Abstract
Introduction: Vernakalant is a new, safe and effective drug used intravenously. It has proven to be more rapid in converting recent onset atrial fibrillation (AF) to sinus rhythm compared to placebo, amiodarone, propafenone and flecainide in clinical studies with few patients. At present no study has been conducted comparing these three drugs with a more substantial number of patients.
The aim of our study is to compare the time to conversion to sinus rhythm, hospital stay and adverse events between vernakalant versus flecainide and propafenone in patients with a recent-onset AF.
Materials and Methods: 150 hemodynamically stable patients with recent onset AF without structural heart disease were prospectively included. A single oral dose of propafenone 600 mg was administered to 50 patients; 50 patients received intravenous vernakalant; and 50 patients received a single oral dose of flecainide 300 mg. Clinical and laboratory variables were recorded.
Results: Baseline characteristics were similar in the three groups.Time to conversion to sinus rhythm was 12 minutes in the vernakalant group versus 151 minutes in the propafenone group and 162 minutes in flecainide group (p< 0.01)
The hospital stay was 243 minutes in the vernakalant group versus 422 minutes in the propafenone group and 410 minutes in flecainide group (p<0.01) (Figure 2).
No adverse events were reported.
Conclusion: The time to conversion to sinus rhythm and hospital stay were statistically shorter in vernakalant group compared to flecainide and to propafenone. There were no adverse events in the three groups.
Keywords: Atrial fibrillation, Propafenone, Flecainide, Vernakalant
Introduction
Recent onset atrial fibrillation (AF) is a frequent cause for presentation to the emergency department.[1,2] Conversion of recent onset AF to sinus rhythm with antiarrhythmic drugs reduces the risk of hemodynamic instability,hospitalizations, and atrial remodelingseen with persistent AF.[3,4]
Boriani et al compared oral loading dose of propafenone 600 mg with intravenous propafenone and placebo.At 8 hours either intravenous or oral propafenone were effective in almost two thirds of the patients with a statistical difference versus placebo.[5]
Khan showed that a single oral dose of flecainide 300 mg had a similar time to conversion of AF to sinus rhythm versus intravenous class IC drugs.[6]
This is the reason why an oral loading dose of propafenone 600 mg or a single dose of flecainide 300 mg are used in our center as in other places around the world for conversion of recent onset AF in patients without structural heart disease.
Vernakalant is a new, safe and effective drug used intravenously for conversion AF that has been studied in patients with and without structural heart disease; including those after cardiovascular surgery.[7-10]
Vernakalant has proven to be more rapid in converting recent onset AF to sinus rhythm compared with propafenone and flecainide in small studies of no more than 51 patients.[11,12,13]
Until now, no study has been conducted comparing these three drugs in a more substantial number of patients.
The aim of our study is to compare the time to conversion to sinus rhythm, hospital stay and adverse events between vernakalant versus flecainide and propafenone in patients with recent-onset AF. Based upon the small studies as well as non-direct comparison data, we expect to prove that vernakalant will prove superior
Materials and Methods
This is a prospective observational study which included 150 patients.
50 patients with, hemodynamically stable, symptomatic, recent onset AF (lasting less than 48 hours) without structural heart disease underwent pharmacological cardioversion and received an initial intravenous dose of vernakalant, 3.0mg/kg over 10minutes. After a 15 minute observation period, if conversion to sinus rhythm did not occur, a second 10 minute infusion of vernakalant at a dose of 2mg/kg was administered.
50 additional patients received a single oral dose of flecainide 300 mg and 50 patients received a single oral dose of propafenone 600mg.
All patients received the assigned pharmacological cardioversion agents. If patients persisted with AF after attempted pharmacological cardioversion, electrical cardioversion was performed at 2 hours after intravenous vernakalant or at 8 hours after oral propafenone or flecainide.
Inclusion Criteria: Patients>18 years, with AF lasting less than 48 hours and documented by electrocardiogram, weight between 45and136kg, systolic blood pressure>90mmHg and<160mmHg and diastolic blood pressure<95mmHg (all chosen based uponsafety considerations).
