Abstract
Atrial fibrillation frequently complicates myocardial infarction. Patients with atrial fibrillation complicating acute coronary syndrome have increased morbidity and mortality relative to patients that remain in normal sinus rhythm. No studies have identified a mortality benefit to rhythm control compared with rate control in the setting of acute coronary syndrome. Stroke prevention should be pursued with oral anticoagulation therapy, although the majority of patients with atrial fibrillation associated with acute coronary syndrome receive only antiplatelet therapy. There are several novel oral anticoagulant therapies now available, but these agents have not been well studied in combination with dual antiplatelet therapy. Therefore, warfarin as part of triple therapy is the most conservative approach until additional data becomes available.
Introduction
Scope of the Problem
Atrial fibrillation (AF) is the most common arrhythmia and accounts for one-third of hospitalizations for rhythm disorders.[1] The prevalence of AF in the United States is 0.89% and increases with age, such that approximately 70% of cases of AF are in patients between 65 and 85 years of age.[2] With the aging of the population, the number of patients with AF is expected to increase 150% by 2050, with more than 50% of patients being over the age of 80.[3-8] The increasing burden of AF is expected to lead to a higher incidence of stroke, as patients with AF have a five to seven fold greater risk than the general population.[9-11] Strokes secondary to AF have a worse prognosis than in patients without AF.[12,13] Moreover, AF is an independent risk factor for mortality with an adjusted odds ratio of 1.5 in men and 1.9 in women in the Framingham population.[14] Each year there are more than one million hospitalizations for Acute Coronary Syndrome (ACS) in the US. Despite a decrease in the proportion of ST-segment elevation myocardial infarctions (STEMI) over the past 10 years, 29% of ACS episodes are STEMI events.[15,16] The incidence of non-STEMI has increased, particularly following the introduction of highly sensitive troponin.[17,18] Although mortality has decreased over the past two decades, 30-day mortality remains significant at 8%.[7,19]
AF is a known, common complication of ACS. There are multiple mechanisms for induction of AF during myocardial infarction (see Figure 1). Animal models of atrial ischemia have shown that there is an increase in spontaneous atrial ectopic activity and in slowing of atrial conduction, leading to initiation and sustained reentry of AF.[20,21] Canines with atrial ischemia develop gap junction uncoupling that facilitates AF.[22] Other infarct related causes of AF include pericarditis,[23,24] hypoxia,[25,26] sinus node ischemia,[27] ventricular dysfunction,[28] and increase in atrial pressure.[29] While myocardial ischemia promotes AF, the ventricular irregularity caused by AF can initiate or exaggerate existing subendocardial ischemia by creating a myocardial oxygen demand mismatch.[30]
Figure 1. Schematic representation of mechanisms of AF in the setting of ACS.
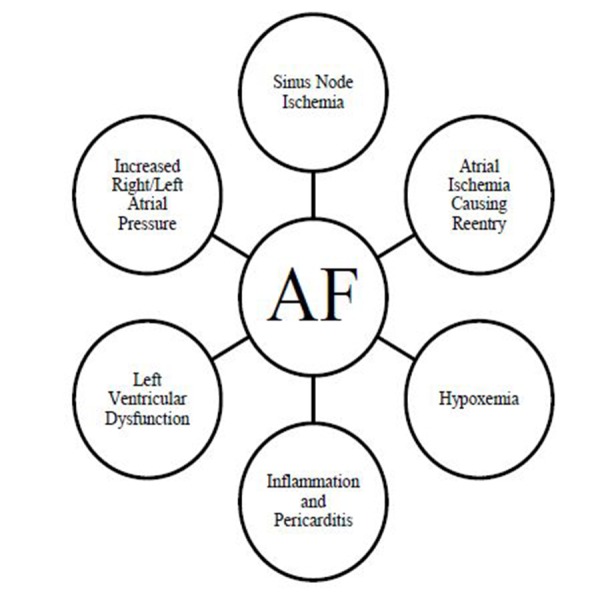
Abbreviations: RA, right atrium; LA, left atrium; TV, Tricuspid valve; MV, mitral valve; PV, pulmonary vein; SVC, superior vena cava; IVC inferior vena cava; LAA, left atrial appendage
Incidence of AF after ACS
In the pre-thrombolytic era approximately one in ten patients with ACS developed AF.[31-34] As shown in Table 1, the incidence of AF in the post-thrombolytic era has been more varied, ranging between 3-25%,[35-56] as has been described in previous review and systematic review articles.[57,58] At the higher end, a community cohort study of 3220 patients identified an incidence of 25%, and the majority (54%) of patients developed AF more than 30-days out from their ACS event.[35] Overall, in the post-thrombolytic era, the mean incidence of AF complicating ACS, after adjusting for study size, was 8.8%. One of the limitations of these observational studies is the unknown rate of pre-existing, undiagnosed AF. Estimates of pre-existing AF have ranged from 1.1% to 11% with a mean of 3.6%, after adjusting for study size. Lopes, et al. conducted a pooled analysis of 120,566 patients from ten randomized clinical trials (GUSTO-I, GUSTO-IIb, GUSTO-III, ASSENT-2, ASSENT-3, ASSENT-3 Plus, PURSUIT, PARAGON-A, PARAGON-B, and SYNERGY). In a substudy of 40,000 patients for whom baseline electrocardiograms were available, pre-existing AF was identified in nearly 1 in 5 patients (18%).[49]
Table 1. Incidence of AF after ACS in Post-thrombolytic Era.
