Abstract
Atrial fibrillation significantly contributes to mortality and morbidity through increased risk of stroke, heart failure and myocardial infarction. Investigations of mechanisms responsible for the development and maintenance of atrial fibrillation have highlighted the importance of gap junctional remodeling. Connexins 40 and 43, the major atrial gap junctional proteins, undergo considerable alterations in expression and localization in atrial fibrillation, creating an environment conducive to sustained reentry. Atrial fibrillation is initiated and/or maintained in this reentrant substrate. This review will focus on connexin remodeling in the context of underlying mechanism and possible therapeutic target for atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia that contributes to mortality.[1] AF increases mortality risk[2] and increased morbidity due to increased risk of stroke, heart failure and myocardial infarction.[3-5] Many factors predispose patients to the development of AF, such as surgery,[6] hypertension, valve disease, coronary artery disease or heart failure; however the strongest predisposing factor is ischemia.[7] Yet, understanding risk factors has not improved patient survival. Current therapies for AF are suboptimal and their potential pro-arrhythmic effects limit their benefit.[8] Research has focused on understanding the mechanisms underlying AF onset and maintenance in the hope of developing more targeted beneficial treatments. Research has begun to focus on the importance of slowed electrical conduction through atrial tissues in the development and maintenance of AF. Gap junctions, made up of connexins, are principle components responsible for both atrial and ventricular myocardial conduction. In this review, we will focuses on the relationship between connexins and mechanisms responsible for the development and maintenance of AF.
AF Mechanisms
A summary of the current understanding of AF is that initiation occurs through either reentry or focal ectopic firing. In lone AF, continued ectopic firing from pulmonary veins or other sites may play a role in sustaining the arrhythmia as well. In the setting of structural heart disease, most studies suggest that AF is sustained through intra-atrial reentry.[9,1-]
Focal ectopic firing that initiates, and in some circumstances sustains, AF can manifest as early-afterdepolarizations (EADs) or delayed afterdepolarizations (DADs). EADs can occur with action potential duration (APD) prolongation, which allows sodium or L-type Ca2+ channels to recover from inactivation to depolarize the membrane. This mechanism may explain the increased prevalence of AF in some long-QT patients.[11] Studies have also shown involvement of the sodium-calcium exchanger in the development of either EADs or DADs.[12] DADs occur when abnormal 2+ release during diastole causes an inward current by activating the Na+-2+ exchanger, depolarizing the membrane. Several reviews have been written on these topics and other ion channel changes, such as increased K+ currents, that occur in the setting of AF.[13,14]
Reentry requires tissue heterogeneities in conduction and repolarization. An important contributor to these elements are atrial structural and electrical remodeling that occur with sustained AF. The phrase ‘AF begets AF’ summarizes how these remodeling events resulting from AF increase the probability of reentry and thereby increase the probability of further AF. Electrical remodeling refers to alterations in ion channels that promote the development of AF. Examples include up-regulation of IK1 and IKACH, which lead to APD shortening.[15,16] Similarly, Ca-channel down-regulation and changes in calcium handling in AF also lead to APD shortening. Most important in structural remodeling is the development of atrial fibrosis, which can interfere with electrical coupling and cause conduction slowing.[17] Both APD shortening and CV slowing are inherent mechanisms in sustaining AF. Wavelength theory states that a reentrant wave is dependent upon conduction velocity (CV)and refractory period. Both decreased CV and shortened APD occur in AF, which leads to maintenance of AF. Drug-induced or gene-therapy induced APD prolongation has proven effective in suppressing AF.[18,19] Recently, we have also shown that enhancing CV through overexpression of connexins 40 or 43 (Cx40, Cx43) reduces the incidence of AF.[10] This highlights the importance of connexin remodeling in the setting of AF.
Connexins
Connexins are ubiquitous proteins that can be found in most organs including atrial and ventricular myocardium and the specialized cardiac conduction system. Each connexin has a general structure of 4 transmembrane helices, 2 extracellular loops, a cytosolic loop and cytosolic N- and C-termini (Figure 1B). Six connexins join together to form a hemichannel, which docks with a hemichannel on an adjacent cell to form a gap junction (Figure 1A). Gap junctions are large conductance channels between adjacent cells that allow for low resistance passage of metabolic substrates and ions from one cell to another (arrow Figure 1C). In the heart, intercellular connectivity through gap junctions is a fundamental element controlling conduction of the electrical impulse across the myocardium. Atrial fibrillation, heart-failure, myocardial ischemia and infarction have all been associated with heterogeneous or decreased expression of Cx43 and decreased CV, suggesting a tight association between connexins and conduction.[20,21] Several isoforms of connexins exist and they are named according to their molecular weight. In the atrial myocardium, Cx40 is the predominant connexin followed by Cx43 and Cx45.[22] Studies have found strong involvement of Cxs 40 and 43 in the development and maintenance of AF but little is reported on Cx45 in AF.
Figure 1. General Connexin Structure Six individual connexins form a connexon which docks with a second connexon, forming a gap junction (A). Connexins have the general structure of 4 transmembrane α-helices, 2 extracellular loops, a cytosolic loop, and a cytosolic N- and C- terminus (B). Connexins are normally located at the polar ends of myocytes in the intercalated disk and allow for the passage of metabolic substances and ions from one myocyte to the next (arrows) (C). In atrial fibrillation, there is a reduction in connexins at the intercalated disk (outlined connexins) and enhanced lateralization of connexins. There can also be alterations in phosphorylation of connexins, resulting in reduced conductance (dashed arrow) (D).
