Abstract
Typical atrial flutter (AFL) or type I AFL is the most common type of macroreentrant atrial tachycardia. Its prevalence increases with age and is more common in men. Ablation of the cavo-tricuspid isthmus (CTI), a critical part of the circuit, is safe and highly effective. Despite the long-term efficacy of this therapy, a significant proportion of patients undergoing CTI ablation will develop atrial fibrillation (AF) during the follow-up and identifying them can have an important impact on their management. PV isolation and typical flutter ablation during the same procedure may be an effective treatment strategy for patients with clinical documentation of both arrhythmias.
Keywords: Atrial Flutter, Atrial fibrillation, Radiofrequency Ablation
Introduction
Typical or common atrial flutter (AFL) is the most common type of macroreentrant atrial tachycardia. Its prevalence increases with age and is 2.5 times more common in men.[1] Typical AFL results from re-entrant activation in the right atrium (RA) around the tricuspid valve annulus and catheter ablation of the cavo-tricuspid isthmus (CVI) – a critical part of the circuit - is a well-established first-line therapy.[2-8] This intervention is safe and highly effective with single procedure efficacy evaluated to be near 95%.[9-10]
Despite the long-term efficacy of this therapy, a significant proportion of patients[4,11-13] will develop atrial fibrillation (AF) during the follow-up. Identifying the patients at risk to develop AF after typical AFL catheter ablation is important, because it will influence their subsequent medical management (e.g. pursuing anticoagulation and/or antiarrhythmics) and they can be informed of the risk of recurrent symptoms and related prognostic implications.
The aim of this report is to review the inter-relationship between typical AFL and AF; the clinical predictors associated with occurence of AF after typical AFL ablation and finally, outline an approach to management of these patients.
The Inter-Relationships of Typical AFL and AF
Typical AFL and AF are both atrial arrhythmias and they frequently coexist in the same patient. Despite the substrate of AF and typical AFL being different in terms of location [(AFL – right atrium (RA) / AF – left atrium (LA)], some observations suggest a mechanistic interaction between them, especially during the initiation of arrhythmia.
Experimental studies in the sterile pericarditis model demonstrated that typical AFL starts preferentially with brief episodes of AF.[14] This was also recognized in humans after open heart surgery[15] or in patients when AFL was induced in the electrophysiology laboratory[16]
In experimental studies, the conversion from AF to typical AFL occurred when a functional line of block was created in the RA free wall, between the two vena cava, along the crista terminalis.[17-18] The length and location of this line of block, with the presence of areas of slow conduction in the RA, was the key to convert AF to sustained typical AFL.
This conversion was analyzed in patients referred for typical AFL ablation.[19] Despite the absence of LA endocardial activation recordings, a characteristic sequence of events was observed in the RA just before the conversion. An organized activation or ¨organized AF¨ along the trabeculated RA was observed, which was preceded and followed by an electrical silent period, a necessary event for conversion to typical AFL. This sequence of activation could be favoured in patients with remodeled RA with pre-existing but incomplete (anisotropic or structural scar-related) intercaval zone of block.
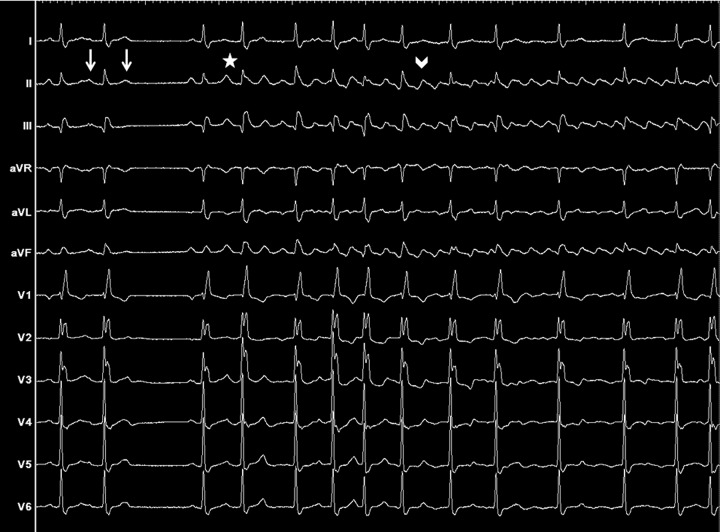
Therefore, one hypothetical scenario may be that short episodes of AF - initiated by rapid firing ectopic foci predominantly of LA origin[20]- create a functional complete intercaval zone of block in patients with a RA substrate - and a period of LA quiescence or slowing favours the occurence of AFL (see figure 1).
