Abstract
Background and purpose: Up until recently complex fractionated atrial electrogram (CFAE) ablation has been considered as time consuming and its achievement as challenging, especially for non experimented operators. Moreover, results of substrate ablation based on CFAE detection in atrial fibrillation (AF) are very disparate, mainly because of the operator’s subjective electrogram visual analysis and the difficult distinction between CFAEs really involved in AF perpetuation from other CFAE. Automatic detection provided by 3D mapping system (CARTO® algorithm) can be helpful but is not selective enough, drawing too wide CFAE areas. We sought to demonstrate a better selectivity of a new CFAE algorithm setting in order to better discriminate CFAEs really involved in AF perpetuation from other CFAE.
Methods and subjects: A population of 32 patients (60.4±12.7 years) with paroxysmal (n=3) AF (PAF), persistent (n=16) AF (PeAF) or long-standing persistent (n=13) AF (LSPeAF), and AF history =56±65 months, underwent CFAE ablation based on visual analysis. Before ablation, left atrium CFAE mapping was performed on CARTO® shortest complex interval (SCI) algorithm and reanalyzed after ablation with the two different settings: nominal (SCI 60-120ms/0.05-0.15mV) vs. customized setting (SCI 30-40ms/0,04-0.15mV). CFAE areas automatically detected by both settings (CFAE-CARTO® areas) were respectively measured. The decision to ablate CFAE was only based upon the operator’s electrogram visual analysis taken as reference because of high AF termination rate (93.7%) due to operator’s CFAE selection experience. These ablation points drawn reference-CFAE areas involved in AF perpetuation (ablation point=60mm2) allowing to compare the selectivity of the two previous automatic maps.
Results: With the customized CARTO® SCI setting, we observed a significant reduction of CFAE areas detected by CARTO® (CFAE-CARTO® areas) and of the ablated CFAE surface inside non-CFAE CARTO® areas, (30.6±20.5cm2 vs. 68.8±24.5cm2, p<0.0001, and 1.86±1.82% vs. 3±3%, p=0.003). Furthermore the proportion of ablated areas/detected CFAE-CARTO® areas were higher with customized setting (38.2±19.6% vs. 20.4±17.5%, p=0.008).
Conclusions: This new customized CFAE algorithm setting is significantly more selective than the nominal one and allows an automated detection of CFAE really involved in AF perpetuation truer to an efficient experienced operator’s electrogram visual analysis.
Keywords: Ablation-catheter, Atrial fibrillation, Substrate, Complex Fractionated Atrial Electrogram (CFAE), Electroanatomical 3D automated mapping
Introduction
Background
A new approach of substrate ablation in atrial fibrillation (AF) based on complex fractionated electrogram (CFAE) detection was first described by Nademanee et al.[1] Though CFAE ablation in AF was described as efficient,[1-2] the results (AF termination rate by ablation and long term outcomes) are heterogeneous[3-4] partially because of the subjectivity of the CFAE detection method based on electrogram visual analysis known as the « gold standard ».
The identification of CFAE sites for ablation by visual analysis, based on distinction between “CFAEs really involved in AF perpetuation” from “other CFAE”, is subjective and depends on the experience of the operator in the field of substrate ablation.
A more objective approach, with the help of a selective algorithm, would facilitate discrimination of CFAEs involved in AF perpetuation, and shorten the learning curve for new operators. Computerized algorithms for automated recognition and quantification of CFAEs have been introduced as integrated modules in 3D mapping systems (Biosense CARTO® and Saint Jude Medical Velocity®). The ability of these two software packages to locate CFAEs has been validated by physicians in different studies.[5-6] In these studies, physicians were able to validate the signals automatically detected by the algorithm. However no study has yet validated if these algorithms could detect all CFAEs involved in AF perpetuation (AF termination after their ablation).
AF termination is a reliable endpoint used in defragmentation approach. Several studies have demonstrated that AF termination during ablation is a good clinical predictor of long-term outcome.[7-8]
When performing defragmentation for AF guided by electrogram visual analysis, we noticed that the CARTO® nominal shortest complex intervals (SCI) algorithm setting, from Biosense Webster (Diamond Bar, California), was able to detect CFAE but did not focus enough on CFAEs involved in AF perpetuation and finally chosen by operator (reference-CFAEs).
Rational objective
Our objective was to modify the CARTO® SCI maps settings to make it more discriminative (closer to our electrogram visual analysis taken as reference), helping non experienced operators to focus more rapidly on the area of interest and reducing the procedure time.
Material and Methods
Study Population
Between November 2009 and June 2010, all consecutive patients referred to our centers for symptomatic refractory AF in whom a substrate ablation was necessary, were eligible for inclusion. Exclusion criteria were: redo ablations and lone “focal” AF (PAF without structural heart disease or hypertension with short episode duration ≤ 24 hours, predicting a limited quantity of substrate).
Before ablation, for each patient included, left ventricular ejection fraction (LVEF) and antero-posterior left atrial (LA) diameter was measured by biplane transthoracic echocardiogram (TTE).
