Abstract
G2 arrest of cells suffering DNA damage in S phase is crucial to avoid their entry into mitosis, with the concomitant risks of oncogenic transformation. According to the current model, signals elicited by DNA damage prevent mitosis by inhibiting both activation and nuclear import of cyclin B1-Cdk1, a master mitotic regulator. We now show that normal human fibroblasts use additional mechanisms to block activation of cyclin B1-Cdk1. In these cells, exposure to nonrepairable DNA damage leads to nuclear accumulation of inactive cyclin B1-Cdk1 complexes. This nuclear retention, which strictly depends on association with endogenous p21, prevents activation of cyclin B1-Cdk1 by Cdc25 and Cdk-activating kinase as well as its recruitment to the centrosome. In p21-deficient normal human fibroblasts and immortal cell lines, cyclin B1 fails to accumulate in the nucleus and could be readily detected at the centrosome in response to DNA damage. Therefore, in normal cells, p21 exerts a dual role in mediating DNA damage-induced cell cycle arrest and exit before mitosis. In addition to blocking pRb phosphorylation, p21 directly prevents mitosis by inactivating and maintaining the inactive state of mitotic cyclin-Cdk complexes. This, with subsequent degradation of mitotic cyclins, further contributes to the establishment of a permanent G2 arrest.
INTRODUCTION
Cyclin B1-Cdk1 is a master mitotic regulator and is therefore an ultimate target toward which most, if not all, antiproliferative signals generated during S and G2 phase converge. In cycling cells, cyclin B1 synthesis increases during late S-early G2 phase (Pines and Hunter, 1989), but the newly formed cyclin B1-Cdk1 complexes are kept inactive by inhibitory phosphorylations on Thr14 and Tyr15 (Norbury et al., 1991) and by active and constant nuclear exclusion via Crm1-mediated nuclear export (Hagting et al., 1998; Yang et al., 1998). The rapid nuclear accumulation of cyclin B1-Cdk1 that occurs in prophase coincides with phosphorylation of cyclin B1 at the cytoplasmic retention sequence, which also contains its nuclear export sequence (Hagting et al., 1999; for review, see Porter and Donoghue, 2003). Nuclear import of cyclin B1-Cdk1 coincides with Cdc25 phosphatase-mediated dephosphorylation of Thr14 and Tyr15 on Cdk1 (Berry and Gould, 1996). Based on these observations, it has been assumed that cytoplasmic cyclin B1-Cdk1 is not active. However, both earlier and most recent work suggest that cyclin B1-Cdk1 might already be activated in the cytoplasm and most probably at the centrosome (Heald et al., 1993; De Souza et al., 2000; Hirota et al., 2003; Jackman et al., 2003).
According to the current model, signals elicited by DNA damage in S and G2 phases prevent mitotic entry by inhibiting both activation (via inactivation of Cdc25) and nuclear import of cyclin B1-Cdk1 (Jin et al., 1998; Yang et al., 1998, 2001; Deming et al., 2001). G2 arrest is initiated via phosphorylation of Cdc25 by Chk1/2 kinases, a process that does not require active p53. On the other hand, the maintenance of G2 arrest is highly p53 dependent and involves at least two of its transcriptional targets, the Cdk inhibitor p21Waf1/Cip1 (p21) and the adaptor protein 14-3-3σ (for reviews, see Ohi and Gould, 1999; Taylor and Stark, 2001). The latter protein has been proposed to mediate nuclear exclusion of cyclin B1-Cdk1, an event thought to be necessary for blocking mitosis (Chan et al., 1999; van Hemert et al., 2001).