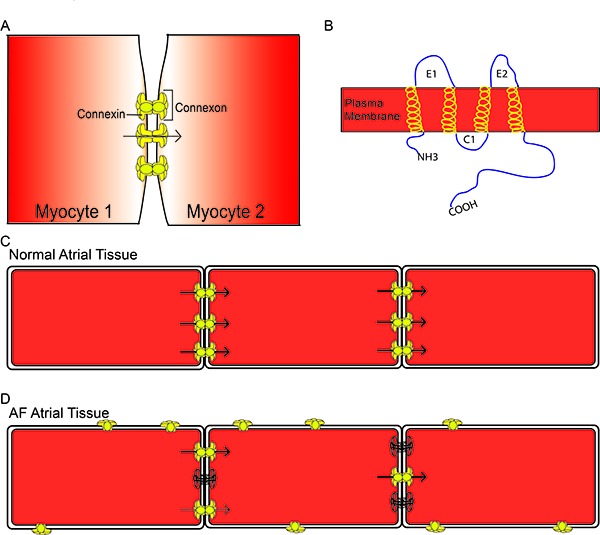
Connexins are highly regulated proteins such that they respond to intracellular voltage changes or acidification, and their function is altered by phosphorylation.[23-26] Alterations in connexin phosphorylation status lead to changes in connexin localization and/ or conductance. Connexins are normally expressed at the polar ends of myocytes in the intercalated disks (Figure 1C). Under stressed conditions such as ischemia or AF, connexins undergo changes in phosphorylation that ultimately lead to changes in connexin localization away from the intercalated disks and changes in gap junctional conductance for the connexins remaining at the disks (Figure 1D).
Connexins Involvement in AF
Connexin 40
Human genetics studies point to a role for Cx40 in the initiation and maintenance of AF. Although the majority of AF is thought to be sporadic and non-familial, connexin mutations have clearly been associated with familial, early onset AF in patients as summarized in Table 1. Human studies have identified Cx40 nonsense mutations, gene promoter polymorphisms and somatic mutations that lead to an overall reduction in Cx40 expression.[27-30]
Table 1. Connexin 40 in Human AF: Examples of studied Cx40 genetic mutations found in AF patients and findings of Cx40 alterations in patients with AF.
| Connexin 40 in Human AF | |||
|---|---|---|---|
| Gene Mutations | Effect on protein expression | AF effect | |
| Nonsense mutations Cx40 promoter SNPs | Stop codon inserted resulting in truncated Cx40 at amino acid 49 -44 Glycine → Alanine | Familial AF with 100% Penetrance | Yang et al 2010 |
| Increased atrial vulnerability (i.e. easy | Firouzi et al 2004 | ||
| Early onset AF | Wirka et al 2011 | ||
| Missense Mutations | TATA box SNP (rs10465885) resulted in decreased Cx40 mRNA expression Proline 88 → Serine or Glycine 38→Aspartate, resulting in no Cx40 at the intercalated disc Alanine 96→Serine resulting in low conductance GJ Methionine 163→Valine, no GJ phenotype observed | All observed in early onset AF | Gollob et al 2006 |
| Patients in AF With mitral valve disease | Increased Expression of Cx40 but with increased lateralized Cx40 and Cx43 Increased Cx40 expression | Study of 12 chronic AF patients | Polontchouk et al 2001 |
| Reduced and heterogeneously expressed Cx40 | Study of 41 lone AF and 36 AF patients with valve disease | Wetzel et al 2005 | |
| Study of 10 patients with valve disease and AF | Nao et al 2003 |
Although Cx40 genetic mutations have been identified in the development of AF, public screening for Cx40 mutations to explain lone AF would not be cost-effective. Evidence suggests family history of AF increases the risk of development of AF[31,32] but Cx40 disease-causing mutations have been infrequent and polymorphisms that change neither Cx40 expression nor function have also been identified.[33] Screening for AF risk genes may prove beneficial in the future, if more Cx40 mutations become linked to development of AF. Currently, Cx40 gene mutations have been identified in only several cases of familial AF.
Although Cx40 genetic mutations have not been identified as the major cause of familial AF, altered Cx40 expression has clearly been observed in patients with AF. Both genetic mutations in Cx40 and non-genetic based AF have led to connexin trafficking defects,reductions in gap junctional conductance and altered expression of Cx40. Nao et al found AF patients with mitral valve regurgitation had heterogeneous reductions in Cx40 expression.[34] In contrast, Polontchouk et al and others found AF patients expressed higher levels of Cx40, but they found increased connexin lateralization (Table 1).[35,36] Although changes in total Cx40 expression differ between studies, common findings between these human AF studies include heterogeneous expression of Cx40 across different regions of the atria and lateralization of Cx40 throughout the atria, both of which could lead to heterogeneous and slow conduction and potentially to reentry.
Several animal models have been developed to study AF, including atrial burst pacing with and without heart failure, and ventricular failure with increased AF inducibility but without sustained AF. Similar to the human data, a common finding across these diverse models has been heterogeneous expression of Cx40. Further supporting a role of Cx40 in AF, Cx40 knock-out mice have abnormal atrial impulse propagation, slowed conduction, decreased safety factor for 1:1 conduction in the atrium and increased arrhythmia inducibility.[37-40] Contrary to adult mice studies, in vitro cultured strands of neonatal atrial myocytes from Cx40 knock-out mice showed enhanced conduction velocity with an associated increase in Cx43 expression at the intercalated. A confounding factor for these cell culture studies was the finding of increased Cx43 in the atrial myocytes isolated from the Cx40 knock-out neonates. The increased conduction velocity may simply be a function of Cx43 levels in that experimental system.
