Abstract
Background
Physical inactivity is associated with poor outcomes in COPD, and as a result, interventions to improve physical activity (PA) are a current research focus. However, many trials have been small and inconclusive.
Objective
The aim of this systematic review and meta-analysis was to study the effects of randomized controlled trials (RCTs) targeting PA in COPD.
Methods
Databases (Physiotherapy Evidence Database [PEDro], Embase, MEDLINE, CINAHL and the Cochrane Central Register for Controlled Trials) were searched using the following keywords: “COPD”, “intervention” and “physical activity” from inception to May 20, 2016; published RCTs that aimed to increase PA in individuals with COPD were included. The PEDro scale was used to rate study quality. Standardized mean differences (effect sizes, ESs) with 95% confidence intervals (CIs) were determined. Effects of included interventions were also measured according to the minimal important difference (MID) in daily steps for COPD (599 daily steps).
Results
A total of 37 RCTs with 4,314 participants (mean forced expiratory volume in one second (FEV1) % predicted 50.5 [SD=10.4]) were identified. Interventions including exercise training (ET; n=3 studies, 103 participants) significantly increased PA levels in COPD compared to standard care (ES [95% CI]; 0.84 [0.44–1.25]). The addition of activity counseling to pulmonary rehabilitation (PR; n=4 studies, 140 participants) showed important effects on PA levels compared to PR alone (0.47 [0.02–0.92]), achieving significant increases that exceeded the MID for daily steps in COPD (mean difference [95% CI], 1,452 daily steps [549–2,356]). Reporting of methodological quality was poor in most included RCTs.
Conclusion
Interventions that included ET and PA counseling during PR were effective strategies to improve PA in COPD.
Keywords: pulmonary disease, chronic obstructive, physical activity, interventions
Introduction
Physical inactivity is a key predictor of increased hospitalization and all-cause mortality in COPD.1,2 A systematic review comparing activity levels in COPD patients with age-matched controls showed a marked reduction in activity duration, intensity and daily step counts, regardless of disease severity.3 Physical activity (PA) levels are strongly correlated with functional exercise capacity.4 In addition, a large 5-year follow-up study showed important associations between levels of PA and health-related quality of life (HRQL) in COPD.5
In light of the strong relationship between PA and health outcomes in COPD, the past decade has witnessed the development of several strategies aiming to improve PA levels in COPD. The number of randomized controlled trials (RCTs) designed to test the efficacy of these interventions is growing rapidly. A recent review that studied the effects of RCTs, non-RCTs and experimental studies of PA interventions concluded that although exercise training (ET) is the most commonly used approach, its effects on PA levels are uncertain.6 The inclusion of non-RCTs in this review is a potential source of bias that might overestimate the true effects of PA interventions.7 To date, attempts to compare effects of interventions on PA levels using meta-analysis of published RCTs exclusively have not been reported. In this systematic review, we aimed to analyze and compare the effects of various PA interventions reported through RCTs in COPD.
Methods
The systematic review protocol is registered in PROSPERO (CRD42013004460, http://www.crd.york.ac.uk/PROSPERO).
Inclusion criteria
Studies that recruited participants diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Diseases (GOLD) guidelines were included.8 In the case of mixed populations, studies were included if 80% or more of individuals had COPD. RCTs published in peer-reviewed journals that aimed to evaluate the efficacy of interventions to improve PA levels were included. These comprised, but were not limited to, pulmonary rehabilitation (PR), ET, pharmaceutical treatment, self-management or written advice. Studies were included if PA was either a primary or a secondary outcome.
The primary outcome of the review was PA measured either objectively (eg, time spent in PA measured by activity monitors) or subjectively (eg, scores of questionnaires). Secondary outcomes of the review were functional and maximal exercise capacity, HRQL, dyspnea and lung function variables.
Search methods
Databases searched were the Cochrane library, Physiotherapy Evidence Database (PEDro), Embase, MEDLINE and CINAHL. The subject headings were “COPD”, “interventions” and “physical activities” (Supplementary materials). Databases were searched through two separate periods: from their inception to the end of January 2015 and from February 2015 to May 20, 2016. In addition, we searched major trial registries including ClinicalTrials.gov, European Union Clinical Trial Register, Australian New Zealand Clinical Trials Registry and WHO International Clinical Trials Registry Platform for relevant projects.
One author (AL) reviewed the title and abstract of trials and two authors (AL and AEH) independently assessed the full text of articles to decide on inclusion. Any disagreement was resolved by discussion. Data were extracted independently by two authors (AL and AEH) using a standardized data extraction sheet (Supplementary materials). Online software was used to extract data from plots and graphs where necessary (www.arohatgi.info/WebPlotDigitizer).
Quality assessment was performed using the PEDro quality scale, which is an 11-item scale that assesses internal and external validity of clinical trials.9 The higher the given score, the better the quality. Cut points of the scale were excellent (9–10), good (6–8), fair (4–5) and poor (≤3).
Data interpretation and synthesis
Comparisons of interest were interventions aimed to improve PA versus usual care (or placebo), interventions aimed to improve PA added to PR versus PR alone, and PA intervention versus another active PA intervention. Effects of interventions on continuous outcome measures were assessed using standardized mean differences (SMDs) and 95% confidence intervals (CIs), to allow comparison of effect size (ES) across interventions. Trials reporting effects of interventions using comparable outcome measures at comparable time points were combined in a meta-analysis. Software used to conduct meta-analysis and compute ESs was Review Manager 5.3 manufactured by the Nordic Cochrane Centre. Based on Cohen’s classifications, an SMD of 0.2 is small, 0.5 is moderate and 0.8 or higher is a large effect.
Meta-analysis was conducted where trials were statistically and clinically homogeneous. Statistical homogeneity was defined as I2≤40%.7 Clinical homogeneity was defined as similarity of study population, intervention components and their comparators and selected outcome measures. In the case of PA levels in an included RCT being measured using more than one variable, the variable that best reflects PA levels was selected for meta-analysis (eg, objectively measured time spent in walking was selected over self-reported PA time,10 and overall PA time was selected over PA time spent in 1 day).11 Objectively and subjectively measured PA variables were not pooled together in a meta-analysis. When available, change scores were extracted to measure the effects of interventions. In the case of a change score not being directly provided, baseline and final scores were used to measure mean differences, and available variables of the change (eg, correlation coefficient of the change) were used to compute standard deviation of the change.7 When necessary, study investigators were contacted for additional information to compute change scores. Final scores at available time points were extracted when change score was unable to be computed.
We intended to perform sensitivity analyses to examine the effects of trial quality by excluding trials without concealed allocation and assessor blinding; however, insufficient data prevented these analyses.
In trials where PA was measured in daily steps, weighted mean differences in steps achieved by interventions were compared. Changes in the number of steps from baseline produced by different interventions were compared to the minimal important difference (MID), which has been estimated to be a change of 599 daily steps.12 MID is defined as a meaningful change or effect that might be considered important to produce sizeable change in vital outcomes.13
Results
The search yielded a total of 4,178 records identified throughout the two separate periods, with 81 full texts reviewed for eligibility. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram (Figure 1) depicts the search process. A total of 37 RCTs identified in 40 separate reports met our selected criteria and were included in the systematic review.
Figure 1.