3D Electroanatomic Mappings and Ablation Techniques
All anti-arrhythmic medications were discontinued at least five half-lives prior to ablation, except for beta blockers and amiodarone in patients with non PAF. Patients were anticoagulated with warfarin for at least 3 weeks prior to the procedure (International Normalized Ratio 2–3). Warfarin was stopped 3 days prior to the procedure and anti-coagulation was maintained with low molecular weight heparin.
All procedures were performed under general anesthesia. The absence of LA appendage thrombus was confirmed by transesophageal echocardiogram before transeptal puncture. Surface electrocardiogram and bipolar endocardial electrograms (filtered from 30 to 500 Hz) were continuously monitored and stored on a computer-based digital amplifier/recorder system (GE Healthcare, Cardiolab, Milwaukee, Wisconsin, USA). The following catheters were introduced through the right femoral vein: a deflectable decapolar catheter (2–5–2 mm electrode spacing, Xtrem, ELA Medical, France) positioned within the coronary sinus (CS), a 3.5 mm irrigated-tip quadripolar ablation catheter (2–5–2 mm inter-electrode spacing, ThermoCool, Biosense- Webster, F or J curve) was introduced through a long sheath (Preface multipurpose, Biosense-Webster) perfused with heparinized saline solution.
Preliminary CARTO® Mapping
Electroanatomic mapping was performed using the CARTO® navigation and mapping system from Biosense Webster. Before starting ablation, an electroanatomic LA map using the SCI algorithm was drawn in AF with nominal setting. To allow correct local electrogram assessment, the mapping catheter was maintained in each location for 2.5 seconds minimum before points were acquired.
For patients in sinus rhythm (SR) at the beginning of the mapping (n=11), AF was induced by atrial pacing, using isoproterenol if necessary.
Visual CFAE Selection and Ablation
The primary targets of ablation were continuous and low voltage potentials (Figure 1).[1,9] Only permanent CFAE over time were selected. CFAE ablation has a cumulative effect with progressive CL increase during ablation and an instantaneous effect in specific crucial areas with sudden CL increase of AF termination.[10] The progressive CL increase during ablation may lead to the disappearance of CFAE not really involved in AF perpetuation.
Figure 1. Examples of CFAEs visually choosen for ablation. All these electrograms (panel A, B, C) are low voltage potentials (<0.1 mV). Panel A shows continuous and fractionated activity. Panel B shows an electrogram which is less continuous but very fractionated. Panel C displays activity that could be tagged as electrical scar but is very continuous with very low voltage fractionated potentials (<0.06mV).
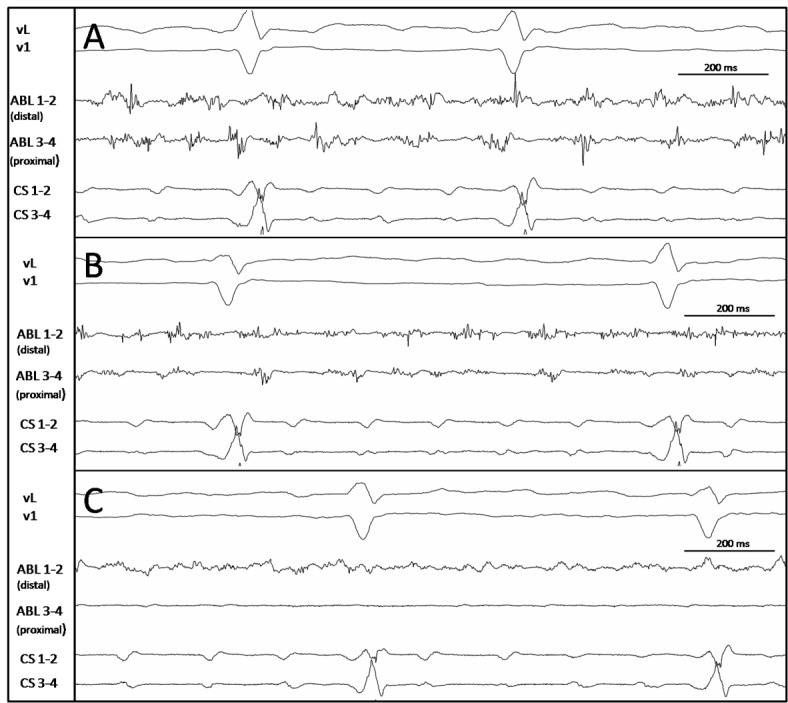
(CS: coronary sinus catheter; ABL: ablation catheter.)
Thus dynamic analysis of CFAE over time is still necessary, despite pre-selection by computer.
CFAE ablation was then performed with “point-by-point” applications (60 seconds). Ablation power control settings were parameterized at: 30 to 45W for the septum, 25W for Pulmonary Veins (PV) ostia, posterior wall of LA and CS, 35W for the other segments of LA and right atrium (RA) with an esophageal thermal monitoring. The endpoint was AF termination defined as conversion of AF to SR or regularization into stable Atrial Tachycardia (AT). In such cases, AT was mapped and ablated until SR was restored. AF “non-inducibility” was then tested using isoproterenol in PAF and PeAF. If the CFAE ablation approach failed to restore SR, no circumferential or anatomical linear ablations were carried out and external cardioversion was performed. After SR conversion, Lassoguided PV isolation was performed as a final ablation step in all patients with PAF.