While there may be some differences in findings between the various experimental models (Table 2), the majority of animal evidence supports alterations in Cx40 expression and/or localization, associated with reduced CV and increased AF vulnerability. These studies corroborate the human data strongly suggesting an integral role of Cx40 in AF.
Table 2. Connexin 40 in animal models of AF.
| Model | Effect | |
|---|---|---|
| Cx40 KO mice | Atrioventricular conduction slows | Simon et al 1998 |
| Atrial Conduction slowing, with high frequency of intra-atrial reentrant tachycardia | Kirchhoff et al 1998 | |
| Altered atrial conduction patterns, decreased right atrial conduction and decreased safety factor for maintaining 1:1 conduction | Bagwe et al 2005 | |
| Loss of right atrial and left atrial CV heterogeneity, Cx40 developmentally required for setting up heterogeneity | ||
| Cx40 KO neonatal cardiomyocytes | Increased propagation velocity in synthetic strands of Cx40 KO cardiomyocytes. This is associated with increased Cx43 in the intercalated disc. | Beauchamp et al 2006 |
| Rapid-pacing induced AF in goat | Total Cx40 expression and CV is unchanged, but heterogeneous expression of Cx40 | Van der Velden et al 1998 |
| Cx40/Cx43 ratio decreases as increased stability of AF | Van der Velden et al 2000 | |
| Rapid-pacing induced AF in pig | Total Cx40 expression same as sinus-rhythm; however, intra-atrialconduction reduced | Igarashi et al 2012 |
Connexin 43
Similar to Cx40, inconsistencies in Cx43 expression between the various studies have been shown (Table 3). Rare familial AFassociated Cx43 mutations have been found that alter connexin trafficking, resulting in a mosaic pattern of connexin expression across the atrium, suggesting that decreases in Cx43 can cause AF.[41] Wetzel et al. found that AF patients with mitral valve disease had increased Cx43 expression.[35,35] Rucker-Martin found no change in overall Cx43 expression but relative increased in the amount of unphosphorylated and/or lateralized Cx43.[42] Importantly, studies have found that total Cx43 expression decreases in heart failure, but the AF studies have generally not had a significant component of heart failure patients in their populations. Overall, the human data on Cx43 is less robust than the data on Cx40, but the available data suggest that alterations in Cx43 expression or function may cause AF, and that AF may reduce the amount of functional, intercalated disklocalized Cx43 if not the total Cx43 amount.
Table 3. Connexin 43 in Human AF: This table lists examples of both genetic mutations and observed Cx43 alterations in patients with AF.
| Gene Mutations | Effect | ||
|---|---|---|---|
| Frameshift Mutation | Nucleotide Deletion resulting in intercellular retention of GJs and dominant-negative down-regulation of both Cx40 and Cx43 | Sporadic nonfamilial case of AF | Thibodeau et al 2010 |
| Patients with AF With mitral valve disease | Increased Cx43 expression | Study of 36 AF patients | Wetzel et al 2005 |
| With dilated atria | Both dilated atria and AF patients had dephosphorylated and lateralized Cx43. | Study of 5 AF patients and 5 patients with dilated atria | Rucker-Martin et al 2006 |
Animal models of AF more strongly support the involvement of Cx43 in AF than the available human data. Several animal models show alterations in Cx43 expression associated with AF (Table 4). In the neonatal mouse in vitro myocyte culture system described above, Beauchamp found atrial strands from Cx43 knock-out mice have shown reduced CV and the substrate for reentry, but their Cx43 findings were confounded by the finding of decreased Cx40 expression in the myocytes isolated from the Cx43 knock-out mice.[43] Further highlighting the importance of Cx43 in atrial conduction, transgenic mice carrying the Cx43 G60S mutant (described in occulodentodigital dysplasia) are highly susceptible to induction of atrial tachycardia and atrial arrhythmias.[44] Similarly, a rapid pacing pig model of AF and severe heart failure had decreased Cx43 expression, decreased phosphorylated Cx43, enhanced lateralized connexins (Figure 2) with CV slowing and persistent AF.[10] In addition gene-therapy expressing Cx43 preserved atrial conduction and prevented the development of AF, further supporting Cx43 as a major contribution to atrial stability.[10]
Table 4. Connexin 43 in Animal Models of AF.