Despite accumulating evidence suggesting that p21 could regulate DNA damage-induced G2 arrest (Bunz et al., 1998; Dulic et al., 1998; Winters et al., 1998; Chan et al., 2000; Flatt et al., 2000; Baus et al., 2003), it is widely assumed that this inhibitor does not play a direct role in inactivating mitotic cyclin-Cdk1 complexes, partly because of its low affinity toward Cdk1 (Harper et al., 1995). Indeed, p21 has been detected only in a minority of cyclin B1-Cdk1 complexes after DNA damage (Levedakou et al., 1995; Barboule et al., 1999; Flatt et al., 2000) or even after overexpression of p53 or p21 (Winters et al., 1998; Smits et al., 2000). This is in striking contrast to another mitotic regulator, cyclin A-Cdk1, which massively associates with p21 in response to DNA damage, suggesting that, at least in normal human fibroblasts (NHFs), Cdk1 could be a major target of this inhibitor (Dulic et al., 1998; Baus et al., 2003). Moreover, it has been shown that overexpression of p21 might block phosphorylation of Cdk1 on Thr161 by CAK (Smits et al., 2000) or even promote nuclear accumulation of cyclin B1 in the absence of mitotic events (Winters et al., 1998; Smits et al., 2000). However, these observations made in transformed or immortalized cell lines, are difficult to reconcile with the current model according to which DNA damage-induced G2 arrest is accomplished by nuclear exclusion of cyclin B1-Cdk1.
In this article, we investigate whether in normal human fibroblasts p21 plays a direct role in controlling cellular distribution and activation of cyclin B1-Cdk1 in response to genotoxic stress.
MATERIALS AND METHODS
Cell Culture, Treatment, and Synchronization
NHFs as well as NHFs expressing HPV16-E6 or -E7 oncogenes were described previously (Baus et al., 2003). The cells were cultured in DMEM supplemented with 10% fetal calf serum. Wild-type and p21-/- human lung fibroblasts (LF1) were cultured in special hypoxic conditions as described previously (Wei et al., 2001).
Drug concentrations and synchronization protocols were as described previously (Baus et al., 2003). Cells were presynchronized by contact inhibition, replated (1/4 dilution) to stimulate cell cycle entry, and synchronized at the G1/S boundary by incubation with 2 mM hydroxyurea (10-12 h). The drugs were added 2 h after release from the G1/S block. Control cells were fixed in methanol 26 h after replating at the time when most control cells had entered mitosis. ICRF-193-treated cells were fixed 3 h later. The cell cycle distribution was determined by flow cytometric analysis (fluorescence-activated cell sorting, FACS) of propidium iodide-stained cells. In some experiments (Figures 2, 5, 6, and 7), drugs were added to asynchronously proliferating cultures as described previously (Baus et al., 2003).
Figure 2.
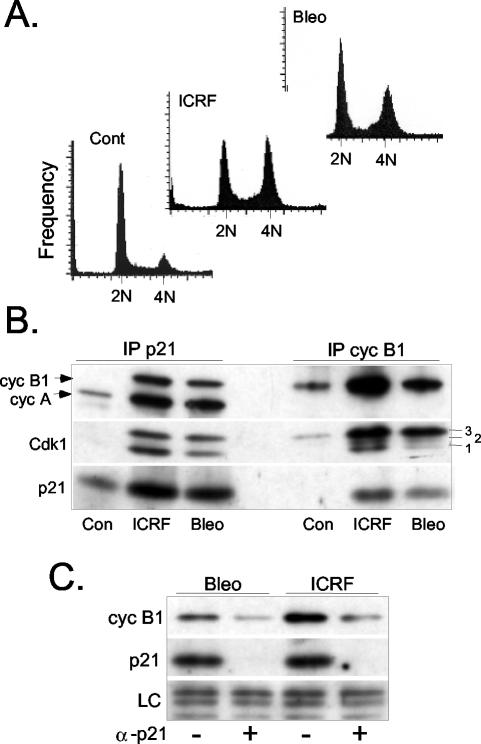
Increasing association between p21 and cyclin B1-Cdk1 after exposure to DNA damage-inducing drugs. (A) Cell cycle distribution of asynchronously growing untreated NHFs (Cont) and cultures exposed to ICRF-193 or bleomycin for 12 h. DNA content was determined by FACS analysis of propidium iodide-stained cells. The same cell cultures were used for the biochemical analysis described below. (B) Western blot analysis showing cyclin B1, cyclin A, Cdk1, and p21 levels in p21 and cyclin B1 immunoprecipitates (IP) isolated from untreated NHFs (Con) and NHFs exposed to ICRF-193 (ICRF) or bleomycin (Bleo). For all immunoprecipitation experiments, the equivalent amounts of cell extract (150 μg) were used, and the samples were analyzed on the same immunoblots. Numbers 1, 2, and 3 indicate differentially phosphorylated Cdk1 isoforms (see text for the details). (C) Western blot analysis showing cyclin B1 levels after p21 immunodepletion in bleomycin- and ICRF-193-treated NHF. LC, loading control.