PRISMA flow diagram for database search and study selection process.
Abbreviations: PA, physical activity; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
Quality assessment
The quality of included studies ranged from fair to good (median PEDro score, interquartile range [IQR] =6 [5–7]; Table 1), with only one study scoring poorly.14 A total of 18 studies (48% of included RCTs) failed to conceal allocation, 27 studies (73% of included RCTs) failed to blind subjects and 23 studies (62% of included RCTs) failed to blind outcome assessors. Another common limitation was the inability to report outcomes for >85% of randomized subjects (n=20, 54%; Table 1).
Table 1.
Qualitative synthesis of included studies using PEDro scale for the quality of RCTs
| Study name | Eligibility criteriaa | Random allocation | Concealed allocation | Baseline similarity | Blinding (subject) | Blinding (therapist) | Blinding (assessor) | Measure for >85% | ITT | Group comparison | Point measure | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies comparing PA interventions versus usual care (or placebo; n=16) | ||||||||||||
| Berry et al15 | * | * | * | * | * | * | * | 6 | ||||
| Borges and Carvalho16 | * | * | * | * | * | * | * | * | * | 8 | ||
| Breyer et al17 | * | * | * | * | * | * | 5 | |||||
| Dal Negro et al46,47 | * | * | * | * | * | * | * | * | * | * | * | 10 |
| Faulkner et al19 | * | * | * | * | * | * | 5 | |||||
| Goris et al48 | * | * | * | * | * | * | * | 6 | ||||
| Hornikx et al31 | * | * | * | * | * | * | * | 6 | ||||
| Hospes et al32 | * | * | * | * | * | * | 5 | |||||
| Jonsdottir et al39 | * | * | * | * | * | * | * | 6 | ||||
| Kruis et al53 | * | * | * | * | * | * | * | * | * | 8 | ||
| Larson et al20 | * | * | * | * | * | * | * | * | * | * | 9 | |
| Sandland et al51 | * | * | * | * | * | * | * | 6 | ||||
| Schuz et al41; Walters et al42 | * | * | * | * | * | * | * | * | * | 8 | ||
| Steele et al37 | * | * | * | * | * | * | 5 | |||||
| Watz et al45 | * | * | * | * | * | * | * | * | * | * | 9 | |
| Wilson et al24 | * | * | * | * | * | * | * | 6 | ||||
| Studies comparing PA interventions added to PR versus PR alone (n=8) | ||||||||||||
| Altenburg et al25 | * | * | * | * | * | * | 5 | |||||
| Burtin et al27 | * | * | * | * | * | * | * | * | 7 | |||
| Cruz et al29 | * | * | * | * | * | * | * | * | 7 | |||
| de Blok et al30 | * | * | * | * | * | 4 | ||||||
| Duiverman et al50 | * | * | * | * | * | 4 | ||||||
| Kawagoshi et al33 | * | * | * | * | * | * | 5 | |||||
| Kesten et al43 | * | * | * | * | * | * | * | 6 | ||||
| Pleguezuelos et al52 | * | * | * | * | * | * | * | 6 | ||||
| Studies comparing PA interventions versus another PA intervention (n=13) | ||||||||||||
| Berry et al26 | * | * | * | * | * | * | * | 6 | ||||
| Casaburi et al49 | * | * | * | * | * | * | 5 | |||||
| Effing et al18 | * | * | * | * | * | * | * | 6 | ||||
| Mendoza et al34 | * | * | * | * | * | * | * | * | 7 | |||
| Moy et al35 | * | * | * | * | * | * | * | 6 | ||||
| Nguyen et al36 | * | * | * | * | * | * | * | * | 7 | |||
| Nguyen et al40 | * | * | * | * | * | * | * | * | 7 | |||
| Pomidori et al21 | * | * | * | * | * | * | 5 | |||||
| Probst et al22 | * | * | * | * | * | * | 5 | |||||
| Sewell et al23 | * | * | * | * | * | * | * | 6 | ||||
| Tabak et al38 | * | * | * | * | * | * | * | * | 7 | |||
| Troosters et al44 | * | * | * | * | * | * | * | * | * | * | * | 10 |
| Vergeret et al14 | * | * | * | * | 3 | |||||||
Notes:
Yes, score = 1.
Item does not contribute to total quality score. The higher the given score, the better the quality. Cut points of the scale were excellent (9–10), good (6–8), fair (4–5) and poor (3).
Abbreviations: ITT, intention to treat; PA, physical activity; PEDro, Physiotherapy Evidence Database; PR, pulmonary rehabilitation; RCTs, randomized controlled trials.
Characteristics of included RCTs
Included RCTs measured the effects of ET (n=10),15–24 activity counseling (n=15),25–40 health monitoring (n=1),41,42 pharmacological treatment (n=3),43–45 nutritional interventions (n=2),46–48 oxygen therapy (n=4),14,49–51 urban walking circuits (n=1)52 and integrated disease management (n=1)53 on PA levels in COPD. PA counseling was delivered either in a center-based setting (n=9),25–27,29,30,32–34,39 through phone calls (n=3)31,36,37 or by internet-mediated approaches (n=3).35,38,40 Studies compared interventions to usual care (n=16),15–17,19,20,24,31,32,37,39,41,42,45–48,51,53 PR programs (n=8)25,27–30,33,43,50,52 or another active intervention (n=13).14,18,21–23,26,34–36,38,40,44,49 Characteristics of included studies are summarized in Table 2.
Table 2.