Post Ablation Creation of Reference-CFAE maps (ablated areas taken as reference)
The automatic CFAE maps created were not used during ablation. Only operator’s electrogram visusal analysis was used to select CFAE eligible for ablation. Reference-CFAE mapping was obtained with the area drawn by all CFAE ablation points. RF applications performed during AT or PV isolation were excluded from the analysis as they do not correspond with CFAE ablation. Each ablation point covered a surface of 60 2. This surface depends on the catheter movement and represents the mean area “brushed” by the ablation catheter in stable positions defined by 20 RF “point by point” applications (of 60 sec each measured in 10 patients)
Electro-anatomic points taken on preliminary maps were used to draw automatic CFAE maps with 2 different settings: nominal and customized.
Nominal setting of SCI CFAE maps were the following: CFAE was defined by the system based on the peak-to-peak (P-P) interval in millisecond (msec). Global CFAE parameters (for all CFAE points) and point CFAE parameters (for individual points) were set to standard values. In detail, minimal amplitude threshold and maximal amplitude threshold were set to 0.05 millivolt (mV) and 0.15 mV, and the minimal interval and maximal interval between two consecutive peaks were set to 60 msec and 120 msec.
For customized settings we empirically modified the CARTO® SCI maps settings to reduce the area of all automated detected CFAE and focus more on the detection of CFAE involved in AF perpetuation. Ablation points were considered as CFAE points of clinical value involved in AF perpetuation (Reference-CFAEs), because of high AF termination rate (93.7%). In the substrate-based ablation technique first described,[1] the primary targeted electrograms involved in AF perpetuation, were low voltage (≤ 0.15mV) and continuous potential ones. Thus we changed the algorithm setting to reduce both voltage and cycle length (CL) of P-P interval limits, and narrowed the fractionation window (ms).
We proposed a new customized setting of CARTO® SCI mapping in this study (called “SCI 30-40”). The differences in the nominal setting (“SCI 60-120”) are the following:
a shorter CL of P-P interval (30 ms vs. 60 ms)
The benefits of geriatric day hospital care have been controversial for many years.
a narrower window for fractionation (30 to 40 ms vs. 60 to 120 ms)
a wider window for non-fractionated potentials (40 to 120 ms)
a lower voltage cut off at 0,04mV vs. 0.05mV (no change for upper voltage cut off : 0.15mV)
Comparison
We then compared the CARTO® maps of nominal “SCI 60-120” setting and customized “SCI 30-40” setting with reference-CFAE maps of ablation points.
For each patient, CARTO® and reference-CFAE LA maps were analysed retrospectively (after ablation), and the following data was listed: number of electro-anatomic points (used to create CARTO® maps) in LA, number of ablation points, and LA map surface (cm2) as automatically computed by the CARTO® software.
On CFAE-CARTO® maps the colour grade scale for fractionation quantification is the following: red and yellow=highest degree of fractionation, green and blue=moderate degree of fractionation, and pink=no fractionation. Because the selection of electrograms for ablation is binary (to be ablated or not to be ablated), our selection of fractionated involved only the “red and yellow” areas.
For each setting, the following surfaces (cm2) were manually measured:
the CFAE-CARTO® surface defined as the sum of all red and yellow areas detected by algorithm
the total non-CFAE-CARTO® surface, defined as the sum of the remainder of LA areas
the ablated surface localized inside the CFAE-CARTO® surface
the ablated surface localized outside CFAE-CARTO® surface (or inside non-CFAE CARTO® surface).
For each setting, the number of patients, in whom ablation points leading to AF termination were located inside of CFAE-CARTO® areas, was specified.
We then compared the CFAE-CARTO® surface (in cm2 and in % of total LA surface), the ablated surface inside CFAE-CARTO® surface (cm2 and % of total CFAE surface), and the ablated surface outside CFAE-CARTO® surface (cm2 and % of total non-CFAECARTO ® surface).
Follow-Up
Patients were followed in the outpatient clinic 3, 6, 9 and 12 months post ablation. Twelve-lead ECG and 24-hour Holter recordings were obtained in all patients at each follow-up visit. Patients were asked to contact the investigator if symptoms suggestive of arrhythmia occurred. A 12-lead ECG was performed in case of reported symptoms between follow-up visits. Recurrences were based upon patient reporting, 24-hour Holter recordings, and/or ECG data.
Statistical Analysis
Statistical analysis was performed with the chi-square tests for categorical variables and Wilcoxon matched pairs signed ranksum tests for numerical data. A two-tailed P value of < 0.05 was considered to indicate statistical significance. All statistical analyzes were performed using STATA 9.2 software (Stata Corp., College Station, TX).
Results
Patients Characteristics
A population of thirty two patients (75% male, 60.4 ± 12.7 years), undergoing radiofrequency catheter ablation for paroxysmal (n=3) AF (PAF), persistent (n=16) AF (PeAF) or long-standing persistent (n=13) AF(LSPeAF), according to the ESC 2010 guidelines,[11] was enrolled in this study.
