Abstract
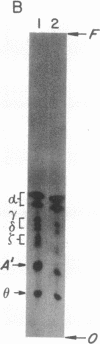
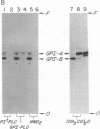
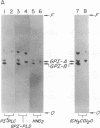
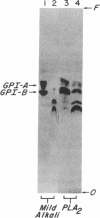
Decay-accelerating factor (DAF) is anchored in cell membranes by a glycosyl-plasmanylinositol (GPI) moiety that is transferred to it en bloc in the rough endoplasmic reticulum. To analyze the biochemical reactions involved in preassembly of this structure, a human hematopoietic cell-free system was employed. Incubation of cell extracts with UDP-[3H]GlcNAc and butanol partitioning of reaction mixtures yielded two products similar in TLC mobility to intermediates described in Trypanosoma brucei. Both species were sensitive to Bacillus thuringiensis phosphatidylinositol-specific phospholipase C, indicative of association of [3H]GlcNAc label with a plasmanylinositol-containing acceptor. In contrast to trypanosome intermediates, which contain phosphatidylinositol (1,2-diacylglycerophosphoinositol), however, alkali treatment and phospholipase A2 digestion generated butanol-phase products characteristic of glycosylated plasmanylinositol (1-alkyl-2-acylglycerophosphoinositol). Kinetic and pulse-chase experiments indicated that the slower-migrating species was a product of the faster and that it, but not the faster, was sensitive to both GPI-specific phospholipase D and nitrous acid deamination, consistent with conversion of GlcNAc- to GlcN-plasmanylinositol. Accordingly, acetic anhydride acetylation retransformed the slower species back to the faster. Further incubation with cell extracts converted the slower species into more polar products. Lysates of normal and of affected blood leukocytes from two paroxysmal nocturnal hemoglobinuria (PNH) patients supported assembly of the two intermediates within 1 min. Thus, the initial enzymes mediating human GPI-anchor assembly are GlcNAc-plasmanylinositol transferase and GlcNAc-plasmanylinositol deacetylase, their substrates contain plasmanylinositols, and the products of their activities are normal in affected PNH cells.
Full text
PDF

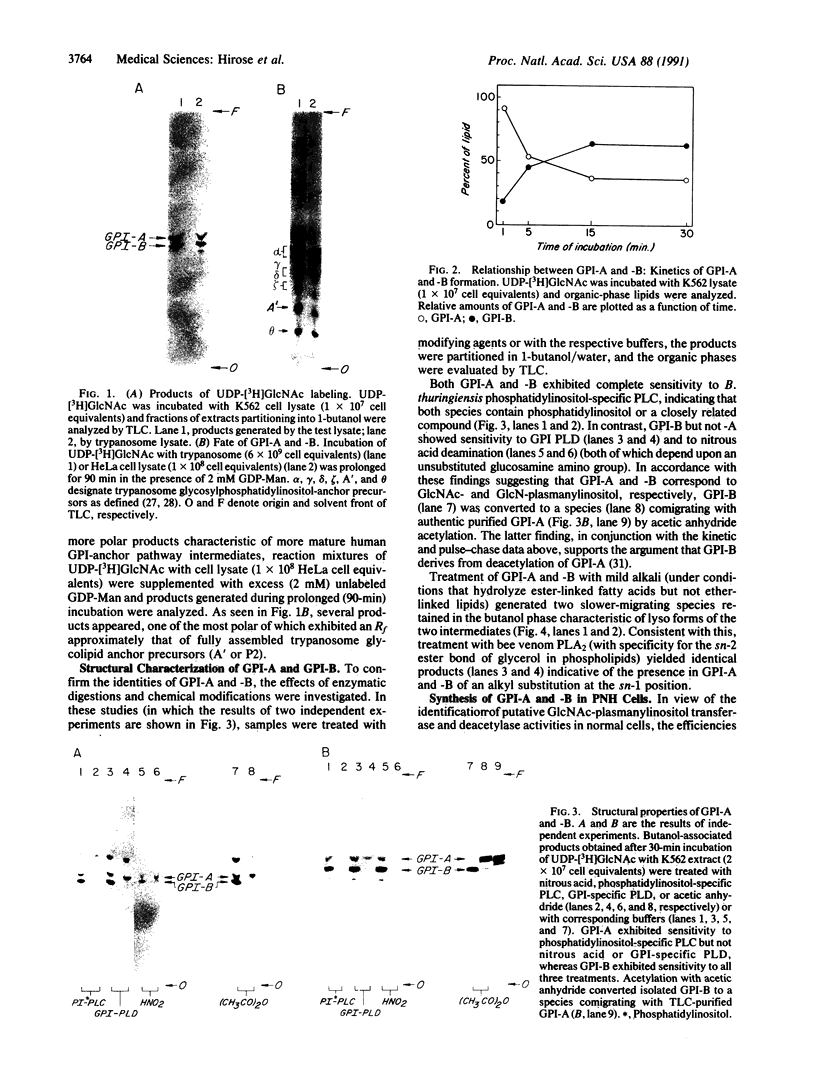
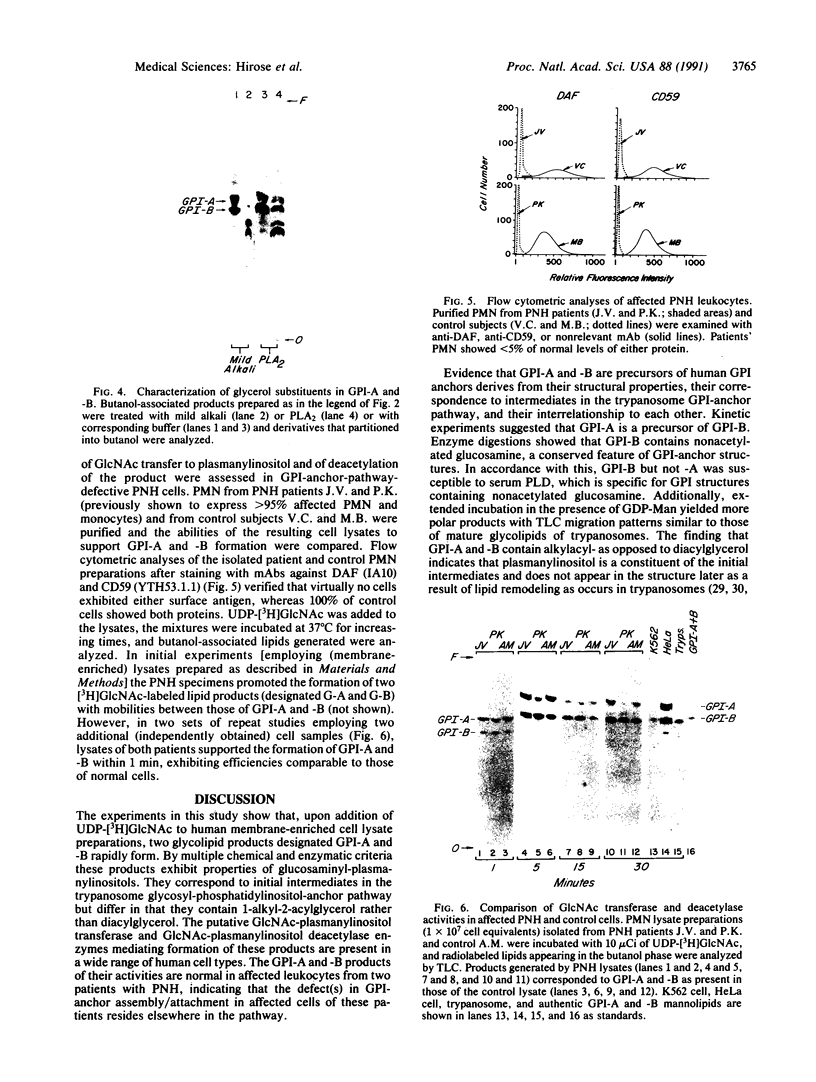

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUDITORE J. V., HARTMANN R. C., FLEXNER J. M., BALCHUM O. J. The erythrocyte acetylcholinesterase enzyme in paroxysmal nocturnal hemoglobinuria. Arch Pathol. 1960 May;69:534–543. [PubMed] [Google Scholar]
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Hazra S. V., Andreson S. W., Medof M. E. Synthesis of aberrant decay-accelerating factor proteins by affected paroxysmal nocturnal hemoglobinuria leukocytes. J Clin Invest. 1990 Jan;85(1):47–54. doi: 10.1172/JCI114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow F. L., Hall S. E., Rosse W. F., Telen M. J. Separation of the acetylcholinesterase-deficient red cells in paroxysmal nocturnal hemoglobinuria. Blood. 1986 Apr;67(4):893–897. [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering T. L., Masterson W. J., Englund P. T., Hart G. W. Biosynthesis of the glycosyl phosphatidylinositol membrane anchor of the trypanosome variant surface glycoprotein. Origin of the non-acetylated glucosamine. J Biol Chem. 1989 Jul 5;264(19):11168–11173. [PubMed] [Google Scholar]