| Author/Study | Publication Date | Treatment of ACS | Patients Included | Incidence of New AF | Incidence of Pre-existing AF |
|---|---|---|---|---|---|
| Jabre | 2011 | 100% Throm bolysis/PCI | 3,220 | 24.69% | 9% |
| Lau/ACACIA | 2009 | 100% Throm bolysis/PCI | 3,393 | 4.96% | 11% |
| Berton | 2009 | 40% Throm bolysis | 505 | 9.10% | 3.60% |
| Lopes | 2008 | N/A | 120,566 | N/A | 7.50% |
| Siu | 2007 | 47% Throm bolysis/PCI | 431 | 13.70% | N/A |
| Kober/VALIANT | 2006 | 50% Throm bolysis/PCI | 14,703 | 12.30% | 2.30% |
| Lehto/OPTIMAAL | 2005 | Lehto/OPTIMAAL | 5,477 | 7.20% | 12% |
| Stenestrand/RIKS-HIA | 2005 | N/A | 82,565 | 7.60% | N/A |
| Laurent/RICO | 2005 | N/A | 1,701 | 7.60% | N/A |
| McMurray/CAPRICORN | 2005 | 46% Throm bolysis/PCI | 1,959 | 2.60% | 9% |
| Kinjo/OACIS | 2003 | 100% PCI | 2,475 | 7.70% | 4.30% |
| Mehta/GRACE | 2003 | 71% Throm bolysis/PCI | 21,785 | 6.20% | 7.90% |
| Goldberg | 2002 | 29% Throm bolysis | 2,596 | 13.20% | N/A |
| Al-Khatib/PURSUIT | 2001 | 100% Eptifibatide,PCI | 9,432 | 6.40% | N/A |
| Pizetti/GISSI-III | 2001 | 50% Throm bolysis | 17,749 | 7.80% | 1.10% |
| Rathore/CCP | 2000 | N/A | 106,780 | 11.30% | 10.80% |
| Wong(17)/GUSTO-III | 2000 | 100% Throm bolysis | 13,858 | 6.50% | N/A |
| Pedersen(33)/TRACE | 1999 | 41% Throm bolysis | 6,676 | 17.10% | 3.90% |
| Eldar | 1998 | 46% Throm bolysis | 2,866 | 8.90% | N/A |
| Crenshaw/GUSTO-I | 1997 | 100% Throm bolysis | 40,891 | 8.00% | 2.50% |
| Sakata | 1997 | 13% PCI | 1,039 | 9.60% | N/A |
| Madias | 1996 | 17% Throm bolysis | 517 | 11.20% | 2.70% |
| Total 461,184 | |||||
| PCI=Percutaneous Coronary Intervention, N/A=Data Not Available | |||||
Timing of AF
The timing of new-onset AF varies following ACS. Among 13,858 STEMI patients treated with thrombolytic therapy in the GUSTO III clinical trial, the median onset of AF was 2 days after ACS,[41] which is similar timing as seen in the non-STEMI population.[37] Madias et al. conducted a single center study of 517 patients and found that AF developed in 43%, 24%, 14%, and 19% of patients at post-ACS days 1, 2, 3, and > 3, respectively.[44] Other studies have suggested a more protracted evolution of new-onset AF. For example, in the OPTIMAAL trial, only 28% of those who developed AF in long-term follow-up (3 years) had AF at 3 months post-ACS.[48] Similarly, the distribution of onset of AF after ACS in Jabre et al. was 30% within 2 days, 16% between 3 and 30 days, and 54% greater than 30 days.[35] A subgroup of the CARISMA trial followed post-MI patients with left ventricular ejection fraction ≤ 40% and an implantable cardiac monitor for 2 years. Of the 101 patients, 39% had an episode of AF: 16% at 2 months, 32% at 12 months, and 29% at 24 months after ACS.[59] These disparate data likely reflect two periods of risk: an acute phase, similar to the risk observed after cardiothoracic surgery, and a longer, chronic risk of AF that is related to progressive risk factors, including left atrial hypertension and heart failure. In support of there being multiple phases to post-ACS AF, a substudy analysis of 1131 patients included in the VALIANT study found a differential response to treatment strategies for AF based upon time from myocardial infarction.[60]
Few data are available regarding the type of AF and subsequent treatment of AF complicating ACS. Larger studies, such as GISSI-III have shown that fewer than 25% of patients with AF complicating ACS return to sinus rhythm prior to hospital discharge.[40] Long-term follow-up suggests that the risk of recurrent AF after ACS is substantial. Asanin et al. followed 320 patients with AF after ACS for a mean of 7 years (5.5 to 8.5 years) to monitor for frequency of recurrence of AF. All patients were in sinus rhythm at discharge of their ACS hospitalization, and 22.5% developed recurrences of AF. Of note in this study, amiodarone was the only antiarrhythmic used, and 10% of patients (more in the recurrence group), received amiodarone.[61] There is no data available regarding the impact of direct current cardioversion on patients with AF in the setting of ACS.
Predictors of AF
associated with the development of AF after ACS (Table 2). Age is the most frequently identified predictive factor, consistent with the prominent age-related incidence of AF in the overall population.[62] Killip classification at presentation is a significant, independent predictor for the development of AF in several cohorts, with odds ratios between 1.58 and 5.55.39, [47,48,61] As expected, the presence of cardiogenic shock (Killip Class IV) carries the greatest risk. Hypertension, female sex, and heart rate are also frequently associated with AF after ACS .[34-51],[53-56] A heart rate > 100 beats per minute was associated with a 3-fold increased risk of AF in the OACIS cohort (OR 3.0 [1.94-4.64]).[47] Finally, among STEMI patients, delayed revascularization (> 4 hours from symptom onset) had a higher incidence of AF.[49] Delayed treatment > 12 hours accentuates risk further (OR 2.19 [1.00-4.79]).[61]
Table 2. 30-day and 1-year postoperative morbidity and mortality.
Higher body mass index, cardiac arrest, creatine kinase level, prior chronic obstructive pulmonary disease, height, history of hyperlipidemia, left main disease, lower ejecton fraction, left ventricular hyperthophy,non-smoker, North American, and STEMI were all listed in 1 study with a frequency of 5%
| Predicators of AF | Frequency in Studies (n=22) | Frequency (%) |
|---|---|---|
| Age | 21 | 95% |
| Killip | 15 | 68% |
| Prior HTN | 10 | 45% |
| Female | 9 | 41% |
| Heart rate | 8 | 36% |
| Prior DM | 5 | 23% |
| Lower SBP | 4 | 18% |
| Prior MI | 4 | 18% |
| Anterior MI | 4 | 14% |
| Caucasian | 3 | 14% |
| Prior CHF | 3 | 14% |
| Less thrombolytics | 3 | 14% |
| Creatinine | 2 | 9% |
| Male | 2 | 9% |
| Prior angina | 2 | 9% |
A single-center study of 1039 patients admitted with ACS found that patients who developed AF within 24 hours of ACS had a higher frequency of proximal RCA lesions (67%) when compared to those with sinus rhythm. Patients with AF at < 24 hours had the most significant elevation in right atrial pressure; right ventricular dilation; and incidence of cardiogenic shock, right ventricular acute myocardial infarction, and high grade atrioventricular block. Patients with onset of AF > 24 hours more frequently had proximal occlusion of the left anterior descending artery, increased wedge pressure, and decreased left ventricular ejection fraction.[55]
AF & Mortality following ACS
AF is associated with higher mortality following ACS (Table 3).[35-49], [53-56] The increased risk of death is observed in-hospital but persists in long-term follow-up. In general, the risk of death at one year is 1.5 to 1.75 times greater when compared to patients without AF.
Table 3. Mortality with AF after ACS.