| Model | Effect | |
|---|---|---|
| Congestive Heart Failure canine | Decrease in phosphorylated Cx43 associated with an increase in lateralized Cx43. Also interstitial fibrosis present, leading to heterogeneous conduction in atria. | Burstein et al 2009 |
| Rapid-pacing induced AF in goat | Total levels of Cx43 and localization unchanged, increase in dephosphorylated Cx43. | Van der Velden et al 1998, 2000 |
| Rapid-pacing induced AF in pig | Reduced and lateralized expression of Cx43. | Igarashi et al 2012 |
| Point-mutation Cx43 G60S mutant (Oculodentodigital dysplasia mouse) | Heterozygous mutant mouse, dominant negative decrease in total Cx43 expression. Results in a 50% reduction of myocyte junctional conductance, highly susceptible to induction of AF/sustained AF. | Tuomi et al 2011 |
| Cx43 KO embryonic cardiomyocytes | No expression of Cx43 but also a decrease in Cx40 expression. Decrease in conduction velocity. | Beauchamp et al 2005 |
| Cx43 heterozygous KO mice | 50% reduction in atrial Cx43 expression, but no change in atrial CV | Thomas et al 1998 |
| Sterile pericarditis in canine | Overall reduction in Cx43 expression, CV slowed and increased sustained atrial arrhythmias (AF or atrial flutter) | Ryu et al 2006 |
Figure 2. Atrial staining for Connexin 40 and 43 Rapid-pacing induced AF in a porcine model showed no alteration in Cx40 expression (yellow); however, there was reduced expression of total and phosphorylated Cx43 and enhanced lateralization of Cx43 (green). This resulted in significantly less Cx43 localized in the intercalated disc (IC-disk), as shown in A with quantification in B. IC-disk was calculated by (IC-disk intensity/total intensity) x 100% Adapted from Igarashi et al 2012.
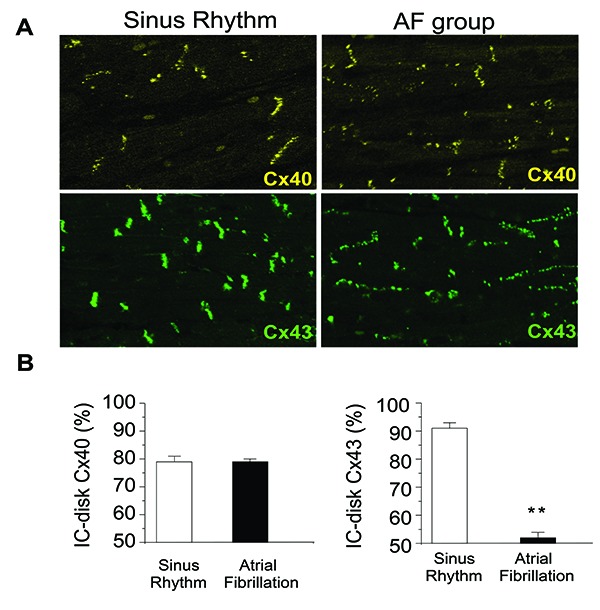
Although strong evidence supports Cx43 involvement in determining atrial conduction properties and potentially increasing vulnerability to development or maintenance of AF, not all studies have shown this. Thomas et al reported no changes in atrial CV or P-wave duration in Cx43 heterozygous knock-out mice.[45] Overall, the data on Cx43 show association of Cx43 mutations with AF in very rare cases, correlation between Cx43 expression and intercalated disk localization and atrial conduction properties in most but not all studies, and a reduction in Cx43 expression resulting from AF in the setting of heart failure but not necessarily in the setting of normal ventricular function.
Therapy for AF
Therapies to terminate or prevent AF include antiarrhythmic drugs, the surgical maze procedure, and ablation either to isolate pulmonary veins or to disrupt intra-atrial reentry. Pulmonary vein isolation is successful in terminating AF in up to 80% of patients, with greater success occurring in patients with paroxysmal, lone AF.[47] Ablation of reentrant circuits is considerably less successful, with long-term freedom from AF less than 50% in patients with dilated atria and persistent or permanent AF.[49] Limitations to ablation success include inadequacies in currently available tools to create complete lines of conduction block and the lack of a generally accepted lesion set based on the underlying mechanisms responsible for maintaining AF.
Better atrial imaging and mapping techniques are being developed to improve ablation efficacy. MRI imaging has successfully identified gaps through scarred atrial regions which provide the site of reentry for AF, allowing for more targeted ablations.[50,51] In contrast, another more recent study did not identify visible reentrant pathways on MRI even though reentrant circuits were found within the scarred regions.[52] Similarly, Focal Impulse and Rotor Modulation (FIRM) techniques to identify rotors and focal sources of AF show promise in creating smaller ablation lesions while still providing AF termination.[53,54] In canine models, extensive ablation of both the right and left atrium was required to get 100% reduction of AF. However this was associated with decreased Cx43 expression in areas near the ablation site.[55] This finding suggests that less extensive ablation may have the unintended consequence of worsening conduction heterogeneity, which may be proarrhythmic.
Research has focused on developing therapies that target both connexin and ion channel remodeling, in hopes of altering the underlying mechanism thereby preventing development of sustained AF. In canines, bepridil was found to reduce AF inducibility and duration, while simultaneously preventing SCN5A and L-type Ca2+ channel reductions.[56] Pairing bepridil with olmesartan also prevented Cx43 down-regulation and reduced tissue fibrosis, both resulting in improved conduction velocity.[57] This bepridil/olmesartan strategy successfully prevented AF inducibility, but results of this strategy for patients with already remodeled atria are unclear. A similar study using amiodarone showed reverse-remodeling, which may be a more relevant end-point.[58] Of course, many patients with AF are already treated with amiodarone. Since this drug is not curative, more extensive investigation is needed to develop a definitive therapy. Recently relaxin, an anti-fibrotic hormone, showed promising atrial fibrosis reverse-remodeling in spontaneous hypertensive rats; however, large animal studies need to be done to evaluate translational possibilities.[59] Similarly, metoprolol was found to antagonize connexin lateralization and conduction slowing in AF,[60] however, metoprolol also is not curative for AF. These drug data emphasize the complexity of the situation and the need for extensive analysis in realistic test models.