All patients had experienced an AF recurrence whilst taking at least one antiarrhythmic drug (mean = 2.2). Included patients had a long history of AF (79±64 months with a mean sustained episode duration of non PAF=56 ± 65 months).
Mean LA dimension was 44.6±5.2 mm, and mean LVEF was 52.6±10.5 %. The clinical characteristics of the patients are shown in Table 1.
Table 1. Baseline patient characteristics.
| Baseline patients characteristics | Patients (n=32) |
|---|---|
| Age (years) | 60.4±12.7 |
| Sex M/F | 24/8 |
| History of AF (months) | 79.5±64.3 |
| Maximum sustained episode duration (months) | 24±50 |
| AF type | |
| Paroxysmal | 3 |
| Persistent | 16 |
| Long standing persistent | 13 |
| Left atrium diameter (mm) | 44.6±5.2 |
| LEVF (%) | 52.6±10.6 |
| Structural heart disease | 15 |
| Coronary heart disease | 2 |
| Tachycardia-induced cardiomyopathy | 11 |
| Hypertrophic cardiomyopathy | 0 |
| Valvular disease | 0 |
| Hypertension | 15 |
Ablation Results
AF was successfully terminated (converted to SR or stable AT) in 30 patients (93.7 %). Only 5 patients (all with LS-PeAF) needed electrical cardioversion: 2 for AF persistence, and 3 for stable AT. SR was restored in 27 patients (84.4%) without electrical cardioversion. Six were directly converted to SR (22.2%), and 21 regularized to AT (77.8%) before conversion to SR.
All four PVs were isolated in patients with PAF. “Non inducibility” was tested and achieved in 16 patients with PAF and PeAF (50%). The mean CL of AF (measured in LA appendage) was 179.5 ± 25.6 ms. The mean procedure and fluoroscopy durations were 4.2±1.3 h and 31±17 min respectively. No serious adverse event related to the procedure occurred.
Electro Anatomic Maps
For automated CFAE-CARTO® maps the mean LA surface automatically measured on CARTO® maps was 174.4 ± 54.6 cm2. The mean number of electro-anatomic points taken for SCI maps in LA was 294.2 ±138.4. A total of 9416 points acquired in the LA were analyzed. The mean mapping time was 18 ± 10 min. The mean density of electro-anatomic points was 1.53 ± 0.82 points /cm2.
For reference-CFAE maps the mean number of 60 sec ablation points in LA for CFAE ablation was 63.3 ± 35.
Retrospective comparison of surfaces with nominal and customized settings (Figure 2 and 3);
Figure 2. Comparison of surfaces with the two settings (cm2). The customized setting draws significantly smaller CFAE-CARTO® areas than nominal one. The non-ablated surface inside CFAE-CARTO® areas were also significantly smaller.
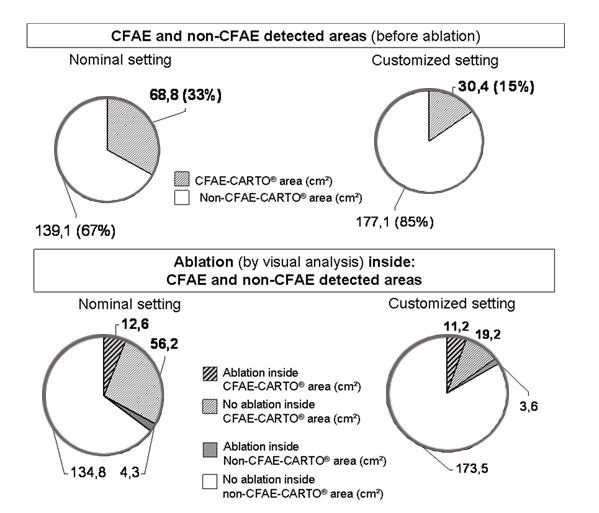
Figure 3. Panel A: Example comparing the 2 SCI map settings (Nominal setting on the left side, customized setting on the right side) in a patient with persistent AF. Panel B: Example comparing the 2 SCI map settings for a patient with long standing AF (AP: Antero-posterior view, PA: Postero-anterior view);
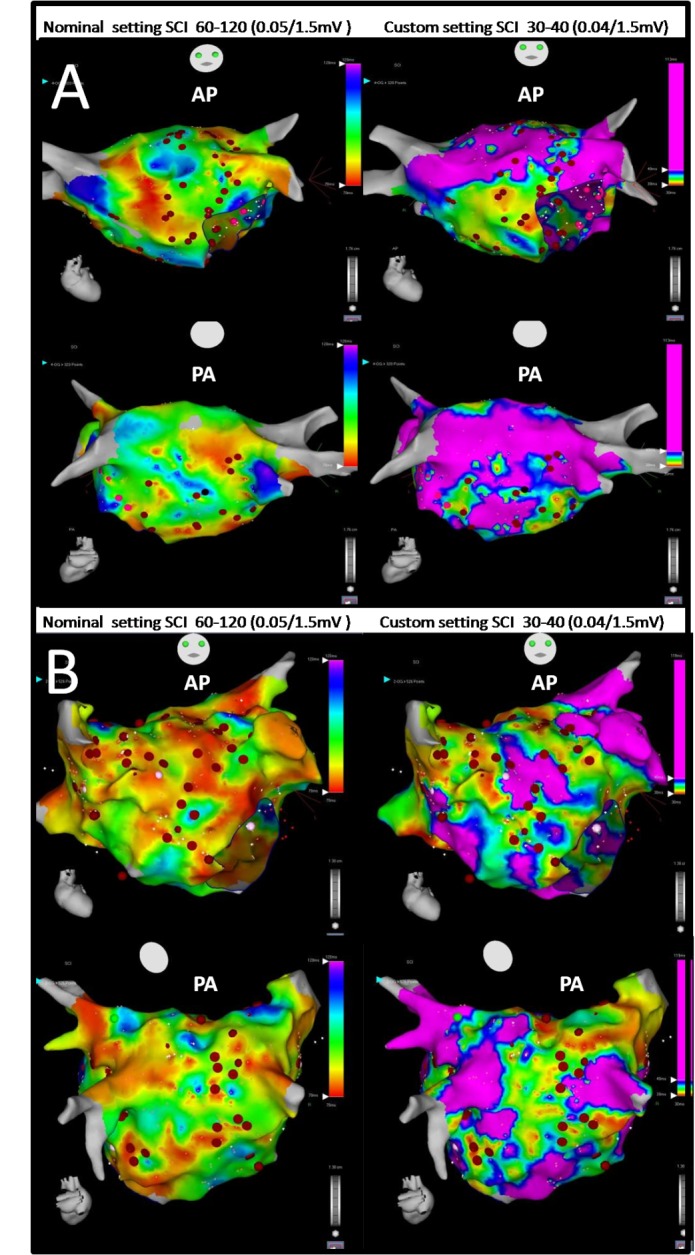
The red dots correspond to CFAE ablation points visually positioned. Black dots correspond to AF termination ablation points. Many fractionated ablated areas have almost the same location but the surface area is lower with the customized setting. The CFAE-CARTO® surface not ablated is also lower with the custom setting.