Characteristics of included studies
| Author | Year | Country | n (I/C) | Age, years; mean (SD) | FEV1 % predicted L; mean (SD) | Gender (male/female) | Intervention arm | Control arm | Time points/outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Studies comparing PA interventions versus usual care (or placebo; n=16) | |||||||||
| Berry et al15 | 2003 | USA | 70/70 | 67.7 (5.8) | 58.4 (18.1) | 78/62 | Exercise maintenance (1 h ×3 times/W supervised ET sessions after 3M of ET program pre-randomization – 15M) | UC (PA advice after 3M of ET program pre-randomization – 15M) | 15M: subjective activity score, FEV1, FVC and FER. 6M, 12M, 15M: 6MWD |
| Borges and Carvalho16 | 2014 | Brazil | 15/14 | 65.9 (10.9) | 40.4 (14.3) | 18/11 | Whole-body ET (≥3 ET sessions starting at day 3 of admission, mean sessions =5.6) | UC (daily chest physiotherapy and O2 therapy) | 1M post-discharge: daily walking, standing, sitting and lying time, FEV1 % predicted. 1M post-discharge: 6MWD, SGRQ |
| Breyer et al17 | 2010 | Austria | 30/30 | 60.3 (8.5) | 46.3 (17.6) | 27/33 | Nordic walking (3 times/W walking with power poles ET sessions and 1 time/W EDU sessions – 3M) | UC (1 time/W EDU sessions – 3M) | 3M, 6M, 9M: movement intensity, 6MWD, SF36 |
| Dal Negro et al46,47 | 2012 and 2010 | Italy | 44/44 | 74 (6.7) | FEV1 =0.8 (0.3) | 61/27 | Daily EAA dietetics (4 g ×2 times/day – 12W) | Placebo dietetics (4 g ×2 times/day – 12W) | 1M, 3M: daily steps and energy expenditure, FEV1 (l/sec). 3M: SGRQ |
| Faulkner et al19 | 2010 | UK | 6/8 | – | – | – | Health-enhancing PA program (3 times/W supervised ET and EDU sessions with exercise diary – 8W) | UC (8W) | 9W: subjective 7-day total PA, ISWT, CRQ, MRC, FEV1 % predicted |
| Goris et al48 | 2003 | the Netherlands | 11/9 | 62 (11) | 40 (16) | 11/9 | Respifor® (Nutricia Advanced Medical Nutrition, Schiphol, The Netherlands) nutritional supplement (375 mL ×3 times/day with nutritional consultation sessions – 3M) | UC (3M) | 1M, 3M post-discharge: daily PA levels and energy expenditure |
| Hornikx et al31 | 2009 | Belgium | 15/15 | 67 (6.5) | 43 (18) | 17/12 | PA counseling and GS (3 times/W phone calls – 1M) | UC (1M) | 1M: daily steps, walking time and movement intensity during walking, 6MWD, MRC |
| Hospes et al32 | 2009 | the Netherlands | 18/17 | 62.2 (8.6) | 64.7 (16.1) | 21/14 | Individualized exercise counseling (30 min × 5 sessions using MI – 12W) | UC (12W) | 12W: daily steps, 6MWD, SGRQ, CCQ, SF36 |
| Jonsdottir et al39 | 2015 | Iceland | 48/52 | 59 (4.5) | 57.6 (17.7) | 46/54 | Partnership with families’ self-management (≥30 min ×4 RN/patient/family consultations, 6M of SC treatment and one interdisciplinary team/patient/family meeting – 6M) |
UC (6M) | 12M: weekly IPAQ subscales: total, vigorous, moderate and walking, SGRQ |
| Kruis et al53 (clustered RCT) | 2014 | the Netherlands | 554/532 | 68.3 (11.2) | 67.8 (20.4) | 585/501 | Primary care practitioners training for integrated disease management: spirometry use, MI, SC and exercise advice (24M) | UC (24M) | 12M: daily IPAQ, SGRQ, CCQ, SF36, MRC |
| Larson et al 20 | 2014 | USA | 15/14 | 71 (7.8) | 58.5 (18.5) | – | Self-efficacy and UBRT (15 min ×4 times/M EDU sessions – 4M) | UC (gentle chair exercise – 4M) | 4M: daily MVPA, LPA, and sedentary time |
| Sandland et al51 | 2008 | UK | 10/10 | 73.4 (6.8) | 43.6 (22.5) | 14/6 | Ambulatory O2 (3.1 kg cylinder O2 with a backpack – 8W) | Ambulatory air (3.1 kg cylinder placebo with a backpack – 8W) | 8W: daily activity counts, ISWT, ESWT, CRQ |
| Schuz et al41 (Walters et al42) | 2015 (2013) | Australia | 90/92 | 67.7 (7.8) | 55.2 (13.4) | 96/86 | Health monitoring (16×30 min phone calls by trained community nurses – 12M) | UC (12 times/M social phone calls) | 6M, 12M: daily steps, SGRQ, SF36 |
| Steele et al37 | 2008 | USA | 52/54 | – | 40.4 (17.8) | – | Exercise adherence counseling (12 phone calls, 1 home visit and ≥20 min ×4 times/W maintenance home ET sessions after 8W of PR pre-randomization – 20W) | UC (after 8W of PR pre-randomization – 20W) | 20W, 12M: daily PA activity, SR daily exercise time, 6MWD, SOLDQ, SF36, SOB levels, FEV1 % predicted |
| Watz et al45 (crossover RCT) | 2014 | Germany | 129 | 61.4 (8.9) | 64.02 (9.38) | 87/42 | Indacaterol (Onbrez®; Novartis Pharmaceuticals, Basel, Switzerland) 150 μg (1 time/day – 21 days) | Placebo (1 time/day – 21 days) | 3W: daily steps, MVPA time and PA levels, adverse events |
| Wilson et al24 | 2015 | UK | 73/75 | 68.3 (12.4) | 41 (16) | 91/57 | PR maintenance (1 h ×3 personalized ET sessions and 1 h ×3 EDU sessions – 12M) | UC (PA advice – 12M) | 12M: weekly IPAQ, VAS, ISWT, ESWT, CRQ |
| Studies comparing PA interventions added to PR versus PR alone (n=8) | |||||||||
| Altenburg et al25 | 2014 | the Netherlands | 31/30 | 54 (9.6) | 43 (25.9) | 32/25 | PA counseling (30 min ×5 sessions using MI – 12W) added to PR (2 h ×3 times/W – 9W) | PR (2 h ×3 times/W – 9W) | 3M, 15M: daily steps and steps equivalent*, 6MWD, CRQ, CCQ |
| Burtin et al27 | 2015 | Belgium | 40/40 | 66.5 (7.5) | 45.5 (16.03) | 66/14 | Individualized PA counseling (30 min ×8 sessions using MI – 6M) added to multidisciplinary PR (average 1.5 h ×2.5 times/W – 6M) | Multidisciplinary PR (average 1.5 h ×Δ2.5 times/W – 6M) | 3M, 6M: daily steps, walking and MVPA time, 6MWD, CRQ |
| Cruz et al29 | 2016 | Portugal | 16/16 | 66.5 (8.4) | 66.9 (20.1) | 27/5 | PA-focused behavioral counseling (average 25 min ×8 sessions using SCT: SE, MI and pedometer and diary feedback – 6M) added to PR (1 h ×3 times/W ET and 1.5 h ×1 time/W EDU sessions – 3M) | PR (1 h ×3 times/W ET and 1.5 h ×1 time/W EDU sessions – 3M) | 3M, 6M: daily steps and MVPA, PA and sedentary time, 6MWD, SGRQ |
| de Blok et al30 | 2006 | the Netherlands | 10/11 | 64.0 (11.3) | 47.3 (17.9) | 9/12 | Lifestyle PA counseling (30 min ×4 sessions using MI – 9W) added to PR (9W) | PR (9W) | 9W: daily steps, SGRQ, SF36 |
| Duiverman et al50 | 2008 | the Netherlands | 31/35 | 62 (8.6) | – | 35/31 | Nocturnal noninvasive positive pressure ventilation during multidisciplinary PR (1 h ×3 times/W ET sessions – 12W) | Multidisciplinary PR (1 h ×3 times/W ET sessions – 12W) | 3M: daily steps, 6MWD, ESWT, FEV1 (l/sec) |