Targeted connexin therapies are in early stages of development. We explored the possibility that targeted overexpression of either connexin 40 or 43 in the atrium would improve CV and prevent AF in our porcine AF-heart failure model.[10,61] The model combined 42-hz burst atrial pacing used in the goat lone AF model with an uncontrolled ventricular response giving tachycardia-induced ventricular failure. We delivered the gene for either Cx40 or Cx43 using an epicardial painting method that we had previous validated to delivery genes densely and homogeneously to the atria.[62] In sinus rhythm animals, we saw no change in the already normal CV with either Cx40 or Cx43 overexpression. Since connexin expression and post-translational processing were normal, we interpreted these data to suggest that gap junctional conductance was not the limiting factor to CV under normal circumstances. In the AF-heart failure animals, we saw normalization of CV and prevention of AF with gene transfer of either connexin. These data linked connexin expression to CV alterations in AF and demonstrated that CV played a role in AF development and maintenance. Bikou et al. found similar results with Cx43 overexpression using a more localized inject and shock delivery method in the pig AF-heart failure model.[61]
Drug therapies targeting connexins have also been found to enhance atrial CV.[63] Rotigaptide is a small peptide that has been show to antagonize CV slowing in a variety of situations. Shiroshita- Takishita et al. found improved conduction in 3 canine models of atrial remodeling; atrial tachypacing, ventricular tachypacing and acute atrial ischemia, but AF vulnerability was reduced only in acute ischemia.[64] Similarly, Haugan et al showed that rotigaptide enhanced atrial CV but did not reduce AF inducibility in a rabbit model of chronic volume overload.[65] Similarly, Guerra et al. found rotigaptide effective in prevention of AF in mitral valve regurgitation but not congestive heart failure.[66] These studies suggest that interaction between connexin expression and function and AF development may vary by model. A limitation of this conclusion is that rotigaptide’s mechanism of action is not fully understood. Further studies need to be performed to understand the possibilities for gap junctiontargeted therapies for either prevention or treatment of AF.
Conclusions
Connexins are highly dynamic proteins that are important in myocardial conduction. Overall, inconsistencies exist over the exact pattern of connexin expression changes in the setting of AF. Although less common, genetic mutations in Cx40 and Cx43 have been found as the definitive cause of AF in both human studies and animal models. Importantly, both animal studies and human studies of AF commonly show connexin phosphorylation is altered, connexins become lateralized and connexins are heterogeneously expressed. Alterations in connexin phosphorylation and localization have been associated with decreased atrial conduction. Heterogeneous and slowed atrial conduction creates the substrate conducive to reentry. Taken together, connexin remodeling is a key mechanism in the maintenance of sustained AF. Several drug-therapies, genetherapies and small peptides targeting connexin remodeling have proven beneficial in antagonizing connexin dephosphorylation and down-regulation in AF, leading to enhanced CV and reduced AF vulnerability, verifying the importance of connexins in the initiation and maintenance of AF. Future studies need to be done to assess the potential role of these therapies in the setting of atrial fibrosis, structural remodeling or pre-existing AF.
Disclosures
None.
References
- 1.Miyasaka Yoko, Barnes Marion E, Gersh Bernard J, Cha Stephen S, Bailey Kent R, Abhayaratna Walter P, Seward James B, Tsang Teresa S M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114 (2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E J, Wolf P A, D'Agostino R B, Silbershatz H, Kannel W B, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 08;98 (10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Anter Elad, Jessup Mariell, Callans David J. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009 May 12;119 (18):2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 4.Krahn A D, Manfreda J, Tate R B, Mathewson F A, Cuddy T E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am. J. Med. 1995 May;98 (5):476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 5.Polimeni Licia, Perri Ludovica, Saliola Mirella, Basili Stefania, Violi Francesco. The risk of myocardial infarction in patients with atrial fibrillation: an unresolved issue. Intern Emerg Med. 2010 Apr;5 (2):91–4. doi: 10.1007/s11739-010-0352-2. [DOI] [PubMed] [Google Scholar]
- 6.Auer Johann, Weber Thomas, Berent Robert, Ng Choi-Keung, Lamm Gudrun, Eber Bernd. Risk factors of postoperative atrial fibrillation after cardiac surgery. J Card Surg. 2005 Sep 13;20 (5):425–31. doi: 10.1111/j.1540-8191.2005.2004123.x. [DOI] [PubMed] [Google Scholar]