Figure 5.
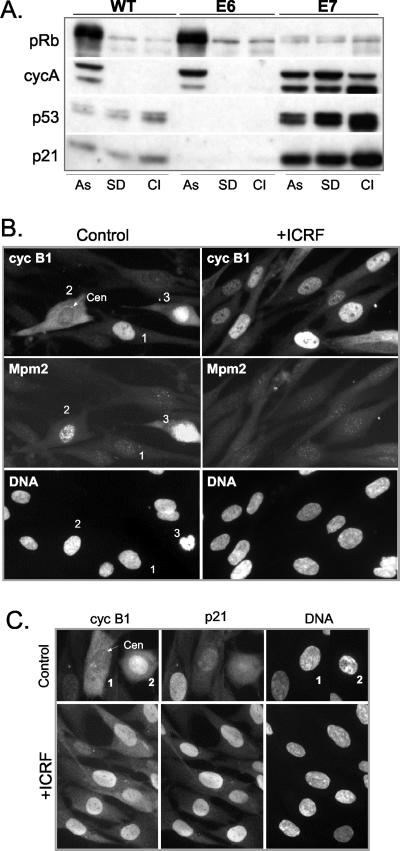
ICRF-193-arrested NHFs expressing HPV16-E7 oncogene accumulate high amounts of cyclin B1 in the nucleus but fail to initiate early mitotic events. (A) Western blot analysis showing the pRb phosphorylation status, and expression of cyclin A, p53, and p21 in asynchronously proliferating (As), serum-deprived (SD), and contact inhibited (CI) wild-type, E6- and E7-expressing NHFs. (B) Colocalization of cyclin B1 and Mpm2 in asynchronous untreated (control) and ICRF-193-treated E7 cells. Cells were fixed in methanol and simultaneously stained with mouse monoclonal anti-Mpm2 (Texas-Red) and with rabbit polyclonal anti-cyclin B1 (fluorescein isothiocyanate, FITC) antibodies. Numbers denote cells arrested in G2 (1) as well as in early (2) and late prophase (3). Arrow depicts the centrosome. Note the centrosomal localization of cyclin B1 in cell 2 exhibiting an elevated Mpm2 signal and nuclear accumulation of cyclin B1 and an accentuated Mpm2 signal in late prophase cell 3 (Figure 3B). (C) Colocalization of cyclin B1 and p21 in untreated (control) and ICRF-193-treated E7 cells. Cells were fixed in methanol and simultaneously stained with mouse monoclonal anti-cyclin B1 (Texas-Red) and with rabbit polyclonal anti-p21 (FITC) antibodies. Numbers denote cells at early (1) and late (2) prophase.
Figure 6.
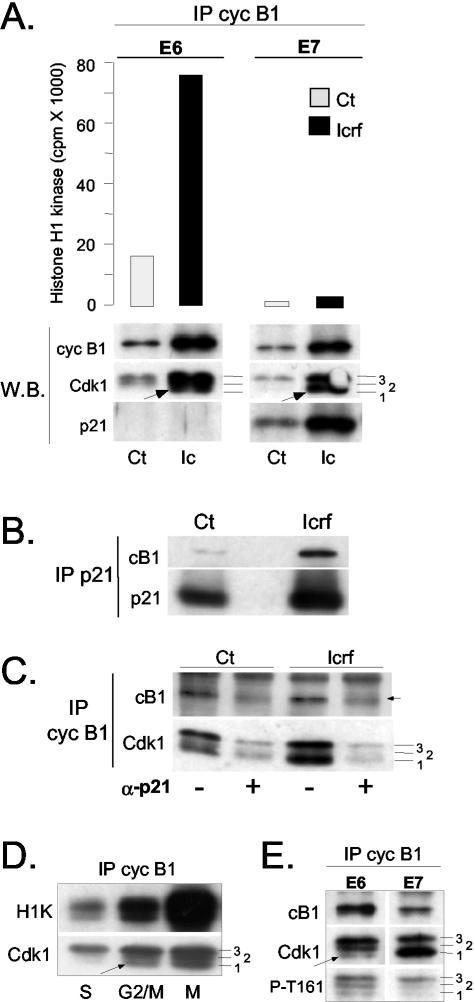
p21 binds to inactive cyclin B1-Cdk1 complexes. (A) Histone H1 kinase assays and Western blot analysis of cyclin B1 immunoprecipitates isolated from asynchronously growing control (Ct) and ICRF-193-treated E6- and E7-expressing NHFs. The same immunoprecipitates were analyzed by Western blot (W.B.) for the presence of cyclin B1, Cdk1 and p21. Numbers denote hyperphosphorylated (3), partially phosphorylated (2), and hypophosphorylated (1) Cdk1 isoforms (see text for more explanation). Arrows point at hypophosphorylated Cdk1 (isoform 1) that specifically accumulates in ICRF-treated cells. Note the difference in the abundance of this isoform between E6 and E7 cells. (B and C) p21 immunodepletion experiments. The protein extracts prepared from untreated (Ct) and ICRF-193-treated cells were immunodepleted for p21-bound complexes as described in MATERIALS AND METHODS. (B) Western blot analysis showing cyclin B1 and p21 levels in p21 immunoprecipitates. (C) Western blot analysis cyclin B1 immunoprecipitates isolated from mock-treated (-) and p21-depleted cell extracts prepared from the same experiment. Numbers 1, 2, and 3 