HR=Hazard Ratio, OR=Odds Ratio, RR=Relative Risk, N/A=Data Not Available, NSTEMI=Non ST-Segment Elevation Myocardial Infraction
| Author/Study | Publication Date | In-hopital Mortality | Follow-up | Risk of Death | Follow-up | Risk of Death |
|---|---|---|---|---|---|---|
| Jabre | 2011 | N/A | 6.6 year | HR 3.77(3.37-4.21) | N/A | N/A |
| Lau/ACACIA | 2009 | OR 2.2(1.0-4.6) | 1 year | HR 1.36(0.84-2.20) | N/A | N/A |
| Berton | 2009 | OR 1.9(0.8 to 4.6) | N/A | N/A | 7 years | OR 1.6(1.2-2.3) |
| Lopes | 2008 | N/A | 7 day | NSTEMI HR 2.30(1.83-2.90), STEMI HR 1.65(1.44- | 1 year | NSTEMI HR 1.67(1.41-1.99), STEMI HR 2.37(1.79- |
| Siu | 2007 | N/A | 2 year | Not Significant | N/A | N/A |
| Kober/VALIANT | 2006 | N/A | 3 year | HR 1.32(1.20-1.45) | N/A | N/A |
| Lehto/OPTIMAAL | 2005 | N/A | 30 day | HR 3.83(1.97-7.43) | 3 years | HR 1.82(1.39-2.39) |
| Kinjo/OACIS | 2003 | HR 1.42(0.88-2.31) | 1 year | HR 3.04(1.24-7.48) | N/A | N/A |
| Mehta/GRACE | 2003 | OR 1.65(1.30-2.09) | N/A | N/A | N/A | N/A |
| Goldberg | 2002 | OR 1.38(0.98-1.94) | 5 year | HR 1.23(0.99-1.52) | N/A | N/A |
| Al-Khatib/PURSUIT | 2001 | N/A | 30 day | HR 4.4(3.3-5.8) | 6 months | HR 3.0(2.4-3.8 |
| Pizetti/GISSI-III | 2001 | RR 1.98(1.67-2.34) | 6 months | OR 1.42(0.88-2.31) | 4 years | RR 1.78(1.60-1.99) |
| Rathore/CCP | 2000 | OR 1.35(1.28-1.42 | 30 day | OR 1.31(1.25-1.37) | 1 year | OR 1.51(1.44-1.58) |
| Wong(17)/GUSTO-III | 2000 | N/A | 30 day | OR 1.49(1.17-1.89) | 1 year | OR 1.64(1.35-2.01) |
| Pedersen(33)/TRACE | 1999 | OR 1.5(1.2-1.9) | 5 year | OR 1.3(1.2-1.4) | N/A | N/A |
| Eldar | 1998 | N/A | 30 day | OR 1.32(0.92-1.87) | 1 year | RR 1.33(1.05-1.87) |
| Crenshaw/GUSTO-I | 1997 | 13.8% AF vs 5.9% no AF | 30 day | OR 1.5(1.2-1.9) | N/A | N/A |
| Sakata | 1997 | 40% AF vs 14% no AF | 8 year | OR3.05(1.85-5.00) | N/A | N/A |
| Madias | 1996 | Not Significant | N/A | N/A | N/A | N/A |
Decreased survival in patients with AF after ACS was first identified in the 1940s, when mortality at 30 days was 89%.31 By 1975, mortality with AF after ACS had improved to 49%, as compared to 16% in patients without AF.[33] Data from the SPRINT trial in the pre-thrombolytic era showed a higher long-term (mean 5.5 years) mortality in patients developing AF after ACS with hazard ratio of 1.28 (1.12-1.46).[34] Eldar et al. completed a prospective study of 25 Coronary Care Units in Israel (2866 patients) in the thrombolytic era. When compared to the historical data from SPRINT, AF patients in the thrombolytic era had improved mortality with a 30 day OR of 0.64 (0.44-0.94) and a 1 year OR of 0.69 (0.54-0.88).[45]
More recently, the TRACE study randomized patients with ACS to ACE-inhibition with trandolapril or placebo. Within TRACE, patients with both AF and depressed left ventricular ejection fraction (< 35%) had a two-fold increase of in-hospital mortality.[63] Patients with AF had a higher mortality at 2 years with adjusted relative risk of 1.33 (1.19-1.49). When examining the relation between AF and cause-specific death, the relative risk of sudden cardiac death and death from other causes were not statistically different at 1.31 (1.07-1.60) and 1.43 (1.21-1.70), respectively.[64] The increase in both cardiac and non-cardiac mortality implies that the impact of AF on mortality is multifactorial.
As might be expected, patients with recurrence of AF have worse prognoses. Patients with recurrent paroxysmal AF after discharge have increased long-term mortality (mean 7-year follow-up) when compared to patients without recurrences (OR of 3.08 [1.45-6.53] and relative risk of 1.52 [1.0-2.31], respectively).[61] Furthermore, persistent AF at discharge is associated with a higher adjusted relative risk of death than paroxysmal AF.[43]
Similar to findings with ventricular arrhythmias after myocardial infarction, mortality is also affected by the timing of AF onset post-ACS. New-onset AF more than 24 hours after ACS is associated with increased mortality at 8-year follow-up compared to AF within 24 hours of ACS (OR 3.7 [1.84-7.52] vs. OR 2.5 [1.23-5.00]).[55] There are conflicting data regarding the relative risks of pre-exisiting versus new-onset AF.[36,39,48,55]
Complications and Length of Stay in Patients with AF
AF complicating ACS is associated with a host of adverse cardiovascular outcomes, including an increased risk of in-hospital stroke, major bleeding, re-infarction, heart failure, and ventricular arrhythmias (Table 4). Multiple studies have documented increased in-hospital stroke among patients with AF after ACS. For example, GUSTO-I demonstrated a statistically significant increase of in-hospital stroke of 3.1% with AF compared to 1.3% without AF, and this was driven mainly by ischemic strokes (1.8% with AF, 0.5% without AF).[42] AF has also been associated with an increased risk of acute renal failure after ACS (OR 2.7 [1.2-6.1]).[36] As shown in Table 4, AF consistently is associated with increased length of stay (range 1.8-4.7 days).
Table 4. Complications Associated with AF after ACS.
HR=Hazard Ratio, OR=Odds Ratio, N/A=Data Not Available, CVA=Cerebrovascular Accident, CHF=Congestive Heart Failure, VT=Ventricular Tachycardia, VF=Ventricular Fibrillation.
| Author/Study | Publication Date | Follow-up | CVA | Length of Hospital Stay | In-Hospital Re-infarction | Major Bleeding | CHF | Cardiogenic Shock | VT | VF |
|---|---|---|---|---|---|---|---|---|---|---|
| Lau/ACACIA | 2009 | N/A | N/A | 9.7 days vs 5.5 days | OR 3.7(2.0-7.0) | OR 5.8(3.1-10.6) | OR 3.1(1.7-5.7) | N/A | N/A | N/A |
| Lopes | 2008 | 30 days | NSTEMI HR 3.45(2.41-4.95), STEMI HR 1.46(1.17- | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Siu | 2007 | 2 year | HR 5.1(2.4-11.2) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kober/VALIANT | 2006 | 3 year | 8.1% AF vs 3.7% no AF | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lehto/OPTIMAAL | 2005 | 30 day | HR 14.6(5.87-36.3) | 14.1 days vs 12.3 days | N/A | N/A | N/A | N/A | N/A | N/A |
| Kinjo/OACIS | 2003 | In-hospital | 2.3% AF vs 0.6% no AF | N/A | N/A | N/A | N/A | 34.8% vs 16.6% | 15.7% vs 6.1% | 27.3% vs 14.7% |
| Mehta/GRACE | 2003 | In-hospital | OR 1.33(0.80-2.20) | 12.5 days vs 7.8 days | OR 2.0(1.37-2.93) | OR 1.64(1.25-2.14) | OR 2.83(2.27-3.52) | OR 2.4(1.88-3.06) | OR 1.97(1.56-1.25) | |
| Goldberg | 2002 | N/A | N/A | N/A | N/A | N/A | 55% vs 27% | 12.8% vs 5.9% | N/A | N/A |
| Al-Khatib/PURSUIT | 2001 | 6 months | HR 2.9(1.7-4.8) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pizetti/GISSI-III | 2001 | In-hospital | Not Significant | 15 days vs 13 days | N/A | N/A | 51.5% vs 23.6% | N/A | 4.3% vs 1.9% | 4.4% vs 2.3% |
| Rathore/CCP | 2000 | In-hospital | 2.8% AF vs 1.7% no AF | 11 days vs 7.6 days | 4.4% vs 3.6% | N/A | 60.1% vs 42.2% | N/A | N/A | N/A |
| Wong(17)/GUSTO-III | 2000 | 30 days | 4% AF vs 2% no AF | N/A | 9% vs 4% | N/A | 44% vs 14% | 14% vs 3% | 10% vs 3% | 10% vs 4% |
| Pedersen(33)/TRACE | 1999 | N/A | N/A | N/A | N/A | N/A | 48% vs 34% | 6% vs 3% | 18% vs 11% | 11% vs 6% |
| Eldar | 1998 | In-hospital | OR 4.6(1.9-10.8) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Crenshaw/GUSTO-I | 1997 | In-hospital | 3.1% AF vs 1.3% no AF | 14.3 days vs 10 days | 8% vs 4% | N/A | 39% vs 14% | 16% vs 5% | 16% vs 5% | 15% vs 6% |
| Sakata | 1997 | N/A | N/A | N/A | N/A | N/A | 63% vs 30% | 44% vs 25% | N/A | N/A |
Management Dilemmas in Patients with AF
Prevention of AF after ACS
Many of the risk factors associated with AF after ACS are modifiable. Optimal management of ACS, including prompt revascularization, beta-blockade, optimal afterload reduction, and aggressive treatment of heart failure are core components of quality ACS care. These same interventions should also help minimize the risk of new-onset AF in both the acute and long-term setting.