- 7.Allessie M A, Boyden P A, Camm A J, Kléber A G, Lab M J, Legato M J, Rosen M R, Schwartz P J, Spooner P M, Van Wagoner D R, Waldo A L. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001 Feb 06;103 (5):769–77. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 8.Corley Scott D, Epstein Andrew E, DiMarco John P, Domanski Michael J, Geller Nancy, Greene H Leon, Josephson Richard A, Kellen Joyce C, Klein Richard C, Krahn Andrew D, Mickel Mary, Mitchell L Brent, Nelson Joy Dalquist, Rosenberg Yves, Schron Eleanor, Shemanski Lynn, Waldo Albert L, Wyse D George. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004 Mar 30;109 (12):1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 9.Atienza Felipe, Almendral Jesús, Moreno Javier, Vaidyanathan Ravi, Talkachou Arkazdi, Kalifa Jérôme, Arenal Angel, Villacastín Julian P, Torrecilla Esteban G, Sánchez Ana, Ploutz-Snyder Robert, Jalife José, Berenfeld Omer. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006 Dec 05;114 (23):2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi Tomonori, Finet J Emanuel, Takeuchi Ayano, Fujino Yoshihisa, Strom Maria, Greener Ian D, Rosenbaum David S, Donahue J Kevin. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012 Jan 17;125 (2):216–25. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson Jonathan N, Tester David J, Perry James, Salisbury Benjamin A, Reed Carol R, Ackerman Michael J. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008 May;5 (5):704–9. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilgemann Donald W. New insights into the molecular and cellular workings of the cardiac Na+/Ca2+ exchanger. Am. J. Physiol., Cell Physiol. 2004 Nov;287 (5):C1167–72. doi: 10.1152/ajpcell.00288.2004. [DOI] [PubMed] [Google Scholar]
- 13.Nattel Stanley. Therapeutic implications of atrial fibrillation mechanisms: can mechanistic insights be used to improve AF management? Cardiovasc. Res. 2002 May;54 (2):347–60. doi: 10.1016/s0008-6363(01)00562-4. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki Yu-ki, Nishida Kunihiro, Kato Takeshi, Nattel Stanley. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011 Nov 15;124 (20):2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 15.Girmatsion Zenawit, Biliczki Peter, Bonauer Angelika, Wimmer-Greinecker Gerhard, Scherer Mirella, Moritz Anton, Bukowska Alicia, Goette Andreas, Nattel Stanley, Hohnloser Stefan H, Ehrlich Joachim R. Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart Rhythm. 2009 Dec;6 (12):1802–9. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Bosch R F, Zeng X, Grammer J B, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 1999 Oct;44 (1):121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 17.Burstein Brett, Comtois Philippe, Michael Georghia, Nishida Kunihiro, Villeneuve Louis, Yeh Yung-Hsin, Nattel Stanley. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ. Res. 2009 Dec 04;105 (12):1213–22. doi: 10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 18.Amit Guy, Kikuchi Kan, Greener Ian D, Yang Lizhu, Novack Victor, Donahue J Kevin. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010 Jun 01;121 (21):2263–70. doi: 10.1161/CIRCULATIONAHA.109.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen O D, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001 Jul 17;104 (3):292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Greener ID ST, Igarashi T, Strom M, Rosenbaum DS, Donahue JK. Connexin 43 gene transfer improves conduction velocity and reduces ventricular arrhythmia susceptibility. Cleveland OH: Case Western Reserve University; . (Provisional Acceptance J Am Coll Cardiol) 2012;0:0–0. [Google Scholar]
- 21.Poelzing Steven, Rosenbaum David S. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004 Oct;287 (4):H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 22.Davis L M, Kanter H L, Beyer E C, Saffitz J E. Distinct gap junction protein phenotypes in cardiac tissues with disparate conduction properties. J. Am. Coll. Cardiol. 1994 Oct;24 (4):1124–32. doi: 10.1016/0735-1097(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 23.Ek-Vitorín J F, Calero G, Morley G E, Coombs W, Taffet S M, Delmar M. PH regulation of connexin43: molecular analysis of the gating particle. Biophys. J. 1996 Sep;71 (3):1273–84. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosinsky Gina E, Solan Joell L, Gaietta Guido M, Ngan Lucy, Lee Grace J, Mackey Mason R, Lampe Paul D. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem. J. 2007 Dec 15;408 (3):375–85. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solan Joell L, Marquez-Rosado Lucrecia, Sorgen Paul L, Thornton Perry J, Gafken Philip R, Lampe Paul D. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J. Cell Biol. 2007 Dec 17;179 (6):1301–9. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solan Joell L, Lampe Paul D. Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 2009 Apr 15;419 (2):261–72. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Yi-Qing, Liu Xu, Zhang Xian-Ling, Wang Xin-Hua, Tan Hong-Wei, Shi Hai-Feng, Jiang Wei-Feng, Fang Wei-Yi. Novel connexin40 missense mutations in patients with familial atrial fibrillation. Europace. 2010 Oct;12 (10):1421–7. doi: 10.1093/europace/euq274. [DOI] [PubMed] [Google Scholar]
- 28.Yang Yi-Qing, Zhang Xian-Ling, Wang Xin-Hua, Tan Hong-Wei, Shi Hai-Feng, Jiang Wei-Feng, Fang Wei-Yi, Liu Xu. Connexin40 nonsense mutation in familial atrial fibrillation. Int. J. Mol. Med. 2010 Oct;26 (4):605–10. doi: 10.3892/ijmm_00000505. [DOI] [PubMed] [Google Scholar]