indicate differentially phosphorylated Cdk1 isoforms. Note that even in untreated E7 cells, a large population of cyclin B1-Cdk1 is bound to p21. In this immunoblot, the cyclin B1 signal (arrow) is obscured by a parasite background band. (D) Histone H1 kinase assays and Western blot analysis of cyclin B1 immunoprecipitates isolated from wild-type NHF synchronized in S, G2/M, and M (+ nocodazole) phases. Numbers 1, 2 and 3 indicate differentially phosphorylated Cdk1 isoforms, whereas the arrow points at the hypophosphorylated and active Cdk1. (E) Western blot analysis comparing cyclin B1 IP isolated from ICRF-193-treated E6 and E7 cultures. Numbers 1, 2, and 3 indicate differentially phosphorylated Cdk1 isoforms. To assess the phosphorylation status of the Cdk1 isoform 1 that accumulates in cyclin B1 IP in ICRF-193-treated E7 cells, immunoblots were probed with an antibody specific for CAK-phosphorylated phospho-threonine 161 (T161-P).
Figure 7.
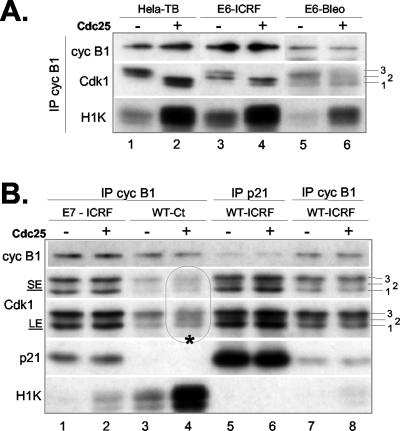
p21 prevents Cdc25-dependent activation of cyclin B1-Cdk1 complexes. Histone H1 kinase assays and Western blot analysis of cyclin B1 immunoprecipitates isolated from p21-deficent cells (HeLa arrested by thymidine block and HPV16-E6-expressing NHFs) (A) and NHFs containing normal (WT) or elevated (HPV16-E7-expressing NHFs) p21 levels (B). The cells were incubated with ICRF-193 (or bleomycin), as described in the legend to Figure 6. Before histone H1 kinase assays (H1K), cyclin B1 immunoprecipitates were treated (+) or not (-) with recombinant Cdc25B as described in MATERIALS AND METHODS. As a control, we also show cyclin B1-Cdk1 complexes from untreated asynchronously growing cells (indicated by asterisk; lanes 3 and 4) that do not contain p21. Lanes 5 and 6 show p21-bound cyclin B1-Cdk1 complexes from ICRF-193-treated cells. Note that these p21 immunoprecipitates contain higher amounts of Cdk1, due to the presence of other mitotic cyclin-Cdk complexes. Immunoblots were first exposed for autoradiography and then incubated with the indicated antibodies to reveal the status of cyclin B1, Cdk1, and p21. Numbers 1, 2, and 3 indicate differentially phosphorylated Cdk1 isoforms. Hyperphosphorylated Cdk1 (isoform 3) is the main Cdc25 target. For better appreciation of different Cdk1 isoforms different ECL exposures are shown (SE, short exposure; LE, long exposure). Autoradiograph B is exposed for longer (24 h) than autoradiograph A (3 h) to visualize a weak cyclin B1-Cdk1 activation in ICRF-193-treated wild-type and E7 cells.