Figure 1. 12-leads ECG from a 77 year-old male with palpitations and without structural heart disease, showing the spontaneous onset of typical atrial flutter. Left : sinus rhythm interrupted by two atrial premature beats (white arrows), one conducted and one blocked. Middle: rapidly firing ectopic foci (white star) arising from the left superior pulmonary vein, inducing a short transitional rhythm corresponding to atrial fibrillation. Right: onset of typical couterclockwise atrial flutter (white arrowhead).
Predictors of AF after Ablation of Typical AFLs
As mentioned earlier, a significant proportion of patients, with successfully ablated typical AFL, will develop subsequent AF. Identifying these patients can have important therapeutical implications, especially in terms of anticoagulation therapy.
There are different clinical predictors associated with the occurence of AF:
Prior history of AF (most significant predictor)
Presence of structural heart disease, especially LA dilatation and/or left ventricular dysfunction[11]
Patients with prior history of AF and/or structural heart disease or those developing typical AFL under Ic antiarrhythmics,[12] are at the greatest risk to develop subsequent AF. In a meta-analysis performed by Péres et al.[22] 52.5% patients with prior history of AF and 47.7% patients with AFL under Ic antiarrhythmics developped AF during an average follow-up of 15 months. Since CTI ablation may prevent only the conversion from AF to AFL and has no effect to the underlying tendency toward fibrillation (PV triggers or LA substrate), it is not surprising that all these patients may develop AF despite successful CTI ablation.
More importantly, even in the subgroup of patient without prior history of AF, the incidence of subsequent AF remains high when the follow-up was long enough; in the meta-analysis cited earlier[22] the occurence of AF continues to rise until reaching the same incidence rate after 5 years of follow-up than patients with prior history of AF. Ellis K. et al[10] observed also in this subgroup of patient, 82% of subsequent AF during a follow-up of nearly 4 years. The persistent risk of AF, even in those patients without the clinical predictors, suggests a potentially important role of AF as a trigger rather than a consequence of typical AFL.
In other words, occurence of AF after typical AFL ablation is relatively common and depends mostly on the duration and intensity of the follow-up. The time of appearance of AF seems to be determined by the presence of underlying electrophysiological and structural substrate in the atria. Patients with a prior history of AF and/or structural heart disease (e.g. LA dilatation, left ventricular dysfunction) will be at risk to develop AF after CTI ablation more earlier than patients with lone AFL, reflecting only a more advanced electrical LA disease in the former patients.
In our center, we retrospectively analyzed patients who had AF ablation therapy between 2002-2011. From a total of 762 patients (68% paroxysmal AF, 32% persistent AF) 7% (51/762) had a previous CTI ablation. Of these 51 patients (35 paroxysmal AF, 16 persistent AF), 65% (33/51) had a prior AF history before developing typical AFL, 31% (16/51) had a left ventricular dysfunction at the time of intervention. Despite the limitations associated with this retrospective analysis, our findings confirm that two-thirds of patients, who develop subsequent AF after CTI ablation, had a prior history of AF.
Clinical and Therapeutical Implications
It is well accepted that ablation of typical AFL is recommended as part of an AF ablation therapy, if documented prior to the intervention or occurring during AF ablation.[23,24] Conversely, in patients without any prior or induced typical AFL, prophylactic CTI ablation does not provide clinical benefit in terms of AFL incidence[25] and could potentially result in procedure-related complications.