- 29.Wirka Robert C, Gore Shamone, Van Wagoner David R, Arking Dan E, Lubitz Steven A, Lunetta Kathryn L, Benjamin Emelia J, Alonso Alvaro, Ellinor Patrick T, Barnard John, Chung Mina K, Smith Jonathan D. A common connexin-40 gene promoter variant affects connexin-40 expression in human atria and is associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Feb;4 (1):87–93. doi: 10.1161/CIRCEP.110.959726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gollob Michael H, Jones Douglas L, Krahn Andrew D, Danis Lynne, Gong Xiang-Qun, Shao Qing, Liu Xiaoqin, Veinot John P, Tang Anthony S L, Stewart Alexandre F R, Tesson Frederique, Klein George J, Yee Raymond, Skanes Allan C, Guiraudon Gerard M, Ebihara Lisa, Bai Donglin. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N. Engl. J. Med. 2006 Jun 22;354 (25):2677–88. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 31.Ellinor Patrick T, Yoerger Danita M, Ruskin Jeremy N, MacRae Calum A. Familial aggregation in lone atrial fibrillation. Hum. Genet. 2005 Nov;118 (2):179–84. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 32.Fox Caroline S, Parise Helen, D'Agostino Ralph B, Lloyd-Jones Donald M, Vasan Ramachandran S, Wang Thomas J, Levy Daniel, Wolf Philip A, Benjamin Emelia J. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004 Jun 16;291 (23):2851–5. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 33.Tchou Gregory D, Wirka Robert C, Van Wagoner David R, Barnard John, Chung Mina K, Smith Jonathan D. Low prevalence of connexin-40 gene variants in atrial tissues and blood from atrial fibrillation subjects. BMC Med. Genet. 2012 Nov 07;13 () doi: 10.1186/1471-2350-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nao Tomoko, Ohkusa Tomoko, Hisamatsu Yuji, Inoue Noriko, Matsumoto Tomo, Yamada Jutaro, Shimizu Akihiko, Yoshiga Yasuhiro, Yamagata Toshihiko, Kobayashi Shigeki, Yano Masafumi, Hamano Kimikazu, Matsuzaki Masunori. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am. J. Cardiol. 2003 Mar 15;91 (6):678–83. doi: 10.1016/s0002-9149(02)03403-3. [DOI] [PubMed] [Google Scholar]
- 35.Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, De Vivie ER, Dhein SJ. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38:883–891. doi: 10.1016/s0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 36.Wetzel U, Boldt A, Lauschke J, Weigl J, Schirdewahn P, Dorszewski A, Doll N, Hindricks G, Dhein S, Kottkamp H. Expression of connexins 40 and 43 in human left atrium in atrial fibrillation of different aetiologies. Heart. 2005 Feb;91 (2):166–70. doi: 10.1136/hrt.2003.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leaf DE, Feig JE, Vasquez C, Riva PL, Yu C, Lader JM, Kontogeorgis A, Baron EL, Peters NS, Fisher EA, Gutstein DE, Morley GE. Connexin40 imparts conduction heterogeneity to atrial tissue. Circulation research. 2008;103:1001–1008. doi: 10.1161/CIRCRESAHA.107.168997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagwe Suveer, Berenfeld Omer, Vaidya Dhananjay, Morley Gregory E, Jalife José. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005 Oct 11;112 (15):2245–53. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon A M, Goodenough D A, Paul D L. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 1998 Feb 26;8 (5):295–8. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 40.Kirchhoff S, Nelles E, Hagendorff A, Krüger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr. Biol. 1998 Feb 26;8 (5):299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- 41.Thibodeau Isabelle L, Xu Ji, Li Qiuju, Liu Gele, Lam Khanh, Veinot John P, Birnie David H, Jones Douglas L, Krahn Andrew D, Lemery Robert, Nicholson Bruce J, Gollob Michael H. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010 Jul 20;122 (3):236–44. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]
- 42.Rucker-Martin Catherine, Milliez Paul, Tan Sisareuth, Decrouy Xavier, Recouvreur Michel, Vranckx Roger, Delcayre Claude, Renaud Jean-François, Dunia Irene, Segretain Dominique, Hatem Stéphane N. Chronic hemodynamic overload of the atria is an important factor for gap junction remodeling in human and rat hearts. Cardiovasc. Res. 2006 Oct 01;72 (1):69–79. doi: 10.1016/j.cardiores.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Beauchamp Philippe, Yamada Kathryn A, Baertschi Alex J, Green Karen, Kanter Evelyn M, Saffitz Jeffrey E, Kléber André G. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ. Res. 2006 Nov 24;99 (11):1216–24. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 44.Tuomi Jari M, Tyml Karel, Jones Douglas L. Atrial tachycardia/fibrillation in the connexin 43 G60S mutant (Oculodentodigital dysplasia) mouse. Am. J. Physiol. Heart Circ. Physiol. 2011 Apr;300 (4):H1402–11. doi: 10.1152/ajpheart.01094.2010. [DOI] [PubMed] [Google Scholar]
- 45.Thomas S A, Schuessler R B, Berul C I, Beardslee M A, Beyer E C, Mendelsohn M E, Saffitz J E. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation. 1998 Feb 24;97 (7):686–91. doi: 10.1161/01.cir.97.7.686. [DOI] [PubMed] [Google Scholar]
- 46.Ryu Kyungmoo, Li Li, Khrestian Celeen M, Matsumoto Naomichi, Sahadevan Jayakumar, Ruehr Mary L, Van Wagoner David R, Efimov Igor R, Waldo Albert L. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2007 Aug;293 (2):H1231–41. doi: 10.1152/ajpheart.00607.2006. [DOI] [PubMed] [Google Scholar]