Where indicated (Figure 4 and Supplemental Figure 2), cells were irradiated (8 Gy) using a 137Cesium source and fixed for immunofluorescence experiments 12 h later.
Figure 4.
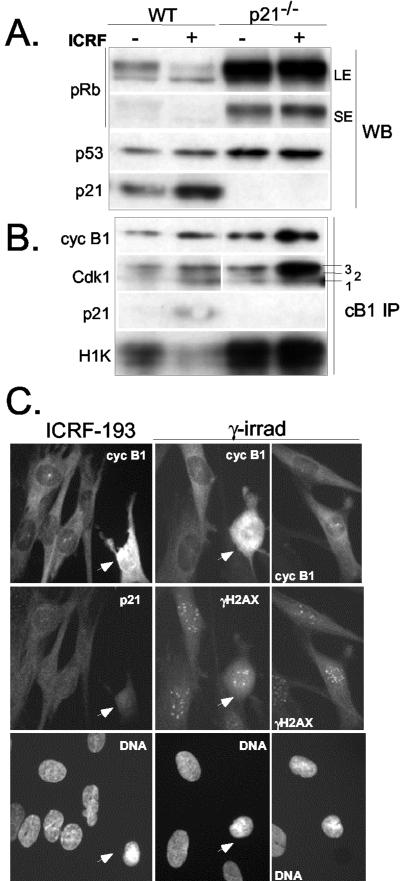
p21-/- fibroblasts fail to accumulate nuclear cyclin B1 in response to ICRF-193 or γ-irradiation. (A) Western blot analysis of total cell extracts showing the pRb phosphorylation status, and expression of p53 and p21 in asynchronously proliferating (-) and ICRF-193-treated (+) wild-type (WT) and isogenic p21-/- NHFs. Two different ECL exposures (SE and LE) were shown to demonstrate the maintenance of pRb hyperphosphorylation in ICRF-193-treated p21-/- NHF. (B) Western blot and histone H1 kinase analysis of cyclin B1 immunoprecipitates (cB1 IP) isolated from the same cell extracts as described above. Although in ICRF-193-treated p21-/- NHFs most of cyclin B1 is associated with hyperphosphorylated Cdk1, the persistence of kinase activity indicates inefficient G2 arrest. (C) Cyclin B1 localization in NHF p21-/- exposed to ICRF-193 and γ-irradiation. Cells also were stained with either anti-p21 (left) or with anti-γ-H2AX antibodies (right) to visualize the foci induced by DNA damage. Arrows point at early mitotic cells showing ongoing DNA condensation.
Immunofluorescence Microscopy
The conditions for cell fixation, double immunolabeling, and mounting were as described previously (Dulic et al., 1998). To detect cyclin B1, γ-tubulin, and Mpm2, the cells were fixed in methanol at -20°C. Primary antibodies to cyclin B1 (monoclonal GNS1, sc-245, and polyclonal sc-752) and polyclonal antibody to p21 (sc-397) were from Santa Cruz Biotechnology (Santa Cruz), polyclonal and monoclonal anti-γ-H2AX antibodies and monoclonal anti-Chk2 antibody were from Upstate Biotechnology (Lake Placid, NY), and monoclonal antibody to Mpm2 and γ-tubulin were purchased from DakoCytomation California (Carpinteria, CA) and Sigma-Aldrich St. Louis, MO), respectively. Antibodies specific to phosphorylated cyclin B1 (P-S133 and P-S126) were generous gifts of Drs. M. Jackman and J. Pines (Jackman et al., 2003). Other antibodies used were described previously (Dulic et al., 1998; Baus et al., 2003).
Immunofluorescence and phase contrast photomicrographs were image-captured and a composite generated using Adobe Macintosh Photoshop or Microsoft PowerPoint software.