In patients presenting primarily with typical AFL, with or without AF, CTI ablation with the endpoint of a bidirectional block by differential pacing[26] must be performed. In the subgroup with sustained typical AFL induced by rapid firing ectopic beats (figure 1) ablation of the firing sites combined with CTI block may be necessary, both for symptom alleviation and for the prevention of AF development.
In the subset of patient with AF developing typical AFL under Ic AAD, it was recently demonstrated that an hybrid therapy (ablation of CTI followed by administration of AAD) was not effective as a long-term therapy, with 90% of patients presenting AF recurrence during a follow-up of 5 years.[27] These patients can be initially managed with AAD but they must be informed that pulmonary vein isolation (PVI) would be necessary for a better long-term rhythm control.
Because AFL and AF are two inter-related arrhythmia and the majority of patients who get a CTI ablation will develop AF. In patients with coexistence of AF and AFL, PVI combined with CTI ablation should be performed as a first-line therapy. This strategy was recently demonstrated to be beneficial in terms of AF recurrence and quality of life.[28] In our center as well, PVI and typical flutter ablation are performed during the same procedure in case of clinical documentation of symptomatic AF and typical flutter.
In terms of surveillance, all CTI ablated patients are advised to perform an arrhythmia follow-up with Holter monitoring at 1 and 3 months followed by 3 to 6 monthly visits to their referring physicians. For patients with lone typical AFL who have no recurrence of arrhythmia, anticoagulation therapy is usually stopped at 3 months.
After CTI ablation, all the patients must be advised of the possibility of recurrent palpitations and require close follow-up. There is no clear strategy concerning the surveillance and the medical management of patients after typical AFL ablation. Continuation of anticoagulation and/or AAD therapy should be chosen on a per patient basis, as well as the choice of performing PVI for those developing clinically significant AF.
Conclusions
Despite the long-term efficacy of CTI ablation for typical AFL, a significant proportion of patients, especially those with prior history of AF and/or structural heart disease, will develop subsequent AF. These patients must be informed about the risk of developing palpitations and other arrhythmia. After successful CTI ablation, close outpatient follow-up with frequent ECG - Holter monitoring must be performed. For the moment on, the pursuit of antiocoagulation and/or antiarrhythmic therapy must be decided in an individual basis, as well as the requirement and timing of AF ablation.
Disclosures
None.
References
- 1.Granada J, Uribe W, Chyou P H, Maassen K, Vierkant R, Smith P N, Hayes J, Eaker E, Vidaillet H. Incidence and predictors of atrial flutter in the general population. J. Am. Coll. Cardiol. 2000 Dec;36 (7):2242–6. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 2.Natale A, Newby K H, Pisanó E, Leonelli F, Fanelli R, Potenza D, Beheiry S, Tomassoni G. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J. Am. Coll. Cardiol. 2000 Jun;35 (7):1898–904. doi: 10.1016/s0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- 3.Cosio F G, López-Gil M, Goicolea A, Arribas F, Barroso J L. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am. J. Cardiol. 1993 Mar 15;71 (8):705–9. doi: 10.1016/0002-9149(93)91014-9. [DOI] [PubMed] [Google Scholar]
- 4.Philippon F, Plumb V J, Epstein A E, Kay G N. The risk of atrial fibrillation following radiofrequency catheter ablation of atrial flutter. Circulation. 1995 Aug 01;92 (3):430–5. doi: 10.1161/01.cir.92.3.430. [DOI] [PubMed] [Google Scholar]