- 47.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò M P, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001 Nov 20;104 (21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 48.Kong Melissa H, Piccini Jonathan P, Bahnson Tristram D. Efficacy of adjunctive ablation of complex fractionated atrial electrograms and pulmonary vein isolation for the treatment of atrial fibrillation: a meta-analysis of randomized controlled trials. Europace. 2011 Feb;13 (2):193–204. doi: 10.1093/europace/euq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neumann T, Wojcik M, Berkowitsch A, Erkapic D, Zaltsberg S, Greiss H, Pajitnev D, Lehinant S, Schmitt J, Hamm CW, Pitschner HF, Kuniss M. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;0:0–0. doi: 10.1093/europace/eut021. [DOI] [PubMed] [Google Scholar]
- 50.Reddy Vivek Y, Schmidt Ehud J, Holmvang Godtfred, Fung Maggie. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J. Cardiovasc. Electrophysiol. 2008 Apr;19 (4):434–7. doi: 10.1111/j.1540-8167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 51.Badger Troy J, Daccarett Marcos, Akoum Nazem W, Adjei-Poku Yaw A, Burgon Nathan S, Haslam Thomas S, Kalvaitis Saul, Kuppahally Suman, Vergara Gaston, McMullen Lori, Anderson Paul A, Kholmovski Eugene, MacLeod Rob S, Marrouche Nassir F. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):249–59. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spragg David D, Khurram Irfan, Zimmerman Stefan L, Yarmohammadi Hirad, Barcelon Bernie, Needleman Matthew, Edwards David, Marine Joseph E, Calkins Hugh, Nazarian Saman. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012 Dec;9 (12):2003–9. doi: 10.1016/j.hrthm.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Shivkumar Kalyanam, Ellenbogen Kenneth A, Hummel John D, Miller John M, Steinberg Jonathan S. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J. Cardiovasc. Electrophysiol. 2012 Dec;23 (12):1277–85. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elvan A, Huang X D, Pressler M L, Zipes D P. Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation. 1997 Sep 02;96 (5):1675–85. doi: 10.1161/01.cir.96.5.1675. [DOI] [PubMed] [Google Scholar]
- 56.Fukaya Hidehira, Niwano Shinichi, Satoh Daisuke, Masaki Yoshihiko, Niwano Hiroe, Kojima Jisho, Moriguchi Masahiko, Izumi Tohru. Inhomogenic effect of bepridil on atrial electrical remodeling in a canine rapid atrial stimulation model. Circ. J. 2008 Feb;72 (2):318–26. doi: 10.1253/circj.72.318. [DOI] [PubMed] [Google Scholar]
- 57.Fukaya Hidehira, Niwano Shinichi, Niwano Hiroe, Masaki Yoshihiko, Kiryu Michiro, Hirasawa Shoji, Sato Daisuke, Moriguchi Masahiko, Izumi Tohru. Combined effects of up- and downstream therapies on atrial fibrillation in a canine rapid stimulation model. Int. J. Cardiol. 2012 May 31;157 (2):197–206. doi: 10.1016/j.ijcard.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Ashikaga Keiichi, Kobayashi Takao, Kimura Masaomi, Owada Shingen, Sasaki Shingo, Iwasa Atsushi, Furukawa Ken-ichi, Motomura Shigeru, Okumura Ken. Effects of amiodarone on electrical and structural remodeling induced in a canine rapid pacing-induced persistent atrial fibrillation model. Eur. J. Pharmacol. 2006 Apr 24;536 (1-2):148–53. doi: 10.1016/j.ejphar.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Parikh A, Patel DR, McTiernan CF, Xiang W, Haney j, Yang L, Lin B, Kaplan AD, Bett G, Rasmusson RL, Shroff SG, Schwartzman DS, Salama G. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy, and increasing conduction velocity and sodium current in spontaneously hypertensive . Circulation research . 2013;0:0–0. doi: 10.1161/CIRCRESAHA.113.301646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhein S, Rothe S, Busch A, Rojas Gomez D M, Boldt A, Reutemann A, Seidel T, Salameh A, Pfannmüller B, Rastan A, Kostelka M, Mohr F W. Effects of metoprolol therapy on cardiac gap junction remodelling and conduction in human chronic atrial fibrillation. Br. J. Pharmacol. 2011 Sep;164 (2b):607–16. doi: 10.1111/j.1476-5381.2011.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bikou Olympia, Thomas Dierk, Trappe Kerstin, Lugenbiel Patrick, Kelemen Kamilla, Koch Martin, Soucek Radim, Voss Frederik, Becker Rüdiger, Katus Hugo A, Bauer Alexander. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc. Res. 2011 Nov 01;92 (2):218–25. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 62.Kikuchi Kan, McDonald Amy D, Sasano Tetsuo, Donahue J Kevin. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005 Jan 25;111 (3):264–70. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- 63.Haugan Ketil, Lam Henrik Rye, Knudsen Carsten Boye, Petersen Jørgen Søberg. Atrial fibrillation in rats induced by rapid transesophageal atrial pacing during brief episodes of asphyxia: a new in vivo model. J. Cardiovasc. Pharmacol. 2004 Jul;44 (1):125–35. doi: 10.1097/00005344-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Shiroshita-Takeshita Akiko, Sakabe Masao, Haugan Ketil, Hennan James K, Nattel Stanley. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007 Jan 23;115 (3):310–8. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 65.Haugan Ketil, Miyamoto Takuya, Takeishi Yasuchika, Kubota Isao, Nakayama Jun, Shimojo Hisashi, Hirose Masamichi. Rotigaptide (ZP123) improves atrial conduction slowing in chronic volume overload-induced dilated atria. Basic Clin. Pharmacol. Toxicol. 2006 Jul;99 (1):71–9. doi: 10.1111/j.1742-7843.2006.pto_432.x. [DOI] [PubMed] [Google Scholar]
- 66.Guerra Jose M, Everett Thomas H, Lee Ken W, Wilson Emily, Olgin Jeffrey E. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006 Jul 11;114 (2):110–8. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]