Immunobiochemistry
Preparation of whole cell lysates from frozen cell pellets, conditions for immunoprecipitation of cyclin complexes, p21 immunodepletion experiments, histone H1 kinase assays, and immunoblotting as well as all the primary and secondary antibodies used in those experiments have been described previously (Dulic et al., 1998; Baus et al., 2003). Secondary antibodies for Western blot analysis were anti-mouse-IgG-horseradish peroxidase (HRP) (DakoCytomation California), anti-rabbit IgG HRP-conjugates (Promega, Madison, WI), and protein A/G coupled to HRP (Pierce Chemical, Rockford, IL). Proteins were visualized by enhanced chemiluminescence (ECL) according to manufacturer's protocol (Amersham Biosciences, Piscataway, NJ).
Recombinant Cdc25B, a gift of Dr. Véronique Baldin (CRBM, Montpellier, France), was prepared and assayed as described previously (Cans et al., 1999).
RESULTS
DNA Damage Induces Premitotic Cyclin B1 Nuclear Accumulation
Our previous work showed that, in response to DNA damage-inducing agents during S phase, NHFs arrest in G2 accumulating inactive cyclin A-Cdk and cyclin B1-Cdk complexes (Baus et al., 2003). This cell cycle arrest was accompanied by strong accumulation of p21 and its association with both Cdk2 and Cdk1. Even though cyclin A-Cdk1/2 complexes were major targets of p21, we repeatedly observed a significant fraction of cyclin B1-Cdk1 complexes that was associated with this inhibitor (Baus et al., 2003). To better understand the role of this latter interaction, we followed by immunofluorescence the localization of p21 and cyclin B1 in synchronized NHF that were specifically arrested in G2 phase in the presence of ICRF-193 (Supplemental Figure 1A), a catalytic inhibitor of DNA topoisomerase II (Roca et al., 1994). Although earlier reports proposed that ICRF-193 does not induce double DNA strand breaks (Downes et al., 1994), distinct nuclear γ-H2AX foci (Rogakou et al., 1999) could be detected in drug-treated cells (Supplemental Figure 2A), thus confirming recent observations (Mikhailov et al., 2002). Moreover, exposure to ICRF-193 induced activation of the checkpoint kinase Chk2, and this occurred regardless of the status of p53 or pRb, as documented by the appearance of an SDS-PAGE mobility shift (Supplemental Figure 2B).
After treatment with ICRF-193, a large population of cells accumulated cyclin B1 in the nucleus without apparent chromosome condensation (Figures 1A and 3B for statistics). Moreover, and in contrast to control G2/M cells (our unpublished data), drug-treated cells invariably contained high p21 levels (Figure 1A). To ensure that these cells were effectively blocked in G2, we determined their cell cycle status with more accuracy by using the Mpm2-specific antibody (Davis et al., 1983; Westendorf et al., 1994). In contrast to the signal obtained with the widely used antibody directed against phospho-histone H3 that stains the cells from prophase onward, the anti-Mpm2 signal significantly increases even before DNA condensation could be observed. Based on the Mpm2 signal, DNA condensation and cyclin B1 localization, we distinguished three different stages in the G2/M transition of NHF. Stage 1 is characterized by cytoplasmic and centrosomal localization of cyclin B1 (Figure 1, B and C, arrows), an elevated Mpm2 signal in comparison with interphase cells (asterisks) and no apparent chromosome condensation. Thus, this stage would correspond to “antephase” or “transition 1,” the terms used previously to describe late G2 phase (Pines and Rieder, 2001). Cells in stage 2 (early prophase) in addition to strong centrosomal staining, accumulate cyclin B1 in the nucleus, display a strong Mpm2 signal and chromosome condensation becomes apparent. Finally, in stage 3, cyclin B1 is mostly in the nucleus and DNA condensation is clearly visible (mid- to late prophase). Off note, most cells in stage 1 were negative for staining with the anti-phospho-histone H3 antibody (our unpublished data).
Figure 1.