- 5.Fischer B, Jaïs P, Shah D, Chouairi S, Haïssaguerre M, Garrigues S, Poquet F, Gencel L, Clémenty J, Marcus F I. Radiofrequency catheter ablation of common atrial flutter in 200 patients. J. Cardiovasc. Electrophysiol. 1996 Dec;7 (12):1225–33. doi: 10.1111/j.1540-8167.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 6.Poty H, Saoudi N, Abdel Aziz A, Nair M, Letac B. Radiofrequency catheter ablation of type 1 atrial flutter. Prediction of late success by electrophysiological criteria. Circulation. 1995 Sep 15;92 (6):1389–92. doi: 10.1161/01.cir.92.6.1389. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa H, Lazzara R, Khastgir T, Beckman K J, McClelland J H, Imai S, Pitha J V, Becker A E, Arruda M, Gonzalez M D, Widman L E, Rome M, Neuhauser J, Wang X, Calame J D, Goudeau M D, Jackman W M. Role of the tricuspid annulus and the eustachian valve/ridge on atrial flutter. Relevance to catheter ablation of the septal isthmus and a new technique for rapid identification of ablation success. Circulation. 1996 Aug 01;94 (3):407–24. doi: 10.1161/01.cir.94.3.407. [DOI] [PubMed] [Google Scholar]
- 8.Chen S A, Chiang C E, Wu T J, Tai C T, Lee S H, Cheng C C, Chiou C W, Ueng K C, Wen Z C, Chang M S. Radiofrequency catheter ablation of common atrial flutter: comparison of electrophysiologically guided focal ablation technique and linear ablation technique. J. Am. Coll. Cardiol. 1996 Mar 15;27 (4):860–8. doi: 10.1016/0735-1097(95)00565-x. [DOI] [PubMed] [Google Scholar]
- 9.Spector Peter, Reynolds Matthew R, Calkins Hugh, Sondhi Manu, Xu Yingxin, Martin Amber, Williams Catherine J, Sledge Isabella. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am. J. Cardiol. 2009 Sep 01;104 (5):671–7. doi: 10.1016/j.amjcard.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Ellis Keith, Wazni Oussama, Marrouche Nassir, Martin David, Gillinov Marc, McCarthy Patrick, Saad Eduardo B, Bhargava Mandeep, Schweikert Robert, Saliba Walid, Bash Dianna, Rossillo Antonio, Erciyes Demet, Tchou Patrick, Natale Andrea. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J. Cardiovasc. Electrophysiol. 2007 Aug;18 (8):799–802. doi: 10.1111/j.1540-8167.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 11.Paydak H, Kall J G, Burke M C, Rubenstein D, Kopp D E, Verdino R J, Wilber D J. Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants, and clinical course. Circulation. 1998 Jul 28;98 (4):315–22. doi: 10.1161/01.cir.98.4.315. [DOI] [PubMed] [Google Scholar]
- 12.Bertaglia Emanuele, Bonso Aldo, Zoppo Franco, Proclemer Alessandro, Verlato Roberto, Corò Leonardo, Mantovan Roberto, Themistoclakis Sakis, Raviele Antonio, Pascotto Pietro. Different clinical courses and predictors of atrial fibrillation occurrence after transisthmic ablation in patients with preablation lone atrial flutter, coexistent atrial fibrillation, and drug induced atrial flutter. Pacing Clin Electrophysiol. 2004 Nov;27 (11):1507–12. doi: 10.1111/j.1540-8159.2004.00668.x. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh Ming-Hsiung, Tai Ching-Tai, Chiang Chern-En, Tsai Chin-Feng, Yu Wen-Chung, Chen Yi-Jen, Ding Yu-An, Chen Shih-Ann. Recurrent atrial flutter and atrial fibrillation after catheter ablation of the cavotricuspid isthmus: a very long-term follow-up of 333 patients. J Interv Card Electrophysiol. 2002 Dec;7 (3):225–31. doi: 10.1023/a:1021392105994. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu A, Nozaki A, Rudy Y, Waldo A L. Onset of induced atrial flutter in the canine pericarditis model. J. Am. Coll. Cardiol. 1991 Apr;17 (5):1223–34. doi: 10.1016/0735-1097(91)90857-6. [DOI] [PubMed] [Google Scholar]
- 15.Waldo A L, Cooper T B. Spontaneous onset of type I atrial flutter in patients. J. Am. Coll. Cardiol. 1996 Sep;28 (3):707–12. doi: 10.1016/0735-1097(96)00223-9. [DOI] [PubMed] [Google Scholar]
