. Effect of atrial fibrillation on coronary circulation and blood flow distribution across the left ventricular wall in anesthetized open-chest dogs. Jpn. Circ. J. 1978 Apr;42 (4):417–23. doi: 10.1253/jcj.42.417. [DOI] [PubMed] [Google Scholar]
- 3.Wichmann J, Ertl G, Rudolph G, Kochsiek K. Effect of experimentally induced atrial fibrillation on coronary circulation in dogs. Basic Res. Cardiol. 1983 Sep 1;78 (5):473–91. doi: 10.1007/BF01906459. [DOI] [PubMed] [Google Scholar]
- 4.Range Felix T, Schäfers Michael, Acil Tayfun, Schäfers Klaus P, Kies Peter, Paul Matthias, Hermann Sven, Brisse Betty, Breithardt Günter, Schober Otmar, Wichter Thomas. Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur. Heart J. 2007 Sep;28 (18):2223–30. doi: 10.1093/eurheartj/ehm246. [DOI] [PubMed] [Google Scholar]
- 5.Range Felix T, Paul Matthias, Schäfers Klaus P, Acil Tayfun, Kies Peter, Hermann Sven, Schober Otmar, Breithardt Günter, Wichter Thomas, Schäfers Michael A. Myocardial perfusion in nonischemic dilated cardiomyopathy with and without atrial fibrillation. J. Nucl. Med. 2009 Mar;50 (3):390–6. doi: 10.2967/jnumed.108.055665. [DOI] [PubMed] [Google Scholar]
- 6.Smit Marcelle D, Tio René A, Slart Riemer H J A, Zijlstra Felix, Van Gelder Isabelle C. Myocardial perfusion imaging does not adequately assess the risk of coronary artery disease in patients with atrial fibrillation. Europace. 2010 May;12 (5):643–8. doi: 10.1093/europace/eup404. [DOI] [PubMed] [Google Scholar]
- 7.Kochiadakis G E, Skalidis E I, Kalebubas M D, Igoumenidis N E, Chrysostomakis S I, Kanoupakis E M, Simantirakis E N, Vardas P E. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur. Heart J. 2002 May;23 (9):734–41. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 8.Wasmund Stephen L, Li Jian-Ming, Page Richard L, Joglar Jose A, Kowal Robert C, Smith Michael L, Hamdan Mohamed H. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003 Apr 22;107 (15):2011–5. doi: 10.1161/01.CIR.0000064900.76674.CC. [DOI] [PubMed] [Google Scholar]
- 9.Ertl G, Wichmann J, Kaufmann M, Kochsiek K. Alpha-receptor constriction induced by atrial fibrillation during maximal coronary dilatation. Basic Res. Cardiol. 1986 Jan 1;81 (1):29–39. doi: 10.1007/BF01907425. [DOI] [PubMed] [Google Scholar]
- 10.Tuinenburg A E, Van Veldhuisen D J, Boomsma F, Van Den Berg M P, De Kam P J, Crijns H J. Comparison of plasma neurohormones in congestive heart failure patients with atrial fibrillation versus patients with sinus rhythm. Am. J. Cardiol. 1998 May 15;81 (10):1207–10. doi: 10.1016/s0002-9149(98)00092-7. [DOI] [PubMed] [Google Scholar]