The GISSI-III trial randomized patients to lisinopril and nitrates versus placebo, and there was a 24% reduction in AF seen in the treatment arm (OR 0.76 [0.65-0.89].[40] ACE inhibition has also been shown to decrease arrhythmic death post MI.[38,[65] Randomized data have also shown that beta-blockade with carvedilol decreased the frequency of AF post-MI (HR 0.41 [0.25-0.68]), including new-onset AF (HR 0.51 [0.28-0.93]).[52] While disappointing in primary prevention of AF outside of ACS, statin therapy has been associated with lower odds of AF after ACS, including data from the Veterans Administration (adjusted OR of 0.57 [0.39-0.83]).[66,67]
Rate & Rhythm Control
Randomized clinical trials have failed to identify a superior survival advantage with either a rate versus rhythm control strategy.[68,69] The PIAF trial compared rate control with diltiazem and rhythm control with amiodarone in 252 patients to detect changes in symptoms related to AF. While there was no symptomatic benefit with rhythm control in the PIAF trial, there was better exercise tolerance, as measured by 6 minute walk test.[70] The ACC/AHA guidelines for the management of AF discuss class I indications in the setting of an acute myocardial infarction: direct-current cardioversion in the setting of hemodynamic instability or ongoing ischemia, intravenous amiodarone for treatment of rapid ventricular response with depressed ejection fraction, and intravenous beta blockers or calcium channel blockers for treatment of rapid ventricular response with preserved ejection fraction.[1] Vaughan-Williams Class IC medications have a Class III recommendation (evidence of harm) due to increased mortality in the CAST trials.[71,72]
The preferred antiarrhythmics for AF post-myocardial infarction are amiodarone and sotalol (in the absence of congestive heart failure given its beta blocking properties). In a subgroup analysis of VALIANT, patients treated with anti-arrhythmic drugs in the immediate peri-infarct period had a higher risk of death than patients treated with a “rate” control strategy. These findings did not extend past 45 days.[60] The DIAMOND-MI trial determined that there was no mortality benefit to treating patients with dofetilide after myocardial infarction in the presence of impaired left ventricular function.[53] AF was successfully treated with dofetilide in this patient population; therefore, it is a reasonable second line agent. While rarely used, Vaughan-Williams class IA agents are recommended as third line therapy in ACS patients.[1] In general, observational data from ACS trials have failed to identify a survival advantage with antiarrhythmic therapy for the maintenance of sinus rhythm.[73]
Stroke Prevention
Even transient AF, has been associated with a significantly increased risk of ischemic stroke (10.2% vs 1.8%) at 1-year.[54] The ACC/AHA guidelines for the management of STEMI give a class I recommendation to use of oral anticoagulation (OAC) in patients with persistent or paroxysmal AF.[74] A consensus document by the European Society of Cardiology Working Group on Thrombosis gave a class IIa recommendation to OAC in combination with aspirin and clopidogrel for AF patients with NSTEMI.[75] Despite these recommendations, only a minority (13.5-29%) of patients with AF complicating ACS are being discharged on OAC.[37,38] In the VALIANT trial only 25% of patients with AF were on OAC at 1-year follow-up after the ACS event.[53] Lopes et al. conducted an analysis with 23,208 patients from three IIb/IIIa trials. Only 13.5% of patients with AF complicating ACS were discharged on warfarin, and consistent with other observational studies, warfarin was independently associated with a lower risk of death or myocardial infarction (HR 0.29 [0.15-0.98]).[50,76]
Jang et al. conducted a study of 362 patients with AF and ACS who were treated with PCI. The average CHADS2 score was 1.6 ± 1.2. Warfarin was prescribed to 23% of patients, including warfarin, aspirin, and clopidogrel (so called “triple therapy” in 22%) and warfarin and clopidogrel (1%). While hampered by a small sample size and low statistical power, there was no statistically significant difference between the OAC and no OAC groups in death, stroke, or major adverse cardiac events, but there was a 5-fold increase in major bleeding (10.7% in OAC group and 2.2% in non-OAC group, p = 0.002).[77] A meta-analysis of nine clinical trials, including 1996 patients on chronic OAC showed that major adverse cardiovascular events were significantly reduced in patients taking aspirin, clopidogrel, and OAC (triple therapy: OR 0.60 [0.42-0.86]). Patients on triple therapy did have more frequent major bleeding at 6-months (OR 2.12 [1.05-4.29]).[78] A second meta-analysis found that triple therapy was associated with a significantly lower incidence of ischemic stroke (OR 0.29 [0.15-0.58]). The triple therapy patients had a twofold increase in major bleeding, and the incidence of death and myocardial infarction were statistically similar between the two groups.[79]
Several novel oral anticoagulants have emerged as alternatives to warfarin. Dabigatran 150 mg twice daily was found to have superior efficacy for the prevention of stroke and systemic embolism with similar risks of major bleeding when compared to dose-adjusted warfarin in an open-label trial.[80] Importantly, when considering its use in patients with AF after ACS, dabigatran may be associated with a small increased risk of MI compared with warfarin. A meta-analysis of 7 trials including 30,514 patients found an increased risk of MI in those treated with dabigatran (1.2 vs. 0.8%; OR 1.33 [1.03-1.71]).[81] A similar trend was seen when ximelgatran was compared with warfarin for the treatment of AF.[82,83] Rivaroxaban once daily was non-inferior to warfarin for the prevention of stroke and systemic embolization and the composite of major and non-major clinically relevant bleeding in the ROCKET AF trial.[84] Finally, apixaban was studied in the ARISTOTLE trial, which showed superiority to warfarin with respect to stroke or systemic embolism, along with decreased major bleeding (HR 0.69 [0.60-0.80]).[85] Importantly, all three of the novel oral anticoagulants lead to significant reductions in intracranial hemorrhage.[80,84,85] Data on a fourth novel oral agent ,edoxaban, will be forthcoming from the ENGAGE AF-TIMI 48 trial, however, these data are not yet available.[86]
Several studies have investigated the use of novel oral anticoagulants in the treatment of patients with ACS (regardless of AF status). Using the same dose of apixaban as the ARISTOTLE trial, APPRAISE-2 evaluated the use of apixaban on top of antiplatelet therapy: aspirin (16% of patients) or aspirin and clopidogrel (81% of patients) for the prevention of recurrent ischemic events. In APPRAISE-2 apixaban increased major bleeding (HR 2.59 [1.50-4.46]), including more frequent fatal and intracranial bleeding events.[87] ATLAS ACS 2-TIMI 51 evaluated the use of rivaroxaban with antiplatelet therapy (99% on aspirin and 93% on clopidogrel). Notably, the doses of rivaroxaban used in ATLAS were much smaller than those used in ROCKET-AF (2.5 and 5 mg twice daily versus 20 mg daily). Those randomized to low-dose rivarrivaroxaban had a 16% reduction in the composite efficacy endpoint (cardiovascular death/myocardial infarction/stroke). While patients treated with rivaroxaban experienced increased major and intracranial bleeding, there was no excess fatal bleeding.[88] Neither of these ACS trials were designed to investigate the impact of triple therapy on stroke or survival for AF patients after ACS and/or PCI.
At present the 2011 ACC/AHA guideline update and a position paper by European Society of Cardiology cite the lack of data and uncertainty regarding combination therapy in patients with AF who undergo PCI.[89,90] Randomized trials evaluating combination oral anticoagulation and antiplatelet therapy after PCI and ACS are needed; however, the design and execution of these trials will be challenging. Given the increased risk of intracranial hemorrhage in APPRAISE-2 and the differences in dosing and patient populations (AF versus ACS) across these trials, the devil we know (warfarin) may be better than the devil we do not (novel OACs) when prescribing triple therapy. Until more data are available, the most conservative approach will be to restrict triple therapy to the use of warfarin. It is also important to limit the duration of triple therapy by using bare metal stents unless there is a significant benefit to drug eluting stents (class IIa recommendation).[75] Finally, as new antiplatelet agents and new oral anticoagulants become engrained in clinical use, best practice patterns for their dosing and associated methods of percutaneous coronary access (femoral vs radial) will require further investigation.