| Kawagoshi et al33 | 2015 | Japan | 12/15 | 74.5 (8.4) | 59.4 (21.5) | 24/3 | Pedometer feedback (1 time/M sessions – 12M) added to multidisciplinary home-based PR (1 time/day home ET, 45 min ×1 time/M EDU and 2 h ×2 times/M supervised ET sessions – 12M) | Multidisciplinary home-based PR (1 time/day home ET, 45 min ×1 time/M EDU and 2 h ×2 times/M supervised ET sessions – 12M) | 12M: daily walking, standing, sitting and lying down time, 6MWD, CRQ, MRC |
| Kesten et al43 | 2008 | USA | 25/21 | 67.4 (7.2) | 33.4 (12.4) | 20/26 | Tiotropium (18 μg ×1 time/day – 25W) added to PR (0.5 h ×3 times/W – 8W) | Placebo (18 μg ×1 time/day – 25W) added to PR (0.5 h ×3 times/W – 8W) | 13W, 17W, 21W, 25W: subjective activity time in 2W, endurance time (min) |
| Pleguezuelos et al52 | 2013 | Spain | 34/37 | 70.4 (2.5) | 53.4 (2.9) | – | Urban walking circuit promotion (leaflets distributed and reviewed 2 times/M – 9M) following PR (1 h ×3 times/W ET sessions – 12W) | UC (generic PA advice reviewed 2 times/M – 9M) following PR (1 h × 3 times/W ET sessions – 12W) | 12M: subjective daily walking time, weekly number of days walked, 6MWD |
| Studies comparing PA interventions versus another PA intervention (n=13) | |||||||||
| Berry et al26 | 2010 | USA | 89/87 | 66 (10) | 51.5 (19.3) | 95/81 | Lifestyle activity program (1 h ×3 times/W ET sessions, group discussions, individualized counseling and 15 min follow-up phone calls – 3M) |
ET (1 h ×3 times/W ET sessions – 3M) | 3M, 6M, 12M: subjective weekly MVPA levels, 6MWD, SF36, CRQ. 12M: adverse events |
| Casaburi et al49 | 2012 | USA | 11/11 | 66.9 (9.2) | 31.3 (10) | – | Lightweight ambulatory O2 (1.6 kg aluminum cylinders – 6M) | E-cylinder ambulatory O2 (10 kg towed on a cart cylinders – 6M) | 3M, 6M: midday PA time |
| Effing et al18 (2×2 factorial RCT) | 2011 | the Netherlands | 77/76 | 63.4 (7.9) | 50 (15.6) | 89/64 | COPE active (average 2.5 times/W supervised ET and 1 time/W home-based ET sessions – 11M) added to self-management (1 h ×6 individualized SC counseling, 2 h ×4 behavioral change group discussions and 1 EDU session – 3M) | Self-management (1 h ×6 individualized SC counseling, 2 h ×4 behavioral change group discussions and 1 EDU session – 3M) | 7M, 12M: daily steps, ISWT, ESWT, CRQ, CCQ |
| Mendoza et al34 | 2015 | Chile | 52/50 | 68.7 (8.5) | 66.1 (19.4) | 62/40 | PA counseling (30 min ×1 time/M individualized pedometer and exercise diary feedback sessions – 3M) | Standardized counseling (30 min × 1 time/M individualized exercise diary feedback sessions – 3M) | 3M: daily steps, 6MWD, SGRQ, mMRC |
| Moy et al35 | 2015 | USA | 154/84 | 67 (9) | – | 223/15 | Internet-mediated pedometer-based program (1 time/W GS and pedometer feedback and online social support – 4M) | Pedometer reporting (1 time/M pedometer and adverse events outcomes reporting to HCP) – 4M | 4M: daily steps, SGRQ |
| Nguyen et al36 | 2009 | USA | 8/9 | 68.2 (11) | 40.9 (17.7) | 6/11 | Cell phone-mediated exercise persistence program (individualized PA advice followed by 1 time/W reinforcement, GS and activity feedback – 6M) | Cell phone-mediated self-monitoring program (individualized PA advice followed by 1 time/day activity recording – 6M) | 3M, 6M: daily steps and % of sedentary and MVPA time, 6MWD, incremental cycle endurance, SGRQ, SF36 |
| Nguyen et al40 | 2013 | USA | 43/41 | 68.5 (9.6) | 51.6 (20.1) | 49/35 | Internet-mediated dyspnea self-management program (1.5–2 h dyspnea management face-to-face consultation, 30 min ×4 times/W individualized home ET sessions, 1 time/W in M01 followed by 2 times/M from M2 to M11 exercise GS feedback and structured EDU sessions – 12M) | General EDU (initial home visit, 2 times/M phone calls and 1 time/M EDU sessions – 12M) | 3M, 6M, 12M: subjective weekly exercise duration, 6MWD, CRQ, SF36 |
| Pomidori et al21 | 2012 | Italy | 18/18 | 72 (8.2) | 48 (12.4) | 27/9 | Home-based walking (20–30 min ×4 times/W walking at a fixed speed paced by metronome, 4 times/W supervised sessions in M1 and 2 times/M phone calls support in M2 to M6 – 6M) | Home-based walking (20–30 min × 4 times/W walking a fixed distance, 4 times/W supervised sessions in M1 and 2 times/M phone calls support in M2 to M6 – 6M) | 6M, 12M: daily METs, METs peak and MVPA time, SGRQ. 12M: 6MWD |
| Probst et al22 | 2011 | Brazil | 20/20 | 66 (8.6) | 39.5 (13.3) | 21/19 | ET (1 h ×3 times/W high-intensity and whole-body ET sessions – 12W) | ET (1 h ×3 times/W light-intensity and breathing ET sessions – 12W) | 12W: daily steps, walking, sitting, standing, lying and activities cost >3 METs time. Total and activities cost >3 MET energy expenditure, 6MWD, maximum work load, endurance time, SGRQ, MRC |
| Sewell et al23 | 2005 | UK | 90/90 | 68.3 (8.6) | – | 99/81 | Individually targeted ET (2 h ×2 times/W individualized ET and EDU sessions – 7W) | General ET (2 h ×2 times/W standardized ET and EDU sessions – 7W) | 7W: daily activity counts, ISWT, ESWT, CRQ |
| Tabak et al38 | 2014 | the Netherlands | 14/16 | 66.6 (7.4) | 52.8 (14.1) | 19/11 | Tele-rehabilitation program (1 time/W internet-mediated pedometer feedback and 90 min ×2 self-management sessions – 4W) added to ET (1 time/W ET sessions – 4W) | ET (1 time/W ET sessions – 4W) | 3W: daily steps, CCQ, MRC |
| Troosters et al44 | 2014 | Belgium | 238/219 | 61.7 (8.4) | 65.7 (8.2) | 312/145 | Tiotropium HandiHaler 18 μg and salbutamol(1 time/day – 24W) added to individualized activity plan and exercise diary feedback (20 min ×1 time/M sessions using MI – 24W) | Placebo HandiHaler and salbutamol (1 time/day – 24W) added to individualized activity plan and exercise diary feedback (20 min ×1 time/M sessions using MI – 24W) | 24W: daily steps and age-appropriate LPA and MVPA time, FEV1 (l/sec) |
| Vergeret et al14 | 1989 | France | 84/75 | 61.9 (7.8) | – | 139/20 | LTOT with portable O2 (>15 h/day O2 concentrators plus gaseous or liquid O2 – 12M) | LTOT with fixed O2 (>15 h/day O2 concentrators – 12M) | 12M: subjective daily indoor and outdoor rest and activities time, subjective daily outdoor distance walked |
Note:
steps equivalent=steps + metabolic equivalent.