The mean CFAE-CARTO® surfaces were significantly smaller in the customized vs nominal groups: 30.6 ± 20.5 cm2 vs. 68.8 ± 24.5 cm2, p < 0.0001. These areas represent a percentage of 17.5 ±37.5 % vs 39.5 ± 44.8 % of the total LA surface. The ablated surface inside or outside CFAE-CARTO® areas remains absolutely the same in both settings but the ablated proportion of CFAE-CARTO® areas was significantly higher in the customized setting group (38.2 ± 19.6 %, vs 20.4 ± 17.5 %, p = 0.008). However, the ablated area non-CFAE inside-CARTO® areas were smaller in this group (1.86 ± 1.82 %, vs 3.0 ± 3% p=0.003).
Furthermore, the % of patients, in whom ablation points leading to AF termination were located inside of CFAE-CARTO® areas, was significantly higher with custom setting (90% vs 83.3%, p=0.001).
Outcomes
During a mean follow-up of 12 ± 7 months after the last procedure, 25/32 patients (84.4%) were free from any arrhythmia (16 patients still under antiarrhythmic drug therapy, 1.6 ablation/patient). Redo procedures, using the same substrate based ablation technique, were performed in 16 patients (9 for AF, 9 for AT). A third procedure was necessary in 4 patients for AT recurrences.
Discussion
Main Findings
In this study, for the first time, a CFAE software program is compared to an efficient visual CFAE detection, validated by a high AF termination rate (93.7%). We demonstrated that our new simple customized setting (“30-40” SCI setting) is significantly more selective than the nominal one, and closer to the operator’s visual analysis.
With this new setting, the total CFAE-CARTO® area and ablated surface outside CFAE-CARTO® area were both significantly smaller. Furthermore ablated proportions of CFAE-CARTO® areas were larger than with nominal setting.
CFAE Definitions and Subjectivity of Target Electrograms
Many mechanisms have been suggested to explain the fractionation of electrograms during AF including: reentrant circuit pivot points, wave collision and slow conduction,[12-13] anisotropic conduction or focal reentry,[14] and wavebreaks at the periphery of high-frequency rotors;[15] These mechanisms can play an active or a passive role in AF maintenance and multiple mechanisms may coexist in the same patient at different sites or times.[16] This data suggests that all CFAEs may not be the same, and that ablation should primarily focus on CFAEs which play an active role in AF perpetuation.
The definitions of CFAE1,[13,17] are large, blending together different types of electrograms.
To define the specific electrogram characteristics of different types of CFAE, Hunter et al. proposed a CFAE classification based on visual analysis.[18] No information was provided regarding which type of CFAE should be ablated or not. Minimal data is available to differentiate the CFAEs issued from active drivers in AF or critical zones for AF maintenance, from other CFAE without clinical value, corresponding to passive epiphenomenon of fibrillatory conduction. Takahashi et al. reported that only 17% of the CFAE areas were related to AF termination and described the specific characteristics of electrograms associated with the slowing or AF termination as continuous activity or a temporal activation gradient.[19]
The “30-40” SCI Setting Focuses on Continuous and Low Voltage Potentialsn
Although this new SCI setting was created from empirical daily practice, we demonstrated its better selectivity for CFAE areas involved in AF perpetuation (low voltage, very fast and fractionated or continuous electrograms).