Conclusions
AF is a common complication of ACS, and it is an independent predictor of mortality and in-hospital complications. Despite guideline recommendations and known mortality benefits, oral anticoagulation remains suboptimal in patients with AF complicating ACS. While we have a wealth of data regarding the epidemiology and outcomes associated with AF after ACS, we have little to no contemporary clinical trial data to guide therapeutic decisions in patients with AF complicating ACS. While preventing stroke, controlling heart rate, and improving quality of life remain inviolable goals in the treatment of AF, we lack clinical trials that address the most common therapeutic choices in each of these treatment strategies after ACS. Despite the obvious challenges to their design, funding, and completion, randomized trials dedicated to the management of AF after ACS are clearly needed.
Disclosures
Jonathan P. Piccini receives research support from Boston Scientific, Johnson & Johnson, and Bayer Healthcare and serves as a consultant to Forest Laboratories, Johnson & Johnson, Medtronic, and Sanofi-Aventis.
Kent R. Nillson receives research support from Medtronic and receives honoraria from Jansen Pharmaceuticals.
The remaining authors have no financial disclosures to report
References
- 1.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffery L, Antman Elliott M, Halperin Jonathan L, Hunt Sharon Ann, Nishimura Rick, Ornato Joseph P, Page Richard L, Riegel Barbara, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm A John, Dean Veronica, Deckers Jaap W, Despres Catherine, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais Joao, Osterspey Ady, Tamargo Juan Luis, Zamorano José Luis. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114 (7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg W M, Blackshear J L, Laupacis A, Kronmal R, Hart R G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995 Mar 13;155 (5):469–73. [PubMed] [Google Scholar]
- 3.Novaro Gian M, Asher Craig R, Bhatt Deepak L, Moliterno David J, Harrington Robert A, Lincoff A Michael, Newby L Kristin, Tcheng James E, Hsu Amy P, Pinski Sergio L. Meta-analysis comparing reported frequency of atrial fibrillation after acute coronary syndromes in Asians versus whites. Am. J. Cardiol. 2008 Feb 15;101 (4):506–9. doi: 10.1016/j.amjcard.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 4.Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 09;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Furberg C D, Psaty B M, Manolio T A, Gardin J M, Smith V E, Rautaharju P M. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am. J. Cardiol. 1994 Aug 01;74 (3):236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 6.Psaty B M, Manolio T A, Kuller L H, Kronmal R A, Cushman M, Fried L P, White R, Furberg C D, Rautaharju P M. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997 Oct 07;96 (7):2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka Yoko, Barnes Marion E, Gersh Bernard J, Cha Stephen S, Bailey Kent R, Abhayaratna Walter P, Seward James B, Tsang Teresa S M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114 (2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin E J, Levy D, Vaziri S M, D'Agostino R B, Belanger A J, Wolf P A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994 Mar 16;271 (11):840–4. [PubMed] [Google Scholar]
- 9.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch. Intern. Med. 1987 Sep;147 (9):1561–4. [PubMed] [Google Scholar]
- 10.Wolf P A, Dawber T R, Thomas H E, Kannel W B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978 Oct;28 (10):973–7. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 11.Flegel K M, Shipley M J, Rose G. Risk of stroke in non-rheumatic atrial fibrillation. Lancet. 1987 Mar 07;1 (8532):526–9. doi: 10.1016/s0140-6736(87)90174-7. [DOI] [PubMed] [Google Scholar]
- 12.Lin H J, Wolf P A, Kelly-Hayes M, Beiser A S, Kase C S, Benjamin E J, D'Agostino R B. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996 Oct;27 (10):1760–4. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka Yoko, Barnes Marion E, Bailey Kent R, Cha Stephen S, Gersh Bernard J, Seward James B, Tsang Teresa S M. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J. Am. Coll. Cardiol. 2007 Mar 06;49 (9):986–92. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin E J, Wolf P A, D'Agostino R B, Silbershatz H, Kannel W B, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 08;98 (10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 15.Roger Véronique L, Go Alan S, Lloyd-Jones Donald M, Benjamin Emelia J, Berry Jarett D, Borden William B, Bravata Dawn M, Dai Shifan, Ford Earl S, Fox Caroline S, Fullerton Heather J, Gillespie Cathleen, Hailpern Susan M, Heit John A, Howard Virginia J, Kissela Brett M, Kittner Steven J, Lackland Daniel T, Lichtman Judith H, Lisabeth Lynda D, Makuc Diane M, Marcus Gregory M, Marelli Ariane, Matchar David B, Moy Claudia S, Mozaffarian Dariush, Mussolino Michael E, Nichol Graham, Paynter Nina P, Soliman Elsayed Z, Sorlie Paul D, Sotoodehnia Nona, Turan Tanya N, Virani Salim S, Wong Nathan D, Woo Daniel, Turner Melanie B. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 03;125 (1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roe Matthew T, Parsons Lori S, Pollack Charles V, Canto John G, Barron Hal V, Every Nathan R, Rogers William J, Peterson Eric D. Quality of care by classification of myocardial infarction: treatment patterns for ST-segment elevation vs non-ST-segment elevation myocardial infarction. Arch. Intern. Med. 2005 Jul 25;165 (14):1630–6. doi: 10.1001/archinte.165.14.1630. [DOI] [PubMed] [Google Scholar]
- 17.Yeh Robert W, Sidney Stephen, Chandra Malini, Sorel Michael, Selby Joseph V, Go Alan S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010 Jun 10;362 (23):2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 18.Roger Véronique L, Weston Susan A, Gerber Yariv, Killian Jill M, Dunlay Shannon M, Jaffe Allan S, Bell Malcolm R, Kors Jan, Yawn Barbara P, Jacobsen Steven J. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010 Feb 23;121 (7):863–9. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabel Elizabeth G, Braunwald Eugene. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012 Jan 05;366 (1):54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 20.Sinno Hani, Derakhchan Katayoun, Libersan Danielle, Merhi Yahye, Leung Tack Ki, Nattel Stanley. Atrial ischemia promotes atrial fibrillation in dogs. Circulation. 2003 Apr 15;107 (14):1930–6. doi: 10.1161/01.CIR.0000058743.15215.03. [DOI] [PubMed] [Google Scholar]
- 21.Nishida Kunihiro, Qi Xiao Yan, Wakili Reza, Comtois Philippe, Chartier Denis, Harada Masahide, Iwasaki Yu-ki, Romeo Philippe, Maguy Ange, Dobrev Dobromir, Michael Georghia, Talajic Mario, Nattel Stanley. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011 Jan 18;123 (2):137–46. doi: 10.1161/CIRCULATIONAHA.110.972778. [DOI] [PubMed] [Google Scholar]