Exclusion Criteria: Pregnancy, atrial flutter, sinus node disease, QRS duration longer than 140ms in non-paced beats,QT interval>440ms, heart failure or acute coronary syndrome. The latter four were exclusions because of class IC contraindications; flutter was excluded because of its known lack of response to vernakalant; sinus node disease was excluded for safety reasons and pregnancy was excluded for ethical reasons.
Clinical, laboratory and electrocardiographic variables wererecorded. All the patients had continuous electrocardiographicmonitoring. Color Doppler echocardiography with measurementof structural and functional parameters was performed to all the patients.
The patients received anticoagulation therapy after discharge according the recommendation of the CHA2DS2-VASc score, but without antiarrhythmics drugs.
Adverse Event Definitions: death, sustained hypotension (systolic blood pressure ≤ 90 mmHg), bradycardia<40 beats per minute, QT interval > 440 ms, ventricular arrhythmia (≥triplets), or any other event that required or prolonged hospitalization were considered serious adverse events. Other events not meeting the criteria of seriousness, such as taste disorders, cough, nausea, or dizziness were not considered serious adverse events.The patients received anticoagulation therapy after discharge according the recommendation of the CHA2DS2-VASc score, but without antiarrhythmics drugs.
Statistical Analysis
All calculations were performed using Statistix 8.0 software package.
Continuous variables were expressed as median with the corresponding interquartile range (p25-p75) and were compared using the Mann Whitney test. Rates were expressed as percentages and were compared using the chi square test with Fisher’s correction, if applicable. Time taken for conversion to sinus rhythm was illustrated on a graph using the Kaplan-Meier method.
This investigation was in accordance with the Declaration of Helsink
Results
One hundred and fifty patients were included. The median age was 64 years (54-70) and 69% were men.
No significant differences were found between the baselinecharacteristics and previous historys of atrial fibrillation, invasiveprocedures, or previous medications in the three groups (Table 1) (Table 2).
Table 1. Baseline characteristics.
Note: BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure
| Variable | Propafenone | Vernakalant | Flecainide |
|---|---|---|---|
| Male gender, % | 60 | 60 | 70 |
| Age, years | 66 (54-68) | 68 (56-70) | 64 (55-67) |
| BMI, kg/m2 | 26 (24-29) | 27 (25-29.2) | 26 (23-28) |
| SBP, mm Hg | 130 (120-142) | 127 (121.5-130) | 129 (119-135) |
| DBP, mm Hg | 72 (67.7-81.5) | 75 (69-80) | 73 (67-75) |
| Cardiovascular risk factors | |||
| Diabetes, % | 20 | 30 | 30 |
| Hypertension, % | 30 | 30 | 50 |
| Current or former smokers, % | 50 | 50 | 70 |
| Dyslipidemia, % | 40 | 50 | 70 |
| Thyroid disorders, % | 14 | 12 | 20 |
| Rate ventricular response per min | 150 (145-159) | 159 (150-165) | 161 (147-166) |
Table 2. History of AF and medication.
Note: AF: Atrial Fibrillation
| Variable | Propafenone | Vernakalant | Flecainide |
|---|---|---|---|
| Previous AF,% | 14 | 12 | 20 |
| Previous AF ablation, % | 10 | 10 | 20 |
| Previous treatment | |||
| Beta blockers, % | 10 | 10 | 10 |
| Calcium channel blockers, % | 0 | 2 | 0 |
| Propafenone/Flecainide, % | 12 | 10 | 20 |
| Amiodarone, % | 14 | 10 | 20 |
| Anticoagulation, % | 0 | 2 | 10 |
Time to conversion to sinus rhythm was 12 minutes in the vernakalant group versus 151 minutes (interquartile range [IQR], 125-325) in the propafenone group and 162 minutes (IQR, 130-315) in flecainide group (p< 0.01) (Figure 1).
Figure 1. Time to conversion of AF to sinus rhythm .