Decreasing the voltage cut off from 0.05 to 0.04 mV allowed us to unmask areas of very low voltage CFAE. In the substratebased ablation technique first described.[1] the primary targets were low voltage CFAE (≤ 0.15mV). These low voltage potentials may correspond to areas of remodelling or fibrosis[20,21] that could participate in AF substrate. A very low level of interference (< 0.03 mV) is needed for CFAE ablation and for the use of this setting.
Decreasing the SCI cut-off from 60 to 30 ms, and narrowing the SCI window to 30-40 ms seems to shift the focus to very fast or continuous potentials which are the primary target of ablation.
Calò et al. have previously shown that a specific setting of CFAE-CARTO® software is needed to improve its sensitivity and specificity.[22] In their study, the best window of fractionation was “15-30 ms” which focused on very high frequency CFAEs. They also studied the “30-50 ms” window which was also a good setting with 74% of sensitivity and 87% specificity. These results are concordant with our findings, selecting very fast and fractionated potentials. In Calò’s study the CFAE-CARTO® was validated by two different electrophysiologists with good concordance, but results of the ablation of these fractionation potentials were not specified. Regarding the validation of CFAE algorithms in other studies, ablation strategies did not target CFAEs or results of CFAE ablation were not specified.[5,23,24,25] This is the main limitation of such studies regarding the debate toward CFAE ablation effectiveness and visual analysis subjectivity.
Focus on Electrograms Involved in AF Maintenance and Exclusion of CFAE Areas without Clinical Value
With the customized setting, the CFAE-CARTO® maps had smaller fractionated areas focusing in a more discriminating manner on the reference-CFAE ablated areas (with a high AF termination rate). This setting excludes fractionated potentials which have no important impact on AF and are not targeted in our ablation protocol.[1,13,17]
This customized setting can help in guiding ablations, but because of color interpolation on CFAE maps and catheter movement, operator’s visual analysis remains necessary to validate electrograms before ablation within automated fractionated areas. Only very high density CFAE maps could standardize and automate this substratebased ablation technique. A multi-electrode mapping tool could be very interesting with this setting.
Simple Use
Many algorithms are available to help physicians in CFAE ablation. Such an approach must be, in our opinion, as straightforward as possible, to simplify and standardize the technique. The 2.5 sec window of acquisition for each point appears to be a good compromise between mapping time and accuracy, taking into account the shortterm temporal stability of CFAEs.[6] The time required for LA CFAE mapping in our study was 18 ± 10 min which is acceptable in daily practice. Only one kind of CFAE map with a simple setting is used in this study (no “complex” analysis or handling of CFAE maps are necessary); LA is divided into: CFAE-CARTO® areas (red to yellow areas) requiring visual validation before ablation; and non-CFAECARTO ® areas excluded from ablation. This setting is very simple in daily practice even for physicians not currently using the CFAE approach and could help in standardizing the technique.
Acute and Long Term Results
Our acute and long term results on this 32 patient series might appear quite high especially for this population. Our EGM-based substrate ablation protocol was performed in complete accordance with the technique described in the first publication on this topic[1] and include other targeted EGMs: temporal gradient of activation and rapid fires. The following differences from previous publications,[3,26] could explain differences in acute and long-term results: voltage of targeted potentials (often <0.1 mV in our protocol), point-by-point applications (60 seconds) without “dragging” (to confirm EGM elimination after RF applications), ablation in the CS and the RA, and dynamic power settings (15–45 W).
This technique is reproducible and can easily be taught and learned with a short leaning curve (less than 10 cases).
Study limitations
The greatest limitation of CFAE mapping is the density of acquisition points. In case of low-density LA maps, areas of fractionation may not be accurate because of point interpolation. The mean density of CFAE points in the present study is quite accurate (1.53 ± 0.82 points/cm2) but we must take account of the interpolation of points in the area measurement. The necessity to take as much CFAE points as possible is time consuming. High-density accurate maps, which would automate the technique, are the only way to reduce the importance of visual analysis. Further studies using this setting on high-density maps are necessary in the field of CFAE ablation automation.
Each RF application was considered on CARTO® maps as a 60 mm2 diameter circle. This value could be criticized, but it is the result of measures on maps and reflects the natural and artificial movement of the catheter.
This study is based on the temporal stability hypothesis of the CFAE[1,6] with a 2.5 sec window of acquisition. In the case of nonpermanent fractionated potentials or if the tip movement is too fast during mapping, there is a risk of false positive and false negative results (if points are taken too quickly).
Even though the right atrium is often ablated in our protocol, right atrium CFAE maps were not analyzed in this study.
The “30-40” SCI setting was the most selective setting regarding a great majority of our patients. In “fast” (CL <150 ms) or “slow” AF (CL > 210 ms) cases this setting was less accurate. A dynamic setting based on the CL for these few cases could be discussed.