- 22.Shiroshita-Takeshita Akiko, Sakabe Masao, Haugan Ketil, Hennan James K, Nattel Stanley. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007 Jan 23;115 (3):310–8. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 23.Tofler G H, Muller J E, Stone P H, Willich S N, Davis V G, Poole W K, Robertson T, Braunwald E. Pericarditis in acute myocardial infarction: characterization and clinical significance. Am. Heart J. 1989 Jan;117 (1):86–92. doi: 10.1016/0002-8703(89)90660-1. [DOI] [PubMed] [Google Scholar]
- 24.Nagahama Y, Sugiura T, Takehana K, Hatada K, Inada M, Iwasaka T. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur. Heart J. 1998 Feb;19 (2):287–92. doi: 10.1053/euhj.1997.0744. [DOI] [PubMed] [Google Scholar]
- 25.Fearon I M, Palmer A C, Balmforth A J, Ball S G, Mikala G, Schwartz A, Peers C. Hypoxia inhibits the recombinant alpha 1C subunit of the human cardiac L-type Ca2+ channel. J. Physiol. (Lond.) 1997 May 01;500 ( Pt 3) ():551–6. doi: 10.1113/jphysiol.1997.sp022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichlmaier A M, Lang V, Harringer W, Heublein B, Schaldach M, Haverich A. Prediction of the onset of atrial fibrillation after cardiac surgery using the monophasic action potential. Heart. 1998 Nov;80 (5):467–72. doi: 10.1136/hrt.80.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyriakidis M, Barbetseas J, Antonopoulos A, Skouros C, Tentolouris C, Toutouzas P. Early atrial arrhythmias in acute myocardial infarction. Role of the sinus node artery. Chest. 1992 Apr;101 (4):944–7. doi: 10.1378/chest.101.4.944. [DOI] [PubMed] [Google Scholar]
- 28.Rechavia E, Strasberg B, Mager A, Zafrir N, Kusniec J, Sagie A, Sclarovsky S. The incidence of atrial arrhythmias during inferior wall myocardial infarction with and without right ventricular involvement. Am. Heart J. 1992 Aug;124 (2):387–91. doi: 10.1016/0002-8703(92)90602-r. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura T, Iwasaka T, Takahashi N, Yuasa F, Takeuchi M, Hasegawa T, Matsutani M, Inada M. Factors associated with atrial fibrillation in Q wave anterior myocardial infarction. Am. Heart J. 1991 May;121 (5):1409–12. doi: 10.1016/0002-8703(91)90146-9. [DOI] [PubMed] [Google Scholar]
- 30.Kochiadakis G E, Skalidis E I, Kalebubas M D, Igoumenidis N E, Chrysostomakis S I, Kanoupakis E M, Simantirakis E N, Vardas P E. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur. Heart J. 2002 May;23 (9):734–41. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 31.Askey JM, Neurath O. The prognostic significance of auricular fibrillation in association with myocardial infarction. American Heart Journal. 1945;29:575–580. [Google Scholar]
- 32.DeSanctis R W, Block P, Hutter A M. Tachyarrhythmias in myocardial infarction. Circulation. 1972 Mar;45 (3):681–702. doi: 10.1161/01.cir.45.3.681. [DOI] [PubMed] [Google Scholar]
- 33.Cristal N, Szwarcberg J, Gueron M. Supraventricular arrhythmias in acute myocardial infarction. Prognostic importance of clinical setting; mechanism of production. Ann. Intern. Med. 1975 Jan;82 (1):35–9. doi: 10.7326/0003-4819-82-1-35. [DOI] [PubMed] [Google Scholar]
- 34.Behar S, Zahavi Z, Goldbourt U, Reicher-Reiss H. Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. European heart journal. 1992;0:0–0. doi: 10.1093/oxfordjournals.eurheartj.a060046. [DOI] [PubMed] [Google Scholar]
- 35.Jabre Patricia, Jouven Xavier, Adnet Frédéric, Thabut Gabriel, Bielinski Suzette J, Weston Susan A, Roger Véronique L. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011 May 17;123 (19):2094–100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau Dennis H, Huynh Luan T, Chew Derek P, Astley Carolyn M, Soman Ashish, Sanders Prashanthan. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am. J. Cardiol. 2009 Nov 15;104 (10):1317–23. doi: 10.1016/j.amjcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 37.Al-Khatib S M, Pieper K S, Lee K L, Mahaffey K W, Hochman J S, Pepine C J, Kopecky S L, Akkerhuis M, Stepinska J, Simoons M L, Topol E J, Califf R M, Harrington R A. Atrial fibrillation and mortality among patients with acute coronary syndromes without ST-segment elevation: results from the PURSUIT trial. Am. J. Cardiol. 2001 Jul 01;88 (1):A7, 76–9. doi: 10.1016/s0002-9149(01)01593-4. [DOI] [PubMed] [Google Scholar]
- 38.Berton G, Cordiano R, Cucchini F, Cavuto F, Pellegrinet M, Palatini P. Atrial fibrillation during acute myocardial infarction: association with all-cause mortality and sudden death after 7-year of follow-up. Int. J. Clin. Pract. 2009 May;63 (5):712–21. doi: 10.1111/j.1742-1241.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 39.Rathore S S, Berger A K, Weinfurt K P, Schulman K A, Oetgen W J, Gersh B J, Solomon A J. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000 Mar 07;101 (9):969–74. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 40.Pizzetti F, Turazza F M, Franzosi M G, Barlera S, Ledda A, Maggioni A P, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart. 2001 Nov;86 (5):527–32. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong C K, White H D, Wilcox R G, Criger D A, Califf R M, Topol E J, Ohman E M. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am. Heart J. 2000 Dec;140 (6):878–85. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 42.Crenshaw B S, Ward S R, Granger C B, Stebbins A L, Topol E J, Califf R M. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 1997 Aug;30 (2):406–13. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen O D, Bagger H, Køber L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur. Heart J. 1999 May;20 (10):748–54. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 44.Madias J E, Patel D C, Singh D. Atrial fibrillation in acute myocardial infarction: a prospective study based on data from a consecutive series of patients admitted to the coronary care unit. Clin Cardiol. 1996 Mar;19 (3):180–6. doi: 10.1002/clc.4960190309. [DOI] [PubMed] [Google Scholar]
- 45.Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, Behar S. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998 Mar 17;97 (10):965–70. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg Robert J, Yarzebski Jorge, Lessard Darleen, Wu Jacqueline, Gore Joel M. Recent trends in the incidence rates of and death rates from atrial fibrillation complicating initial acute myocardial infarction: a community-wide perspective. Am. Heart J. 2002 Mar;143 (3):519–27. doi: 10.1067/mhj.2002.120410. [DOI] [PubMed] [Google Scholar]
- 47.Kinjo Kunihiro, Sato Hiroshi, Sato Hideyuki, Ohnishi Yozo, Hishida Eiji, Nakatani Daisaku, Mizuno Hiroya, Fukunami Masatake, Koretsune Yukihiro, Takeda Hiroshi, Hori Masatsugu. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am. J. Cardiol. 2003 Nov 15;92 (10):1150–4. doi: 10.1016/j.amjcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Lehto Mika, Snapinn Steven, Dickstein Kenneth, Swedberg Karl, Nieminen Markku S. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. Eur. Heart J. 2005 Feb;26 (4):350–6. doi: 10.1093/eurheartj/ehi064. [DOI] [PubMed] [Google Scholar]