Abbreviations: C, control arm; CCQ, Clinical COPD Questionnaire; COPE, community based physiotherapeutic exercise; CRQ, Chronic Respiratory Questionnaire; EAA, essential amino acid; EDU, education; ESWT, endurance shuttle walk test; ET, exercise training; FER, forced expiratory ratio; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GS, goal setting; HCP, health care professionals; I, intervention arm; IPAQ, International PA Questionnaire; ISWT, incremental shuttle walk test; LPA, light PA; LTOT, long-term oxygen therapy; M, month(s); MET, metabolic equivalent task; MI, motivational interviewing; mMRC, Modified Medical Research Council; MRC, Medical Research Council; MVPA, moderate to vigorous PA; 6MWD, 6-minute walk distance; PA, physical activity; PR, pulmonary rehabilitation; RCT, randomized controlled trial; RN, registered nurse; SC, smoking cessation; SCT, social cognitive therapy; SE, self-efficacy; SF36, 36-Item Short Form Survey; SGRQ, Saint George Respiratory Questionnaire; SOB, shortness of breath; SOLDQ, Seattle Obstructive Lung Disease Questionnaire; SR, self-reported; UBRT, upper-body resistance training; UC, usual care; VAS, Visual Activity Scale; W, week(s).
Characteristics of included subjects
This review included a total of 4,314 participants (males = 58%). Mean age of included participants was 66.2 (SD 4.3) years with a mean forced expiratory volume in one second (FEV1) % predicted of 50.5 (10.4). All participants had a confirmed diagnosis of COPD according to the GOLD guidelines.8
Characteristics of PA measurement tools
RCTs assessed PA using several outcome measures. Objective measures included accelerometers (n=18, 50%),16,17,20–23,27,29,33,36,37,41,44–49,51 pedometers (n=8, 22%)18,25,30,32,34,35,38,50 and an activity monitor (n=1).31 PA was also measured subjectively using validated tools such as Seven-Day PA Recall Questionnaire (n=1),19 PA Scale for the Elderly (PASE) questionnaire (n=1),15 Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (n=1)26 and the International PA Questionnaire (IPAQ; n=3).24,39,53 Nonvalidated tools to measure PA included exercise diary cards (n=1)52 and trial-designed activity questionnaires (n=3).14,40,43
Analysis of included interventions
Effects of PA interventions on PA levels
Studies comparing PA interventions versus usual care or placebo
When comparing PA counseling alone to usual care, there were small and nonsignificant effects of counseling on PA for both objective measures (n=2 studies, SMD [95% CI] 0.23 [−0.26 to 0.72], time point 1–3 months; Figure 2)31,32 and subjective measures (n=2 studies, −0.27 [−0.04 to 0.58], 12 months; Figure 2).37,39 Four months of ET produced moderate to large statistically significant effects on PA (n=3 studies, 0.84 [0.44–1.25]; Figure 2),17,19,20 whereas long-term maintenance exercise programs showed small nonsignificant effects on PA at 12 and 15 months post-intervention (n=2 studies, 0.15 [−0.17 to 0.47]; Figure 2).15,24 None of the studies included in the meta-analyses had blinded the assessors or the therapists from study groups. One study of essential amino acid (EAA) supplementation46,47 reported an increase in steps favoring the intervention arm at 3 months (577.6 steps, P=0.02); however, insufficient sample size details prevented ES calculations (Table S1). Important increases in daily steps were also reported in a crossover RCT in favor of the once-daily long-acting beta agonist, indacaterol 150 μg,45 compared to placebo at 3 weeks (0.32 [0.02–0.63]; Table S1).
Figure 2.
Studies comparing PA interventions versus usual care.
Abbreviations: CI, confidence interval; ET, exercise training; IV, independent variable; PA, physical activity; SMD, standardized mean difference.
Studies comparing PA interventions added to PR versus PR alone
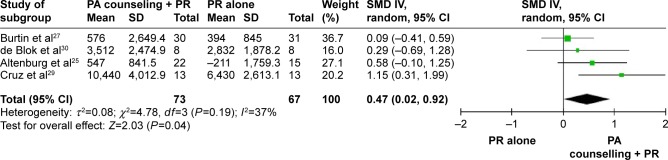
Adding activity counseling to PR produced short-term improvements in PA at 3 months compared to PR alone (n=4 studies, 0.47 [0.02–0.92]; Figures 3 and 4).25,27–30 Two of the four studies included in this meta-analysis had assessor blinding and concealed allocation.27,29 When analyzing sustainability of results, pooled meta-analysis showed small to moderate long-term effects of PA counseling added to PR that trended to statistical significance versus PR alone (n=4, 0.28 [−0.08 to 0.31]; I2, 60%; Figure 4).25,27,29,33 Of the included studies pooled in this analysis, the only study with assessor blinding did not report similar long-term effects on steps at 6 months post-intervention (n=1 study, 0.25 [−0.81 to 0.31]; Figure 4).27 Introducing an urban walking circuit promotion program for 12 months after a 3-month course of PR improved baseline walking time when compared to usual care after PR (1.36 [0.84–1.88]; Table S2).52
Figure 3.
Studies comparing PA counseling added to PR versus PR (short-term effect).
Abbreviations: CI, confidence interval; IV, independent variable; PA, physical activity; PR, pulmonary rehabilitation; SMD, standardized mean difference.
Figure 4.
Studies comparing PA counseling added to PR versus PR (long-term effect).
Abbreviations: CI, confidence interval; IV, independent variable; PA, physical activity; PR, pulmonary rehabilitation; SMD, standardized mean difference.
Studies comparing PA interventions versus another intervention
ET: Although 3 months of high-intensity ET had similar effects to light-intensity training on daily steps and walking time (−0.13 [−0.76 to 0.49] and 0.26 [−0.36 to 0.89], respectively; Table 3), effects on time spent in moderate to vigorous physical activity (MVPA) favored the high-intensity training group at 3 months (1.16 [0.49–1.84]; Table 3).22 When comparing 6 months of home-based walking with fixed speed program to home-based walking with fixed distance,21 significant increases in daily metabolic equivalent tasks (0.94 [0.25–1.63]; Table 3) and daily MVPA time (0.72 [0.04–1.39]; Table 3) were achieved by fixed speed walking at 12 months. Moreover, adding community-based ET to a self-management program showed moderate and significant effects on activity levels at 12 months when compared to self-management alone (0.48 [0.10–0.86]; Table 3).18
Table 3.