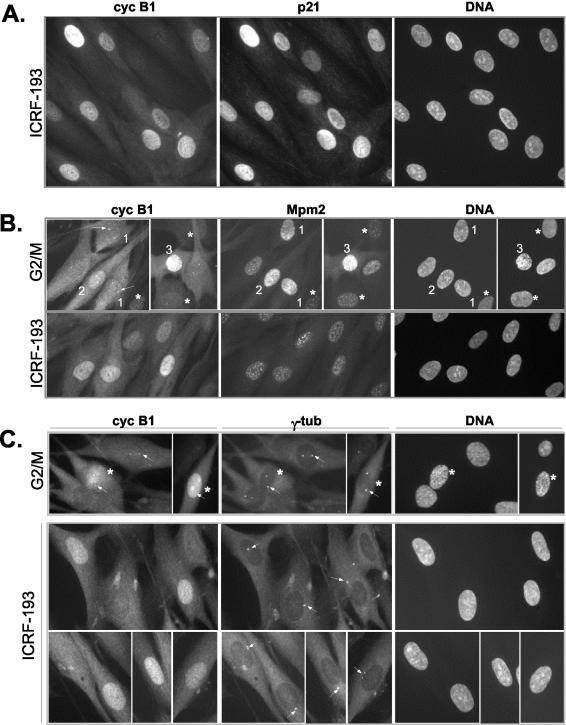
Nuclear accumulation of cyclin B1 in the presence of ICRF-193, a drug that specifically provokes G2 arrest. (A) Colocalization of cyclin B1 and p21 in ICRF-193-treated synchronized NHFs. Cells were synchronized as described in MATERIALS AND METHODS, and the drug was added when the majority of the cells had passed the G1/S boundary. Control cells were fixed when most control cells had entered mitosis, whereas ICRF-193-treated cells were fixed 3 h later. Cell cycle profiles of synchronized control and drug-treated cells are shown in Supplementary Figure 1a. Cells were simultaneously stained with a mouse monoclonal anti-cyclin B1 and with a rabbit polyclonal anti-p21. (B) Indirect immunofluorescence analysis comparing subcellular localization of cyclin B1 and Mpm2 signal in control G2/M-enriched cultures and in ICRF-193-arrested NHFs. Cells were fixed in methanol and simultaneously stained with a mouse monoclonal anti-Mpm2 (Texas-Red) and with a rabbit polyclonal anti-cyclin B1 (fluorescein isothiocyanate, FITC). Cells 1-3 depict different G2/M transition stages as judged by cyclin B1 localization, the intensity of the Mpm2 signals, separation of the centrosomes (arrows), and DNA condensation levels (Hoechst). Note that only cell 3 exhibits significant DNA condensation (late prophase), whereas in the cells exhibiting increased Mpm2 staining (1 and 2) no DNA condensation is readily detectable. Arrows point at cyclin B1 localized at the centrosome. Asterisks depict cells at earlier stages of the cell cycle exhibiting a background Mpm2 signal. (C) Colocalization of cyclin B1 and γ-tubulin in control G2/M and ICRF-193-treated NHFs. Cells were fixed in methanol and simultaneously stained with a mouse monoclonal anti-γ-tubulin (Texas-Red) and with a rabbit polyclonal anti-cyclin B1 (FITC). Cells in prophase with visible DNA condensation are depicted by an asterisk (*). Note the poor separation of centrosomes (arrows) in ICRF-193-arrested cells as compared with control G2/M cells.
Figure 3.
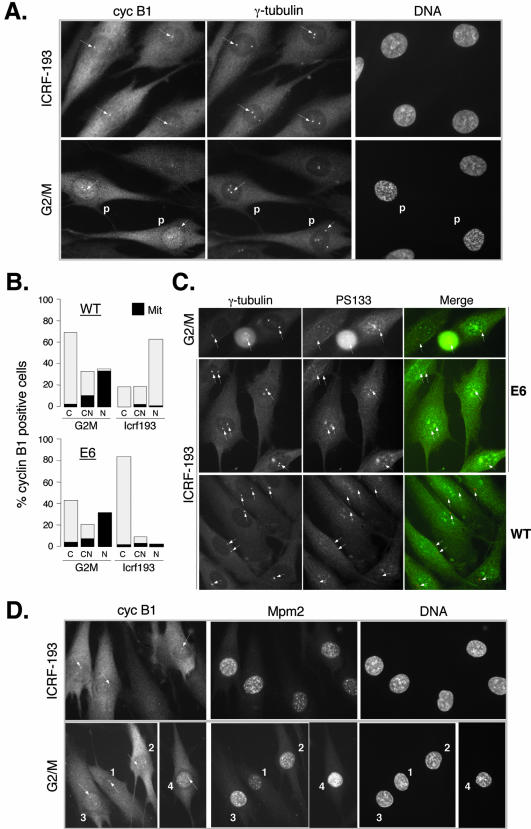
NHF expressing the HPV-16 E6 oncogene undergo early mitotic events after exposure to genotoxic stress. (A) Colocalization of cyclin B1 and γ-tubulin in control G2/M-enriched and ICRF-193-treated E6 expressing fibroblasts. Cells were synchronized as specified in the legend of Figure 1, and their cell cycle profile is shown in Supplementary Figure 1B. Note a strong cyclin B1 signal at the centrosomes (arrows) in ICRF-193-treated cells, similar to one observed in early prophase cells. In control G2/M cells, “P” denotes the cells in advanced prophase showing DNA condensation and exhibiting accumulation of cyclin B1 in the nucleus. Cells were simultaneously stained with mouse monoclonal anti-γ-tubulin (Texas-Red) and with rabbit polyclonal anti-cyclin B1 (fluorescein isothiocyanate, FITC). (B) Statistical analysis of nuclear localization of cyclin B1 in untreated (G2/M transition) and ICRF-193-treated wild-type (WT) and E6-expressing NHFs. Cells expressing cyclin B1 were scored for localization in the cytoplasm (C), both cytoplasm and nucleus (CN), and predominantly