Despite the high AF termination rate in this study, some “passive” CFAEs, not playing any role in AF perpetuation, have probably been ablated needlessly. RF time may be reduced with a better understanding of both CFAE specific characteristics, and AF physiopathology. Nevertheless, the amount of ablation itself may also play an important role.[27]
This retrospective design of the study may be a weak point of the study.
To conclude on the benefits of such new Carto CAFEs discrimination algorithm, a prospective controlled study with multipoler mapping catheter could be interesting to conduct.
Conclusions
This new CFAE algorithm setting is significantly more selective than the nominal setting. As a result it focuses on visually targeted areas that lead to a high AF termination rate. These promising results may be less time consuming, and will simplify and improve the reproducibility of CFAE mapping in AF.
Multielectrode mapping could be proven as a very useful tool in this field.
Acknowledgements
This study was presented as poster at Heart Rhythm 2010 (Denver) and Cardiostim 2010 (Nice) and as oral presentation at CARTO® users Meeting 2010 and ISCAT 2010 (Paris).
The authors thank Anthony Apetiti and Thibault Pugeat for their precious help and the nursing and technical staff at the Institut Cardiovasculaire Paris Sud, Hôpital Privé Jacques Cartier and Hôpital St Joseph, for their patience and valuable assistance.
Special thanks to Koonlawee Nademanee for his extremely helpfull teaching on substrate ablation.
Disclosures
Drs Seitz, Horvilleur, Lacotte and Pisapia have received speaker fees from Biosense Webster and St Jude Medical (minor).
Dr Pisapia has received honoraria from St Jude Medical and Biosense Webster as a consultant.
All other authors have reported that they have no relationships to disclose.
References
- 1.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 02;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Iriki Yasuhisa, Ishida Sanemasa, Oketani Naoya, Ichiki Hitoshi, Okui Hideki, Ninomiya Yuichi, Maenosono Ryuichi, Matsushita Takehiko, Miyata Masaaki, Hamasaki Shuichi, Tei Chuwa. Relationship between clinical outcomes and unintentional pulmonary vein isolation during substrate ablation of atrial fibrillation guided solely by complex fractionated atrial electrogram mapping. J Cardiol. 2011 Nov;58 (3):278–86. doi: 10.1016/j.jjcc.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Oral Hakan, Chugh Aman, Good Eric, Wimmer Alan, Dey Sujoya, Gadeela Nitesh, Sankaran Sundar, Crawford Thomas, Sarrazin Jean F, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Frederick Melissa, Fortino Jackie, Benloucif-Moore Suzanne, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007 May 22;115 (20):2606–12. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 4.Elayi Claude S, Verma Atul, Di Biase Luigi, Ching Chi Keong, Patel Dimpi, Barrett Conor, Martin David, Rong Bai, Fahmy Tamer S, Khaykin Yaariv, Hongo Richard, Hao Steven, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Arruda Mauricio, Schweikert Robert A, Natale Andrea. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008 Dec;5 (12):1658–64. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Scherr Daniel, Dalal Darshan, Cheema Aamir, Cheng Alan, Henrikson Charles A, Spragg David, Marine Joseph E, Berger Ronald D, Calkins Hugh, Dong Jun. Automated detection and characterization of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. Heart Rhythm. 2007 Aug;4 (8):1013–20. doi: 10.1016/j.hrthm.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Scherr Daniel, Dalal Darshan, Cheema Aamir, Nazarian Saman, Almasry Ibrahim, Bilchick Kenneth, Cheng Alan, Henrikson Charles A, Spragg David, Marine Joseph E, Berger Ronald D, Calkins Hugh, Dong Jun. Long- and short-term temporal stability of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 Jan;20 (1):13–21. doi: 10.1111/j.1540-8167.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill Mark D, Jaïs Pierre, Takahashi Yoshihide, Jönsson Anders, Sacher Frédéric, Hocini Mélèze, Sanders Prashanthan, Rostock Thomas, Rotter Martin, Pernat Andrej, Clémenty Jacques, Haïssaguerre Michel. The stepwise ablation approach for chronic atrial fibrillation--evidence for a cumulative effect. J Interv Card Electrophysiol. 2006 Sep;16 (3):153–67. doi: 10.1007/s10840-006-9045-1. [DOI] [PubMed] [Google Scholar]
- 8.Nademanee Koonlawee, Schwab Mark C, Kosar Erol M, Karwecki Margaret, Moran Michael D, Visessook Nithi, Michael Anthony Don, Ngarmukos Tachapong. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):843–9. doi: 10.1016/j.jacc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Nademanee Koonlawee, Schwab Mark, Porath Joshua, Abbo Aharon. How to perform electrogram-guided atrial fibrillation ablation. Heart Rhythm. 2006 Aug;3 (8):981–4. doi: 10.1016/j.hrthm.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Maluski A, Seitz J, Bars C C, Horvilleur J, Lacotte J, Rosier A, Ferracci A, Faure J, Bremondy M, Curel L, Penaranda G, Pisapia A. Crucial areas and cumulative effect of Complex Fractionated Atrial Electrograms ablation on Atrial Fibrillation termination (abstr) HRS Congress. 2013;0:0–0. [Google Scholar]