Studies comparing PA interventions versus another active intervention
| Study | Intervention | Control | Outcome (unit) | Time point | Intervention arm
|
Control arm
|
ES (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||||
| Studies that showed effect of ET when compared to another PA intervention (n =3) | |||||||||
| Effing et al18 | ET and self-management | Self-management | Daily steps (n) | Δ12M | 55 | 815.6 (2,659.4) | 55 | −374.8 (2,272.3) | 0.48 (0.10–0.86) |
| Pomidori et al21 | Home-based walking with fixed speed | Home-based walking with fixed distance | Daily METs (3.5 mL/kg⋅min) | Δ12M | 18 | 0.17 (0.14) | 18 | 0.04 (0.13) | 0.94 (0.25–1.63) |
| Daily METs peak (3.5 mL/kg⋅min) | Δ12M | 18 | 0.16 (0.20) | 18 | 0.06 (0.13) | 0.58 (−0.09 to 1.25) | |||
| Daily highest MVPA (>3 METs) time in 7 days (min) | Δ12M | 18 | 35 (32) | 18 | 13 (28) | 0.72 (0.04–1.39) | |||
| Probst et al22 | High-intensity and whole-body ET | Light-intensity and breathing ET | Daily steps (n) | 3M | 20 | 4,179 (646.7) | 20 | 4,278.6 (796) | −0.13 (−0.76 to 0.49) |
| Daily walking (min) | 3M | 20 | 52.7 (40.4) | 20 | 43.5 (26.7) | 0.26 (−0.36 to 0.89) | |||
| Daily activities cost >3 METs (min) | 3M | 20 | 74 (18.7) | 20 | 53.1 (16.4) | 1.16 (0.49–1.84) | |||
| Daily total EE (kcal) | 3M | 20 | 1,312.3 (119.4) | 20 | 1,347 (199) | −0.21 (−0.83 to 0.41) | |||
| Daily activity cost >3 METs EE (kcal) | 3M | 20 | 408 (118.3) | 20 | 343.8 (442.4) | 0.19 (−0.43 to 0.82) | |||
| Studies that showed effects of self-management or counseling models when compared to another PA intervention (n =3) | |||||||||
| Mendoza et al34 | Pedometer-based counseling | Standard counseling | Daily steps (n) | Δ3M | 50 | 3,080 (3,254.8) | 47 | 138.3 (1,950.4) | 1.08 (0.65–1.51) |
| Moy et al35 | Internet-mediated pedometer-based program | Pedometer recording only | Daily steps (n) | Δ4M | 133 | 447 (1,817) | 68 | −346 (1,949) | 0.84 (0.59–1.08) |
| Nguyen et al40 | Internet-mediated dyspnea self-management program | General EDU | SR weekly exercise duration (min) | 12M | 43 | 221 (184) | 41 | 115 (186) | 0.57 (0.13–1.00) |
Note: Δ, change from baseline.
Abbreviations: CI, confidence interval; EDU, education; EE, energy expenditure; ET, exercise training; ES, effect size; M, month(s); METs, metabolic equivalent tasks; min, minutes; MVPA, moderate to vigorous PA; PA, physical activity; SR, self-reported; W, week(s).
Counseling and self-management: Four months of pedometer-based counseling showed important effects on daily steps when delivered via an internet-mediated program (0.84 [0.59–1.08]; Table 3)35 or through 3 months of individualized sessions (1.08 [0.65–1.15]; Table 3)34 when compared to standard counseling. An internet-mediated dyspnea self-management program also showed important increases in exercise duration assessed by a nonvalidated tool compared to internet-mediated education at 12 months (0.57 [0.13–1.00]; Table 3). None of the other interventions in this group showed important effects on PA when compared to other active interventions (eg, individualized ET versus general ET23 and the once-daily long-acting muscarinic antagonist, tiotropium added to PA counseling versus placebo added to PA counseling;44 Table S3).
MID of PA
Limiting the analysis to 13 trials (456 actively treated subjects) that used daily steps to measure the change in PA from baseline, only the studies that tested the addition of activity counseling to PR (n=4 studies)25,27,29,30 showed large effects that exceeded the established MID of 599 daily steps (mean difference [95% CI], 1452 [549–2,356]; Figure 5). While PA counseling alone31,32,34–36 and the addition of nocturnal noninvasive ventilation (NNIV)50 to PR achieved a mean difference in steps that exceeded MID, the ES was small and did not reach statistical significance (PA counseling [n=5 studies]: 930 [293–1,566], NNIV + PR [n=1 study]: 906 [−655 to 2,467]; Figure 5). ET (n=2 studies)18,22 and health monitoring via phone calls (n=1 study)41 achieved differences in steps that did not exceed the MID for daily steps in COPD (Figure 5).
Figure 5.
Comparison between differences in daily steps achieved by PA interventions according to the MID of daily steps in COPD.
Abbreviations: ET, exercise training; MID, minimal important difference; NNIV, nocturnal noninvasive ventilation; PA, physical activity; PR, pulmonary rehabilitation.
Effects of PA interventions on functional exercise capacity
Compared to standard care, ET interventions including maintenance program15 and Nordic walking17 significantly improved 6-minute walk distance (6MWD) after 6 months (0.48 [0.11–0.85] and 0.73 [0.20–1.25]; Table S4), respectively. Exercise adherence counseling also produced important effects on 6MWD at 4 months (0.44 [0.01–0.86]; Table S4).37 However, these effects were not sustained at 12 months when compared to usual care (0.29 [−0.18 to 0.76]; Table S4).37
Adding tiotropium to PR produced moderate effects on treadmill endurance time compared to placebo (0.67 [0.01–1.33]; Table S4).43 Moderate effects on exercise capacity were also achieved after the addition of an urban walking circuit promotion program to usual care 12 months after PR (0.59 [0.12–1.07]; Table S4).52
The addition of self-management to community-based ET moderately improved incremental shuttle walk distance at 12 months (0.43 [0.09–0.77]; Table S4).18 Improvements in endurance shuttle walk time were present after individualized ET compared to general ET at 2 months (0.72 [0.35–1.09]; Table S4).23
Effects of PA interventions on HRQL
Compared to usual care, only Nordic walking enhanced HRQL using the 36-Item Short Form Survey (SF36) physical performance domain at 3 months (1.17 [0.58–1.76]; Table S5).17 Effects were sustained when reassessed 9 months post-intervention (1.61 [0.98–2.24]; Table S5).17
Pedometer-based counseling produced moderate to large effects on HRQL measured using SGRQ at 3 months when compared to standard counseling in two studies (0.43 [0.03–0.83] and 1.04 [0.00–2.07]; Table S5).34,35 One month of activity counseling via tele-rehabilitation produced moderate effects on Clinical COPD Questionnaire when compared to ET (0.53 [1.17–0.22]; Table S5).38
None of the included interventions showed important effects on dyspnea or lung function when compared to their comparators at measured time points (Tables S6 and S7).
Discussion
This systematic review found that when compared to usual care, ET including Nordic walking,17 supervised exercise19 and self-efficacy enhancing exercise20 produce large effects on PA levels in COPD. These effects were not sustained after a long-term exercise maintenance program.15,24 While PA counseling alone failed to improve PA, the addition of counseling to PR programs succeeded in producing an important increase in activity levels25,27,29,30,33 compared to PR alone. Moreover, nutritional46,47 and pharmaceutical45 interventions significantly increased PA compared to placebo; however, insufficient data and poor methodological quality restricted pooled ES calculations for these interventions. Of all included interventions, only individualized and pedometer-based counseling added to multidisciplinary PR program produced changes that exceed the newly established MID in daily steps for COPD.25,27,29,30
While PR alone achieved important increases in activity levels,6 compared to usual care at 4 months, our analysis showed that providing persistent and individualized feedback on activity levels using wearable monitors in conjunction with PR achieved significant effects on activity levels that exceeded effects produced by PR alone after 3–6 months.25,27,29,30 Moreover, the addition of counseling to PR achieved significant differences in daily steps that exceeded the established MID in daily steps at 3 months while PR alone failed to produce comparable outcomes at similar time points. It is important to mention that, of the included studies that evaluated the addition of counseling to PR, three of four studies failed to blind assessors, which indicates a low methodological quality. While this report shows that effects of the combination of PR and activity counseling significantly increased PA in COPD, high-quality research, ideally using large samples followed up for long periods, would shed light on the true effects of this comprehensive strategy.