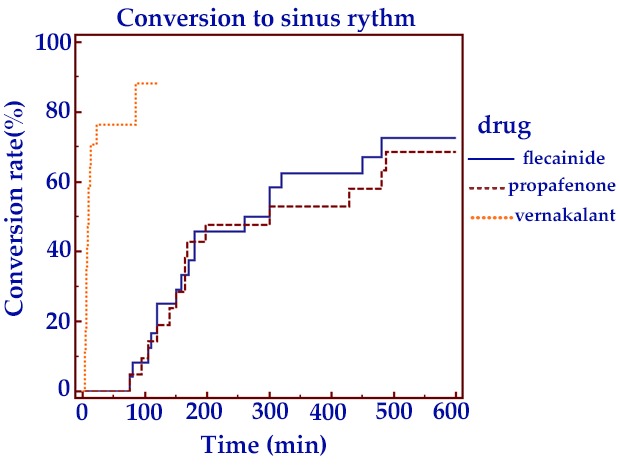
Conversion rate approximated 80% in the propafenone group and,80% in the flecainide group at 8 hours versus 90% in the vernakalantgroup at 2 hours. This difference was not statistically significant at 8hours (p=NS) (Figure 1).
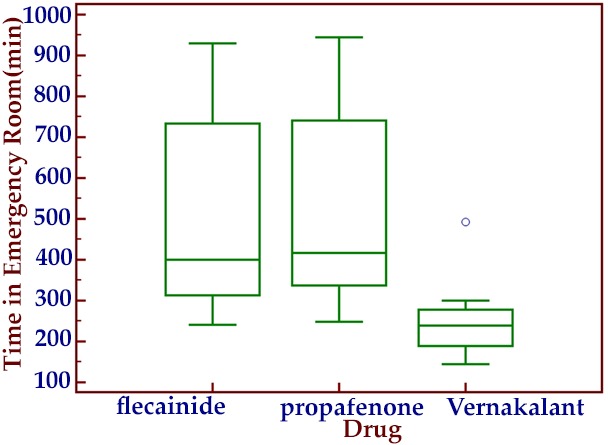
The stay length in emergency care section was 243 minutes (IQR,190-276) in the vernakalant group versus 422 minutes (IQR, 341-739) in the propafenone group and 410 minutes (IQR, 330-727) inflecainide group (p<0.01) (Figure 2).
Figure 2. Hospital stay.
There were no differences in time to conversion to sinus rhythm, hospital stay and adverse events between the groups with and without a prior history of ablation. Similarly, there were no differences intime to conversion in patients in whom a prior antiarrhythmic drug treatment was stopped for inefficacy versus intolerance. However, these numbers may be too small to assess statistically with clinical significance.
No adverse events were reported.
Discussion
Several studies have demonstrated the efficacy of oral propafenonefor conversion of recent onset AF to sinus rhythm.[5] Other studiesshown that oral flecainide has a similar time to conversion to intravenous propafenone or intravenous flecainide
Vernakalant is a novel, relatively atrial-selective antiarrhythmic agent that when used intravenously, prolongs the atrial refractory period but has little effect on ventricular repolarization. It is a multiion channel blocker blocking early-activating potassium channelscombined with concentration-, voltage- and frequency-dependentblockade of sodium channels.[7]
Vernakalant has a rapid distribution and rapid onset of action with a mean half-life elimination of 3 h. Plasma concentrations decline approximately 50% in 10 minutes. Restoration of sinus rhythm occurs within 90 minutes in 50% of cases with a mean time of 8-11 minutes.[7-10]
Vernakalant produced a rapid conversion according to the results of the CRAFT study[7] (versus placebo) or AVRO study,[09] (versusamiodarone).
Although there are two studies with no more of 51 patients indicating that vernakalant is faster for conversion of recent-onset AF than propafenone and flecainide.[11,12,13] there are no studies comparing these three drugs in a more substantial number of patients. Hence, we performed the current investigation.
As with the results in the previous studies with only a few patients, vernakalant achieved a more rapid time to conversion to sinus rhythm and a shorter hospital stay compared with flecainide and with propafenone. At the same time our study saw no adverse events in the three groups.
Study Limitations
Not being a randomized trial is the most important limitation of this study.
Also, a larger sample size might have produced statistically significant differences in the time to conversion and hospital stay length in vernakalant group. Finally, our exclusions preclude extrapolating our data, regarding efficacy rates or safety, to patients unlike the ones included in this study.