- Doering T. L., Masterson W. J., Hart G. W., Englund P. T. Biosynthesis of glycosyl phosphatidylinositol membrane anchors. J Biol Chem. 1990 Jan 15;265(2):611–614. [PubMed] [Google Scholar]
- Fasel N., Rousseaux M., Schaerer E., Medof M. E., Tykocinski M. L., Bron C. In vitro attachment of glycosyl-inositolphospholipid anchor structures to mouse Thy-1 antigen and human decay-accelerating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6858–6862. doi: 10.1073/pnas.86.18.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Ferguson M. A., Homans S. W., Dwek R. A., Rademacher T. W. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science. 1988 Feb 12;239(4841 Pt 1):753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Harada R., Okada N., Fujita T., Okada H. Purification of 1F5 antigen that prevents complement attack on homologous cell membranes. J Immunol. 1990 Mar 1;144(5):1823–1828. [PubMed] [Google Scholar]
- Hideshima T., Okada N., Okada H. Expression of HRF20, a regulatory molecule of complement activation, on peripheral blood mononuclear cells. Immunology. 1990 Mar;69(3):396–401. [PMC free article] [PubMed] [Google Scholar]
- Holguin M. H., Fredrick L. R., Bernshaw N. J., Wilcox L. A., Parker C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Jul;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin M. H., Wilcox L. A., Bernshaw N. J., Rosse W. F., Parker C. J. Relationship between the membrane inhibitor of reactive lysis and the erythrocyte phenotypes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Nov;84(5):1387–1394. doi: 10.1172/JCI114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. L., Hereld D., Bangs J. D., Hart G. W., Englund P. T. Identification of a glycolipid precursor of the Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1986 Sep 15;261(26):12147–12153. [PubMed] [Google Scholar]
- Lewis S. M., Dacie J. V. Neutrophil (leucocyte) alkaline phosphatase in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1965 Sep;11(5):549–556. doi: 10.1111/j.1365-2141.1965.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Masterson W. J., Doering T. L., Hart G. W., Englund P. T. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell. 1989 Mar 10;56(5):793–800. doi: 10.1016/0092-8674(89)90684-3. [DOI] [PubMed] [Google Scholar]
- Masterson W. J., Raper J., Doering T. L., Hart G. W., Englund P. T. Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl phosphatidylinositol membrane anchors. Cell. 1990 Jul 13;62(1):73–80. doi: 10.1016/0092-8674(90)90241-6. [DOI] [PubMed] [Google Scholar]