- 49.Lopes R D, Pieper K S, Horton J R, Al-Khatib S M, Newby L K, Mehta R H, Van de Werf F, Armstrong P W, Mahaffey K W, Harrington R A, Ohman E M, White H D, Wallentin L, Granger C B. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008 Jul;94 (7):867–73. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 50.Stenestrand Ulf, Lindbäck Johan, Wallentin Lars. Anticoagulation therapy in atrial fibrillation in combination with acute myocardial infarction influences long-term outcome: a prospective cohort study from the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Circulation. 2005 Nov 22;112 (21):3225–31. doi: 10.1161/CIRCULATIONAHA.105.552984. [DOI] [PubMed] [Google Scholar]
- 51.Laurent G, Dentan G, Moreau D, Zeller M, Laurent Y, Vincent-Martin M, Lhuillier I, Makki H, Wolf J E, Cottin Y. [Atrial fibrillation during myocardial infarction with and without ST segment elevation]. Arch Mal Coeur Vaiss. 2005 Jun;98 (6):608–14. [PubMed] [Google Scholar]
- 52.McMurray John, Køber Lars, Robertson Michele, Dargie Henry, Colucci Wilson, Lopez-Sendon Jose, Remme Willem, Sharpe D Norman, Ford Ian. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J. Am. Coll. Cardiol. 2005 Feb 15;45 (4):525–30. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 53.Køber Lars, Swedberg Karl, McMurray John J V, Pfeffer Marc A, Velazquez Eric J, Diaz Rafael, Maggioni Aldo P, Mareev Viatcheslav, Opolski Grzegorz, Van de Werf Frans, Zannad Faiez, Ertl Georg, Solomon Scott D, Zelenkofske Steven, Rouleau Jean-Lucien, Leimberger Jeffrey D, Califf Robert M. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur. J. Heart Fail. 2006 Oct;8 (6):591–8. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Siu Chung-Wah, Jim Man-Hong, Ho Hee-Hwa, Miu Raymond, Lee Stephen W L, Lau Chu-Pak, Tse Hung-Fat. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007 Jul;132 (1):44–9. doi: 10.1378/chest.06-2733. [DOI] [PubMed] [Google Scholar]
- 55.Sakata MD K, Kurihara MD H, Iwamori MD K, Maki MD A, Yoshino MD H, Yanagisawa MD A, Ishikawa MD K. Clinical and prognostic significance of atrial fibrillation in acute myocardial infarction. The American journal of cardiology. 1997;80:1522–1527. doi: 10.1016/s0002-9149(97)00746-7. [DOI] [PubMed] [Google Scholar]
- 56.Mehta Rajendra H, Dabbous Omar H, Granger Christopher B, Kuznetsova Polina, Kline-Rogers Eva M, Anderson Frederick A, Fox Keith A A, Gore Joel M, Goldberg Robert J, Eagle Kim A. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am. J. Cardiol. 2003 Nov 01;92 (9):1031–6. doi: 10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Schmitt Joern, Duray Gabor, Gersh Bernard J, Hohnloser Stefan H. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur. Heart J. 2009 May;30 (9):1038–45. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia Gurbir S, Lip Gregory Y H. Atrial fibrillation post-myocardial infarction: frequency, consequences, and management. Curr Heart Fail Rep. 2004 Dec;1 (4):149–55. doi: 10.1007/s11897-004-0002-y. [DOI] [PubMed] [Google Scholar]
- 59.Jons Christian, Jacobsen Uffe G, Joergensen Rikke Moerch, Olsen Niels Thue, Dixen Ulrik, Johannessen Arne, Huikuri Heikki, Messier Marc, McNitt Scott, Thomsen Poul Erik Bloch. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm. 2011 Mar;8 (3):342–8. doi: 10.1016/j.hrthm.2010.09.090. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson Kent R, Al-Khatib Sana M, Zhou Yi, Pieper Karen, White Harvey D, Maggioni Aldo P, Kober Lars, Granger Christopher B, Lewis Eldrin F, McMurray John J V, Califf Robert M, Velazquez Eric J. Atrial fibrillation management strategies and early mortality after myocardial infarction: results from the Valsartan in Acute Myocardial Infarction (VALIANT) Trial. Heart. 2010 Jun;96 (11):838–42. doi: 10.1136/hrt.2009.180182. [DOI] [PubMed] [Google Scholar]
- 61.Asanin Milika, Perunicic Jovan, Mrdovic Igor, Matic Mihailo, Vujisic-Tesic Bosiljka, Arandjelovic Aleksandra, Vojvodic Ana, Marinkovic Jelena, Ostojic Miodrag, Vasiljevic Zorana. Significance of recurrences of new atrial fibrillation in acute myocardial infarction. Int. J. Cardiol. 2006 May 10;109 (2):235–40. doi: 10.1016/j.ijcard.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Piccini Jonathan P, Hammill Bradley G, Sinner Moritz F, Jensen Paul N, Hernandez Adrian F, Heckbert Susan R, Benjamin Emelia J, Curtis Lesley H. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012 Jan;5 (1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedersen Ole Dyg, Bagger Henning, Køber Lars, Torp-Pedersen Christian. Impact of congestive heart failure and left ventricular systolic function on the prognostic significance of atrial fibrillation and atrial flutter following acute myocardial infarction. Int. J. Cardiol. 2005 Apr 08;100 (1):65–71. doi: 10.1016/j.ijcard.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen Ole Dyg, Abildstrøm Steen Z, Ottesen Michael M, Rask-Madsen Christian, Bagger Henning, Køber Lars, Torp-Pedersen Christian. Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction. Eur. Heart J. 2006 Feb;27 (3):290–5. doi: 10.1093/eurheartj/ehi629. [DOI] [PubMed] [Google Scholar]
- 65.Yusuf Salim, Pitt Bertram, Davis Clarence E, Hood William B, Cohn Jay N. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991 Aug 01;325 (5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 66.Ozaydin Mehmet, Turker Yasin, Erdogan Dogan, Karabacak Mustafa, Dogan Abdullah, Varol Ercan, Gonul Emel, Altinbas Ahmet. The association between previous statin use and development of atrial fibrillation in patients presenting with acute coronary syndrome. Int. J. Cardiol. 2010 May 28;141 (2):147–50. doi: 10.1016/j.ijcard.2008.11.172. [DOI] [PubMed] [Google Scholar]
- 67.Ramani Gautam, Zahid Maliha, Good Chester B, Macioce Alanna, Sonel Ali F. Comparison of frequency of new-onset atrial fibrillation or flutter in patients on statins versus not on statins presenting with suspected acute coronary syndrome. Am. J. Cardiol. 2007 Aug 01;100 (3):404–5. doi: 10.1016/j.amjcard.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 68.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 69.Van Gelder Isabelle C, Hagens Vincent E, Bosker Hans A, Kingma J Herre, Kamp Otto, Kingma Tsjerk, Said Salah A, Darmanata Julius I, Timmermans Alphons J M, Tijssen Jan G P, Crijns Harry J G M. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 70.Hohnloser S H, Kuck K H, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000 Nov 25;356 (9244):1789–94. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 71.Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 1989 Aug 10;321 (6):406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 72.Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N. Engl. J. Med. 1992 Jul 23;327 (4):227–33. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 73.Wong C-K, White H D, Wilcox R G, Criger D A, Califf R M, Topol E J, Ohman E M. Management and outcome of patients with atrial fibrillation during acute myocardial infarction: the GUSTO-III experience. Global use of strategies to open occluded coronary arteries. Heart. 2002 Oct;88 (4):357–62. doi: 10.1136/heart.88.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antman Elliott M, Anbe Daniel T, Armstrong Paul Wayne, Bates Eric R, Green Lee A, Hand Mary, Hochman Judith S, Krumholz Harlan M, Kushner Frederick G, Lamas Gervasio A, Mullany Charles J, Ornato Joseph P, Pearle David L, Sloan Michael A, Smith Sidney C, Alpert Joseph S, Anderson Jeffrey L, Faxon David P, Fuster Valentin, Gibbons Raymond J, Gregoratos Gabriel, Halperin Jonathan L, Hiratzka Loren F, Hunt Sharon Ann, Jacobs Alice K. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004 Aug 03;110 (5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 75.Lip Gregory Y H, Huber Kurt, Andreotti Felicita, Arnesen Harald, Airaksinen Juhani K, Cuisset Thomas, Kirchhof Paulus, Marín Francisco. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary--a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2010 Jun;31 (11):1311–8. doi: 10.1093/eurheartj/ehq117. [DOI] [PubMed] [Google Scholar]