Current research concentrates on the importance of lifestyle behavioral change interventions to improve PA.54 Examples of strategies that implement behavioral change are pedometer-based activity counseling, motivational interviewing and well-constructed self-management interventions.55,56 Failure to report robust effects of these interventions may be linked with the wide heterogeneity of self-management interventions. An international expert group has recently published a consensus definition of the construct of self-management.57 Future studies should establish programs based on such robust definitions to improve the likelihood of positive outcomes and ensure that interventions are replicable.
Recommendations to improve PA in COPD inspired by other chronic health conditions stressed the importance of developing “stealth interventions”.58 These interventions aimed to indirectly improve PA by reducing sedentary behavior time and, as a result, improve light-intensity PA. Reductions in sedentary time are known to reduce cardiovascular risk in other groups.59 This is highly relevant to people with COPD, many of whom have cardiovascular comorbidities. Of the studies included in this systematic review, only one studied the effects of PA-focused behavioral counseling using pedometer feedback and motivational interviewing added to 8 months of PR. This study demonstrated significantly reduced sedentary time when compared to PR alone at 3 months (0.96 [0.14–1.78]).29 A recent RCT compared an intervention aimed to increase the time spent in high-intensity activities versus another focused on reducing sedentary time in older adults and suggested that targeting sedentary behavior is more effective in improving physical function.60 Future trials should aim to assess the impact of PA interventions on sedentary time in COPD.
While only ET including Nordic walking,17 community-based18 training and individualized exercise23 programs showed large effects on exercise tolerance in people with COPD, these effects do not appear to have a direct relationship with improvements in PA or other important outcomes such as HRQL or dyspnea. However, PA counseling showed small and insignificant effects on exercise tolerance levels while achieving significant improvements in HRQL.34,35,38 These findings suggest that the integration between ET and activity counseling interventions may be imperative in achieving important effects on patient-focused outcomes in people with COPD.
Limitations of the systematic review
Although the overall quality score of the included trials was moderate, 12 included studies demonstrated low quality as assessed by PEDro scale. Low methodological quality was mainly due to lack of concealed allocation and blinding of assessors, which might affect the strength of evidence summarized in this report. A second limitation was the inability to pool all data in a meta-analysis and compute overall effects. The wide heterogeneity of included interventions, assessment time points and frequency of treatment hindered further analysis attempts. The results reported in this systematic review should be interpreted with caution due to these limitations. It is imperative that future trials in this field select high-quality designs and methodology with long-term follow-ups to report sustainability of true effects on PA in COPD.
Much work is under way to address inactivity in COPD. Recently published protocols describe large RCTs designed to test the effects of home-based PR using motivational interviewing,61 patient-centered home and community-based PA coaching program,62 an integrated pharmacological, ET and self-management program (the PHYSACTO trial)63 and behavioral change self-management program64 on activity levels using high-quality methodology. The outcomes of these refined interventions may provide more certainty regarding the most effective interventions to improve PA in COPD.
Conclusion
This review concluded that while ET alone can improve PA in COPD, greater improvements can be made with the addition of PA counseling. Introducing an activity counseling program in conjunction with PR might be essential to enhance PA in people with COPD. Perhaps this sophisticated model will also produce positive effects on other important COPD outcomes such as exercise capacity and HRQL.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 2.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 3.Vorrink SN, Kort HS, Troosters T, Lammers J-WJ. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):1–8. doi: 10.1186/1465-9921-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 5.Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300. doi: 10.1183/09031936.00021409. [DOI] [PubMed] [Google Scholar]
- 6.Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81. doi: 10.1183/13993003.01744-2015. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.10. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 8.Global Strategy for the Diagnosis, Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (Updated 2016) [Accessed July 18, 2016]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 9.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 10.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Halloran PD, Blackstock F, Shields N, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil. 2014;28(12):1159–1171. doi: 10.1177/0269215514536210. [DOI] [PubMed] [Google Scholar]
- 12.Demeyer H, Burtin C, Hornikx M, et al. The minimal important difference in physical activity in patients with COPD. PLoS One. 2016;11(4):e0154587. doi: 10.1371/journal.pone.0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brozek JL, Guyatt GH, Schunemann HJ. How a well-grounded minimal important difference can enhance transparency of labelling claims and improve interpretation of a patient reported outcome measure. Health Qual Life Outcomes. 2006;4:69. doi: 10.1186/1477-7525-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergeret J, Brambilla C, Mounier L. Portable oxygen therapy: use and benefit in hypoxaemic COPD patients on long-term oxygen therapy. Eur Respir J. 1989;2(1):20–25. [PubMed] [Google Scholar]
- 15.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Jr, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23(1):60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Borges RC, Carvalho CR. Impact of resistance training in chronic obstructive pulmonary disease patients during periods of acute exacerbation. Arch Phys Med Rehabil. 2014;95(9):1638–1645. doi: 10.1016/j.apmr.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Breyer M-K, Breyer-Kohansal R, Funk G-C, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010;11:112. doi: 10.1186/1465-9921-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effing T, Zielhuis G, Kerstjens H, Valk P, Palen J. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med. 2011;105(3):418–426. doi: 10.1016/j.rmed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Faulkner J, Walshaw E, Campbell J, et al. The feasibility of recruiting patients with early COPD to a pilot trial assessing the effects of a physical activity intervention. Prim Care Respir J. 2010;19(2):124–130. doi: 10.4104/pcrj.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson JL, Covey MK, Kapella MC, Alex CG, McAuley E. Self-efficacy enhancing intervention increases light physical activity in people with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1081–1090. doi: 10.2147/COPD.S66846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomidori L, Contoli M, Mandolesi G, Cogo A. A simple method for home exercise training in patients with chronic obstructive pulmonary disease: one-year study. J Cardiopulm Rehabil Prev. 2012;32(1):53–57. doi: 10.1097/HCR.0b013e31823be0ce. [DOI] [PubMed] [Google Scholar]
- 22.Probst VS, Kovelis D, Hernandes NA, Camillo CA, Cavalheri V, Pitta F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir Care. 2011;56(11):1799–1807. doi: 10.4187/respcare.01110. [DOI] [PubMed] [Google Scholar]
- 23.Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128(3):1194–1200. doi: 10.1378/chest.128.3.1194. [DOI] [PubMed] [Google Scholar]