Conclusions:
The time to conversion to sinus rhythm and hospital stay were statistically shorter in vernakalant group compared with flecainide and propafenone.There were no adverse events in the three groups.
Disclosures
None.
References
- 1.Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 09;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Friberg Jens, Buch Pernille, Scharling Henrik, Gadsbphioll Niels, Jensen Gorm B. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003 Nov;14 (6):666–72. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Easley A, Barrington W, Windle J. Evaluation and management of atrial fibrillation in the emergency department. Emerg. Med. Clin. North Am. 1998 May;16 (2):389–403. doi: 10.1016/s0733-8627(05)70008-0. [DOI] [PubMed] [Google Scholar]
- 4.Zimetbaum Peter. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012 Jan 17;125 (2):381–9. doi: 10.1161/CIRCULATIONAHA.111.019927. [DOI] [PubMed] [Google Scholar]
- 5.Boriani G, Capucci A, Lenzi T, Sanguinetti M, Magnani B. Propafenone for conversion of recent-onset atrial fibrillation. A controlled comparison between oral loading dose and intravenous administration. Chest. 1995 Aug;108 (2):355–8. doi: 10.1378/chest.108.2.355. [DOI] [PubMed] [Google Scholar]
- 6.Khan I A. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J. Am. Coll. Cardiol. 2001 Feb;37 (2):542–7. doi: 10.1016/s0735-1097(00)01116-5. [DOI] [PubMed] [Google Scholar]
- 7.Roy Denis, Rowe Brian H, Stiell Ian G, Coutu Benoit, Ip John H, Phaneuf Denis, Lee Jacques, Vidaillet Humberto, Dickinson Garth, Grant Sheila, Ezrin Alan M, Beatch Gregory N. A randomized, controlled trial of RSD1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation. J. Am. Coll. Cardiol. 2004 Dec 21;44 (12):2355–61. doi: 10.1016/j.jacc.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Roy Denis, Pratt Craig M, Torp-Pedersen Christian, Wyse D George, Toft Egon, Juul-Moller Steen, Nielsen Tonny, Rasmussen S Lind, Stiell Ian G, Coutu Benoit, Ip John H, Pritchett Edward L C, Camm A John. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008 Mar 25;117 (12):1518–25. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 9.Camm A John, Capucci Alessandro, Hohnloser Stefan H, Torp-Pedersen Christian, Van Gelder Isabelle C, Mangal Brian, Beatch Gregory. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J. Am. Coll. Cardiol. 2011 Jan 18;57 (3):313–21. doi: 10.1016/j.jacc.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Kowey Peter R, Dorian Paul, Mitchell L Brent, Pratt Craig M, Roy Denis, Schwartz Peter J, Sadowski Jerzy, Sobczyk Dorota, Bochenek Andrzej, Toft Egon. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):652–9. doi: 10.1161/CIRCEP.109.870204. [DOI] [PubMed] [Google Scholar]
- 11.Conde Diego, Costabel Juan Pablo, Aragon Martin, Lambardi Florencia, Klein Andrés, Corrales Barbosa Andrea, Trivi Marcelo, Giniger Alberto. Propafenone versus vernakalant for conversion of recent-onset atrial fibrillation. Cardiovasc Ther. 2013 Dec;31 (6):377–80. doi: 10.1111/1755-5922.12036. [DOI] [PubMed] [Google Scholar]
- 12.Conde Diego, Costabel Juan Pablo, Caro Milagros, Ferro Alejandra, Lambardi Florencia, Corrales Barboza Andrea, Lavalle Cobo Augusto, Trivi Marcelo. Flecainide versus vernakalant for conversion of recent-onset atrial fibrillation. Int. J. Cardiol. 2013 Oct 03;168 (3):2423–5. doi: 10.1016/j.ijcard.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Conde Diego, Costabel Juan Pablo, Aragon Martin, Caro Milagros, Ferro Alejandra, Klein Andres, Trivi Marcelo, Giniger Alberto. Flecainide or propafenone vs. vernakalant for conversion of recent-onset atrial fibrillation. Can J Cardiol. 2013 Oct;29 (10) doi: 10.1016/j.cjca.2013.01.002. [DOI] [PubMed] [Google Scholar]