- 76.Lopes Renato D, Starr Aijing, Pieper Carl F, Al-Khatib Sana M, Newby L Kristin, Mehta Rajendra H, Van de Werf Frans, Mahaffey Kenneth W, Armstrong Paul W, Harrington Robert A, White Harvey D, Wallentin Lars, Granger Christopher B. Warfarin use and outcomes in patients with atrial fibrillation complicating acute coronary syndromes. Am. J. Med. 2010 Feb;123 (2):134–40. doi: 10.1016/j.amjmed.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Jang Sung-Won, Rho Tai-Ho, Kim Dong-Bin, Cho Eun Joo, Kwon Beom-June, Park Hun-Jun, Shin Woo-Seung, Kim Ji-Hoon, Lee Jong-Min, Moon Keon-Woong, Oh Yong-Seog, Yoo Ki-Dong, Youn Ho-Joong, Lee Man-Young, Chung Wook-Sung, Seung Ki-Bae, Kim Jae-Hyung. Optimal antithrombotic strategy in patients with atrial fibrillation after coronary stent implantation. Korean Circ J. 2011 Oct;41 (10):578–82. doi: 10.4070/kcj.2011.41.10.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Hong-Jin, Zheng Zhao-Tong, Wang Zhi-Hao, Li Shao-Hua, Zhang Yun, Zhong Ming, Zhang Wei. "Triple therapy" rather than "triple threat": a meta-analysis of the two antithrombotic regimens after stent implantation in patients receiving long-term oral anticoagulant treatment. Chest. 2011 Feb;139 (2):260–70. doi: 10.1378/chest.09-3083. [DOI] [PubMed] [Google Scholar]
- 79.Gao Fei, Zhou Yu Jie, Wang Zhi Jian, Yang Shi Wei, Nie Bin, Liu Xiao Li, Jia De An, Yan Zhen Xian. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int. J. Cardiol. 2011 Apr 01;148 (1):96–101. doi: 10.1016/j.ijcard.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Connolly Stuart J, Ezekowitz Michael D, Yusuf Salim, Eikelboom John, Oldgren Jonas, Parekh Amit, Pogue Janice, Reilly Paul A, Themeles Ellison, Varrone Jeanne, Wang Susan, Alings Marco, Xavier Denis, Zhu Jun, Diaz Rafael, Lewis Basil S, Darius Harald, Diener Hans-Christoph, Joyner Campbell D, Wallentin Lars. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009 Sep 17;361 (12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 81.Uchino Ken, Hernandez Adrian V. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch. Intern. Med. 2012 Mar 12;172 (5):397–402. doi: 10.1001/archinternmed.2011.1666. [DOI] [PubMed] [Google Scholar]
- 82.Olsson S Bertil. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003 Nov 22;362 (9397):1691–8. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 83.Flaker Greg C, Gruber Michael, Connolly Stuart J, Goldman Steven, Chaparro Sandra, Vahanian Alec, Halinen Matti O, Horrow Jay, Halperin Jonathan L. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am. Heart J. 2006 Nov;152 (5):967–73. doi: 10.1016/j.ahj.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 84.Patel Manesh R, Mahaffey Kenneth W, Garg Jyotsna, Pan Guohua, Singer Daniel E, Hacke Werner, Breithardt Günter, Halperin Jonathan L, Hankey Graeme J, Piccini Jonathan P, Becker Richard C, Nessel Christopher C, Paolini John F, Berkowitz Scott D, Fox Keith A A, Califf Robert M. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011 Sep 08;365 (10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 85.Granger Christopher B, Alexander John H, McMurray John J V, Lopes Renato D, Hylek Elaine M, Hanna Michael, Al-Khalidi Hussein R, Ansell Jack, Atar Dan, Avezum Alvaro, Bahit M Cecilia, Diaz Rafael, Easton J Donald, Ezekowitz Justin A, Flaker Greg, Garcia David, Geraldes Margarida, Gersh Bernard J, Golitsyn Sergey, Goto Shinya, Hermosillo Antonio G, Hohnloser Stefan H, Horowitz John, Mohan Puneet, Jansky Petr, Lewis Basil S, Lopez-Sendon Jose Luis, Pais Prem, Parkhomenko Alexander, Verheugt Freek W A, Zhu Jun, Wallentin Lars. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011 Sep 15;365 (11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 86.Ruff Christian T, Giugliano Robert P, Antman Elliott M, Crugnale Sharon E, Bocanegra Tomas, Mercuri Michele, Hanyok James, Patel Indravadan, Shi Minggao, Salazar Dan, McCabe Carolyn H, Braunwald Eugene. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am. Heart J. 2010 Oct;160 (4):635–41. doi: 10.1016/j.ahj.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 87.Alexander John H, Lopes Renato D, James Stefan, Kilaru Rakhi, He Yaohua, Mohan Puneet, Bhatt Deepak L, Goodman Shaun, Verheugt Freek W, Flather Marcus, Huber Kurt, Liaw Danny, Husted Steen E, Lopez-Sendon Jose, De Caterina Raffaele, Jansky Petr, Darius Harald, Vinereanu Dragos, Cornel Jan H, Cools Frank, Atar Dan, Leiva-Pons Jose Luis, Keltai Matyas, Ogawa Hisao, Pais Prem, Parkhomenko Alexander, Ruzyllo Witold, Diaz Rafael, White Harvey, Ruda Mikhail, Geraldes Margarida, Lawrence Jack, Harrington Robert A, Wallentin Lars. Apixaban with antiplatelet therapy after acute coronary syndrome. N. Engl. J. Med. 2011 Aug 25;365 (8):699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 88.Mega Jessica L, Braunwald Eugene, Wiviott Stephen D, Bassand Jean-Pierre, Bhatt Deepak L, Bode Christoph, Burton Paul, Cohen Marc, Cook-Bruns Nancy, Fox Keith A A, Goto Shinya, Murphy Sabina A, Plotnikov Alexei N, Schneider David, Sun Xiang, Verheugt Freek W A, Gibson C Michael. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012 Jan 05;366 (1):9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 89.Wann L Samuel, Curtis Anne B, January Craig T, Ellenbogen Kenneth A, Lowe James E, Estes N A Mark, Page Richard L, Ezekowitz Michael D, Slotwiner David J, Jackman Warren M, Stevenson William G, Tracy Cynthia M, Fuster Valentin, Rydén Lars E, Cannom David S, Le Heuzey Jean-Yves, Crijns Harry J, Lowe James E, Curtis Anne B, Olsson S Bertil, Ellenbogen Kenneth A, Prystowsky Eric N, Halperin Jonathan L, Tamargo Juan Luis, Kay G Neal, Wann L Samuel, Jacobs Alice K, Anderson Jeffrey L, Albert Nancy, Hochman Judith S, Buller Christopher E, Kushner Frederick G, Creager Mark A, Ohman Erik Magnus, Ettinger Steven M, Stevenson William G, Guyton Robert A, Tarkington Lynn G, Halperin Jonathan L, Yancy Clyde W. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 Jan 04;123 (1):104–23. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 90.De Caterina Raffaele, Husted Steen, Wallentin Lars, Andreotti Felicita, Arnesen Harald, Bachmann Fedor, Baigent Colin, Huber Kurt, Jespersen Jørgen, Kristensen Steen Dalby, Lip Gregory Y H, Morais João, Rasmussen Lars Hvilsted, Siegbahn Agneta, Verheugt Freek W A, Weitz Jeffrey I. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease position paper. J. Am. Coll. Cardiol. 2012 Apr 17;59 (16):1413–25. doi: 10.1016/j.jacc.2012.02.008. [DOI] [PubMed] [Google Scholar]