- 24.Wilson AM, Browne P, Olive S, et al. The effects of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomised controlled trial. BMJ Open. 2015;5(3):e005921. doi: 10.1136/bmjopen-2014-005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altenburg WA, Ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med. 2015;109(1):112–121. doi: 10.1016/j.rmed.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Berry MJ, Rejeski WJ, Miller ME, et al. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med. 2010;104(6):829–839. doi: 10.1016/j.rmed.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burtin C, Langer D, Van Remoortel H, et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS One. 2015;10(12):e0144989. doi: 10.1371/journal.pone.0144989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burtin C, Langer D, van Remoortel H, et al. Correction: physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS One. 2016;11(2):e0148705. doi: 10.1371/journal.pone.0148705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz J, Brooks D, Marques A. Walk2Bactive: a randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13(1):57–66. doi: 10.1177/1479972315619574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Blok BM, de Greef MH, ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns. 2006;61(1):48–55. doi: 10.1016/j.pec.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Hornikx M, Demeyer H, Camillo CA, Janssens W, Troosters T. The effects of a physical activity counseling program after an exacerbation in patients with chronic obstructive pulmonary disease: a randomized controlled pilot study. BMC Pulm Med. 2015;15:136. doi: 10.1186/s12890-015-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hospes G, Bossenbroek L, Ten Hacken NHT, van Hengel P, de Greef MHG. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75(2):274–278. doi: 10.1016/j.pec.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Kawagoshi A, Kiyokawa N, Sugawara K, et al. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(3):364–371. doi: 10.1016/j.rmed.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. 2015;45(2):347–354. doi: 10.1183/09031936.00084514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest. 2015;148(1):128–137. doi: 10.1378/chest.14-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:301–313. doi: 10.2147/copd.s6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele BG, Belza B, Cain KC, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89(3):404–412. doi: 10.1016/j.apmr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:935–944. doi: 10.2147/COPD.S60179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonsdottir H, Amundadottir OR, Gudmundsson G, et al. Effectiveness of a partnership-based self-management programme for patients with mild and moderate chronic obstructive pulmonary disease: a pragmatic randomized controlled trial. J Adv Nurs. 2015;71(11):2634–2649. doi: 10.1111/jan.12728. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen HQ, Donesky D, Reinke LF, et al. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. J Pain Symptom Manage. 2013;46(1):43–55. doi: 10.1016/j.jpainsymman.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuz N, Walters JAE, Cameron-Tucker H, Scott J, Wood-Baker R, Walters EH. Patient anxiety and depression moderate the effects of increased self-management knowledge on physical activity: a secondary analysis of a randomised controlled trial on health-mentoring in COPD. COPD. 2015;12(5):502–509. doi: 10.3109/15412555.2014.995289. [DOI] [PubMed] [Google Scholar]
- 42.Walters J, Cameron-Tucker H, Wills K, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ. 2013;3(9):e003097. doi: 10.1136/bmjopen-2013-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008;3(1):127–136. doi: 10.2147/copd.s2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troosters T, Sciurba FC, Decramer M, et al. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med. 2014;24:14003. doi: 10.1038/npjpcrm.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watz H, Krippner F, Kirsten A, Magnussen H, Vogelmeier C. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease – a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm Med. 2014;14:158. doi: 10.1186/1471-2466-14-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dal Negro RW, Aquilani R, Bertacco S, Boschi F, Micheletto C, Tognella S. Comprehensive effects of supplemented essential amino acids in patients with severe COPD and sarcopenia. Monaldi Arch Chest Dis. 2010;73(1):25–33. doi: 10.4081/monaldi.2010.310. [DOI] [PubMed] [Google Scholar]
- 47.Dal Negro RW, Testa A, Aquilani R, et al. Essential amino acid supplementation in patients with severe COPD: a step towards home rehabilitation. Monaldi Arch Chest Dis. 2012;77(2):67–75. doi: 10.4081/monaldi.2012.154. [DOI] [PubMed] [Google Scholar]
- 48.Goris AHC, Vermeeren MAP, Wouters EFM, Schols A, Westerterp KR. Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementation. Br J Nutr. 2003;89(5):725–729. doi: 10.1079/BJN2003838. [DOI] [PubMed] [Google Scholar]
- 49.Casaburi RPJ, Hecht A, Tiep B, et al. Influence of lightweight ambulatory oxygen on oxygen use and activity patterns of COPD patients receiving long-term oxygen therapy. COPD. 2012;9(1):3–11. doi: 10.3109/15412555.2011.630048. [DOI] [PubMed] [Google Scholar]
- 50.Duiverman ML, Wempe JB, Bladder G, et al. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax. 2008;63(12):1052–1057. doi: 10.1136/thx.2008.099044. [DOI] [PubMed] [Google Scholar]
- 51.Sandland CJ, Morgan MD, Singh SJ. Patterns of domestic activity and ambulatory oxygen usage in COPD. Chest. 2008;134(4):753–760. doi: 10.1378/chest.07-1848. [DOI] [PubMed] [Google Scholar]
- 52.Pleguezuelos E, Perez ME, Guirao L, et al. Improving physical activity in patients with COPD with urban walking circuits. Respir Med. 2013;107(12):1948–1956. doi: 10.1016/j.rmed.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Kruis AL, Boland MR, Assendelft WJ, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ. 2014;349:g5392. doi: 10.1136/bmj.g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh S. One step at a time. lifestyle physical activity interventions. Ann Am Thorac Soc. 2016;13(5):586–587. doi: 10.1513/AnnalsATS.201601-039ED. [DOI] [PubMed] [Google Scholar]
- 55.Cavalheri V, Straker L, Gucciardi DF, Gardiner PA, Hill K. Changing physical activity and sedentary behaviour in people with COPD. Respirology. 2016;21(3):419–426. doi: 10.1111/resp.12680. [DOI] [PubMed] [Google Scholar]
- 56.Leidy NK, Kimel M, Ajagbe L, Kim K, Hamilton A, Becker K. Designing trials of behavioral interventions to increase physical activity in patients with COPD: insights from the chronic disease literature. Respir Med. 2014;108(3):472–481. doi: 10.1016/j.rmed.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Effing TW, Vercoulen JH, Bourbeau J, et al. Definition of a COPD self-management intervention: International Expert Group consensus. Eur Respir J. 2016;48(1):46–54. doi: 10.1183/13993003.00025-2016. [DOI] [PubMed] [Google Scholar]
- 58.Hill K, Gardiner PA, Cavalheri V, Jenkins SC, Healy GN. Physical activity and sedentary behaviour: applying lessons to chronic obstructive pulmonary disease. Intern Med J. 2015;45(5):474–448. doi: 10.1111/imj.12570. [DOI] [PubMed] [Google Scholar]
- 59.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 60.Barone Gibbs B, Brach JS, Byard T, et al. Reducing sedentary behavior versus increasing moderate-to-vigorous intensity physical activity in older adults: a 12-week randomized, clinical trial. J Aging Health. 2016 Mar 3; doi: 10.1177/0898264316635564. Epub. [DOI] [PubMed] [Google Scholar]
- 61.Holland AE, Mahal A, Hill CJ, et al. Benefits and costs of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease – a multi-centre randomised controlled equivalence trial. BMC Pulm Med. 2013;13:57. doi: 10.1186/1471-2466-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen HQ, Bailey A, Coleman KJ, et al. Patient-centered physical activity coaching in COPD (Walk On!): a study protocol for a pragmatic randomized controlled trial. Contemp Clin Trials. 2016;46:18–29. doi: 10.1016/j.cct.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Troosters T, Bourbeau J, Maltais F, et al. Enhancing exercise tolerance and physical activity in COPD with combined pharmacological and non-pharmacological interventions: PHYSACTO randomised, placebo-controlled study design. BMJ Open. 2016;6(4):e010106. doi: 10.1136/bmjopen-2015-010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourbeau J, Lavoie KL, Sedeno M, et al. Behaviour-change intervention in a multicentre, randomised, placebo-controlled COPD study: methodological considerations and implementation. BMJ. 2016;6(4):e010109. doi: 10.1136/bmjopen-2015-010109. [DOI] [PMC free article] [PubMed] [Google Scholar]