- 16.Watson R M, Josephson M E. Atrial flutter. I. Electrophysiologic substrates and modes of initiation and termination. Am. J. Cardiol. 1980 Apr;45 (4):732–41. doi: 10.1016/0002-9149(80)90115-0. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz J, Niwano S, Abe H, Rudy Y, Johnson N J, Waldo A L. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ. Res. 1994 May;74 (5):882–94. doi: 10.1161/01.res.74.5.882. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo K, Uno K, Khrestian C M, Waldo A L. Conduction left-to-right and right-to-left across the crista terminalis. Am. J. Physiol. Heart Circ. Physiol. 2001 Apr;280 (4):H1683–91. doi: 10.1152/ajpheart.2001.280.4.H1683. [DOI] [PubMed] [Google Scholar]
- 19.Roithinger F X, Karch M R, Steiner P R, SippensGroenewegen A, Lesh M D. Relationship between atrial fibrillation and typical atrial flutter in humans: activation sequence changes during spontaneous conversion. Circulation. 1997 Nov 18;96 (10):3484–91. doi: 10.1161/01.cir.96.10.3484. [DOI] [PubMed] [Google Scholar]
- 20.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 21.Tai C T, Chen S A, Chiang C E, Lee S H, Wen Z C, Huang J L, Chen Y J, Yu W C, Feng A N, Lin Y J, Ding Y A, Chang M S. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J. Cardiovasc. Electrophysiol. 1998 Feb;9 (2):115–21. doi: 10.1111/j.1540-8167.1998.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 22.Pérez Francisco J, Schubert Christine M, Parvez Babar, Pathak Vishesh, Ellenbogen Kenneth A, Wood Mark A. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
- 23.Camm A John, Kirchhof Paulus, Lip Gregory Y H, Schotten Ulrich, Savelieva Irene, Ernst Sabine, Van Gelder Isabelle C, Al-Attar Nawwar, Hindricks Gerhard, Prendergast Bernard, Heidbuchel Hein, Alfieri Ottavio, Angelini Annalisa, Atar Dan, Colonna Paolo, De Caterina Raffaele, De Sutter Johan, Goette Andreas, Gorenek Bulent, Heldal Magnus, Hohloser Stefan H, Kolh Philippe, Le Heuzey Jean-Yves, Ponikowski Piotr, Rutten Frans H. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010 Oct;31 (19):2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 24.Scharf Christoph, Veerareddy Srikar, Ozaydin Mehmet, Chugh Aman, Hall Burr, Cheung Peter, Good Eric, Pelosi Frank, Morady Fred, Oral Hakan. Clinical significance of inducible atrial flutter during pulmonary vein isolation in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2004 Jun 02;43 (11):2057–62. doi: 10.1016/j.jacc.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 25.Shah Dipen C, Sunthorn Henri, Burri Haran, Gentil-Baron Pascale. Evaluation of an individualized strategy of cavotricuspid isthmus ablation as an adjunct to atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2007 Sep;18 (9):926–30. doi: 10.1111/j.1540-8167.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 26.Shah D, Haïssaguerre M, Takahashi A, Jaïs P, Hocini M, Clémenty J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000 Sep 26;102 (13):1517–22. doi: 10.1161/01.cir.102.13.1517. [DOI] [PubMed] [Google Scholar]
- 27.Anastasio Nicholas, Frankel David S, Deyell Marc W, Zado Erica, Gerstenfeld Edward P, Dixit Sanjay, Cooper Joshua, Lin David, Marchlinski Francis E, Callans David J. Nearly uniform failure of atrial flutter ablation and continuation of antiarrhythmic agents (hybrid therapy) for the long-term control of atrial fibrillation. J Interv Card Electrophysiol. 2012 Oct;35 (1):57–61. doi: 10.1007/s10840-012-9679-0. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty Sanghamitra, Mohanty Prasant, Di Biase Luigi, Bai Rong, Santangeli Pasquale, Casella Michela, Dello Russo Antonio, Tondo Claudio, Themistoclakis Sakis, Raviele Antonio, Rossillo Antonio, Corrado Andrea, Pelargonio Gemma, Forleo Giovanni, Natale Andrea. Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL). Circulation. 2013 May 07;127 (18):1853–60. doi: 10.1161/CIRCULATIONAHA.113.001855. [DOI] [PubMed] [Google Scholar]