- Mayor S., Menon A. K., Cross G. A., Ferguson M. A., Dwek R. A., Rademacher T. W. Glycolipid precursors for the membrane anchor of Trypanosoma brucei variant surface glycoproteins. I. Can structure of the phosphatidylinositol-specific phospholipase C sensitive and resistant glycolipids. J Biol Chem. 1990 Apr 15;265(11):6164–6173. [PubMed] [Google Scholar]
- Mayor S., Menon A. K., Cross G. A. Glycolipid precursors for the membrane anchor of Trypanosoma brucei variant surface glycoproteins. II. Lipid structures of phosphatidylinositol-specific phospholipase C sensitive and resistant glycolipids. J Biol Chem. 1990 Apr 15;265(11):6174–6181. [PubMed] [Google Scholar]
- Medof M. E., Gottlieb A., Kinoshita T., Hall S., Silber R., Nussenzweig V., Rosse W. F. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987 Jul;80(1):165–174. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Menon A. K., Mayor S., Ferguson M. A., Duszenko M., Cross G. A. Candidate glycophospholipid precursor for the glycosylphosphatidylinositol membrane anchor of Trypanosoma brucei variant surface glycoproteins. J Biol Chem. 1988 Feb 5;263(4):1970–1977. [PubMed] [Google Scholar]
- Menon A. K., Schwarz R. T., Mayor S., Cross G. A. Cell-free synthesis of glycosyl-phosphatidylinositol precursors for the glycolipid membrane anchor of Trypanosoma brucei variant surface glycoproteins. Structural characterization of putative biosynthetic intermediates. J Biol Chem. 1990 Jun 5;265(16):9033–9042. [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Okada H., Nagami Y., Takahashi K., Okada N., Hideshima T., Takizawa H., Kondo J. 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1553–1559. doi: 10.1016/0006-291x(89)90852-8. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Roberts W. L., Santikarn S., Reinhold V. N., Rosenberry T. L. Structural characterization of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase by fast atom bombardment mass spectrometry. J Biol Chem. 1988 Dec 15;263(35):18776–18784. [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rosse W. F. Paroxysmal nocturnal hemoglobinuria and decay-accelerating factor. Annu Rev Med. 1990;41:431–436. doi: 10.1146/annurev.me.41.020190.002243. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Silber R., Low M. G., Springer T. A. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987 Oct 1;166(4):1011–1025. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Tykocinski M. L., Lublin D. M., Holers V. M., Rosse W. F., Atkinson J. P., Medof M. E. Normal polymorphic variations and transcription of the decay accelerating factor gene in paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):880–884. doi: 10.1073/pnas.85.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Takami N., Ogata S., Oda K., Misumi Y., Ikehara Y. Biosynthesis of placental alkaline phosphatase and its post-translational modification by glycophospholipid for membrane-anchoring. J Biol Chem. 1988 Feb 25;263(6):3016–3021. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]