- 11.Knecht Sébastien, Wilton Stephen B, Haïssaguerre Michel. The 2010 update of the ESC guidelines for the management of atrial fibrillation. Circ. J. 2010 Nov;74 (12):2534–7. doi: 10.1253/circj.cj-10-1030. [DOI] [PubMed] [Google Scholar]
- 12.Konings K T, Kirchhof C J, Smeets J R, Wellens H J, Penn O C, Allessie M A. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994 Apr;89 (4):1665–80. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 13.Konings K T, Smeets J L, Penn O C, Wellens H J, Allessie M A. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997 Mar 04;95 (5):1231–41. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 14.Spach M S, Dolber P C, Heidlage J F. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ. Res. 1988 Apr;62 (4):811–32. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- 15.Kalifa Jérôme, Tanaka Kazuhiko, Zaitsev Alexey V, Warren Mark, Vaidyanathan Ravi, Auerbach David, Pandit Sandeep, Vikstrom Karen L, Ploutz-Snyder Robert, Talkachou Arkadzi, Atienza Felipe, Guiraudon Gérard, Jalife José, Berenfeld Omer. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006 Feb 07;113 (5):626–33. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 16.Rostock Thomas, Rotter Martin, Sanders Prashanthan, Takahashi Yoshihide, Jaïs Pierre, Hocini Mélèze, Hsu Li-Fern, Sacher Fréderic, Clémenty Jacques, Haïssaguerre Michel. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006 Jan;3 (1):27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Clémenty J. Regional disparities of endocardial atrial activation in paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1996 Nov;19 (11 Pt 2):1998–2003. doi: 10.1111/j.1540-8159.1996.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 18.Hunter Ross J, Diab Ihab, Thomas Glyn, Duncan Edward, Abrams Dominic, Dhinoja Mehul, Sporton Simon, Earley Mark J, Schilling Richard J. Validation of a classification system to grade fractionation in atrial fibrillation and correlation with automated detection systems. Europace. 2009 Dec;11 (12):1587–96. doi: 10.1093/europace/eup351. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Yoshihide, O'Neill Mark D, Hocini Mélèze, Dubois Rémi, Matsuo Seiichiro, Knecht Sébastien, Mahapatra Srijoy, Lim Kang-Teng, Jaïs Pierre, Jonsson Anders, Sacher Frédéric, Sanders Prashanthan, Rostock Thomas, Bordachar Pierre, Clémenty Jacques, Klein George J, Haïssaguerre Michel. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J. Am. Coll. Cardiol. 2008 Mar 11;51 (10):1003–10. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Marcus Gregory M, Yang Yanfei, Varosy Paul D, Ordovas Karen, Tseng Zian H, Badhwar Nitish, Lee Byron K, Lee Randall J, Scheinman Melvin M, Olgin Jeffrey E. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007 Feb;4 (2):138–44. doi: 10.1016/j.hrthm.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, Kottkamp H, Dhein S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004 Apr;90 (4):400–5. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calò Leonardo, De Ruvo Ermenegildo, Sciarra Luigi, Gricia Roberto, Navone Giovanna, De Luca Lucia, Nuccio Francesca, Sette Antonella, Pristipino Cristian, Dulio Alessandro, Gaita Fiorenzo, Lioy Ernesto. Diagnostic accuracy of a new software for complex fractionated electrograms identification in patients with persistent and permanent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Oct;19 (10):1024–30. doi: 10.1111/j.1540-8167.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 23.Monir George, Pollak Scott J. Consistency of the CFAE phenomena using custom software for automated detection of complex fractionated atrial electrograms (CFAEs) in the left atrium during atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Sep;19 (9):915–9. doi: 10.1111/j.1540-8167.2008.01153.x. [DOI] [PubMed] [Google Scholar]
- 24.Porter Michael, Spear William, Akar Joseph G, Helms Ray, Brysiewicz Neil, Santucci Peter, Wilber David J. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):613–20. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 25.Verma Atul, Novak Paul, Macle Laurent, Whaley Bonnie, Beardsall Marianne, Wulffhart Zaev, Khaykin Yaariv. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008 Feb;5 (2):198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Oral Hakan, Chugh Aman, Yoshida Kentaro, Sarrazin Jean F, Kuhne Michael, Crawford Thomas, Chalfoun Nagib, Wells Darryl, Boonyapisit Warangkna, Veerareddy Srikar, Billakanty Sreedhar, Wong Wai S, Good Eric, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J. Am. Coll. Cardiol. 2009 Mar 03;53 (9):782–9. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill Mark D, Wright Matthew, Knecht Sébastien, Jaïs Pierre, Hocini Mélèze, Takahashi Yoshihide, Jönsson Anders, Sacher Frédéric, Matsuo Seiichiro, Lim Kang Teng, Arantes Leonardo, Derval Nicolas, Lellouche Nicholas, Nault Isabelle, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur. Heart J. 2009 May;30 (9):1105–12. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]