in the nucleus (N). Black color indicates the population of cells exhibiting a visible DNA condensation pattern (visualized by Hoechst staining). The graph represents an average of at least two independent experiments. (C) Recruitment of activated cyclin B1-Cdk1 at the centrosomes in NHFs expressing E6 but not in wild-type NHFs in the presence of ICRF-193. Double immunofluorescence using rabbit polyclonal anti-phospho-serine 133 cyclin B1 (PS133) and mouse monoclonal anti-γ-tubulin antibodies. Micrographs show untreated cells at G2/M transition (E6) and cells exposed to ICRF-193 (WT and E6). (D) Colocalization of cyclin B1 and Mpm2 in untreated (G2/M) and ICRF-193-treated E6 cells. In control G2/M cells, numbers 1-4 depict different G2-prophase stages as judged by cyclin B1 localization, the intensity of the Mpm2 signal, the separation of the centrosomes (arrows), and DNA condensation levels (Hoechst). According to these criteria, cell 1 is in late G2, cells 2 and 3 in early prophase, and cell 4 in midprophase (cyclin B1 is nuclear and DNA is condensed). According to the same criteria, ICRF-193-arrested cells are in early prophase, exhibiting an increased Mpm2 signal and cyclin B1 localized at the centrosome (arrows; cf. also Figure 2A).
According to the Mpm2 signal and the centrosomal status, most of the ICRF-193-treated cells accumulating nuclear cyclin B1 seemed to be arrested in late G2 phase, before stage 1 (Figure 1B). However, a precise cell cycle stage could not be assigned to these cells, because their equivalent could not be observed in an untreated, asynchronously growing population. Note that, unlike untreated cells, where centrosomal cyclin B1 signal strongly increased before nuclear accumulation in early prophase (Figure 1B, arrows), in drug-arrested cells cyclin B1-associated staining of this organelle was much weaker (Figure 1, B and C). Indeed, an antibody raised against phosphorylated Ser133 of cyclin B1 (PS133), which recognizes the cyclin B1-Cdk1 complex activated by Plk1 at centrosomes in very early prophase stages (Jackman et al., 2003), showed minimal staining in ICRF-193-arrested cells (Figure 3C; our unpublished data). This premitotic cyclin B1/p21 nuclear accumulation is not limited to cells treated with ICRF-193 because it could be readily observed in NHF treated with bleomycin or exposed to γ-rays (Supplemental Figure 3, A and C). It represents thus most likely a general response to DNA damage in normal fibroblasts.
Induction of p21-Cyclin B1-Cdk1 Complexes after DNA Damage
Given the striking nuclear colocalization of p21 and cyclin B1, we sought to verify whether, under these experimental conditions, p21 associates significantly with cyclin B1 and Cdk1. We therefore compared p21 and cyclin B1 immunoprecipitates isolated from extracts of untreated cells and those exposed to ICRF-193 and bleomycin for 12 h. Prolonged exposure of NHF to these drugs induced a cell cycle exit that is accompanied with down-regulation of mitotic cyclins (Baus et al., 2003). The cell cycle profile of the cultures used in this experiment is shown in Figure 2A.
Western blot analysis of these immunoprecipitates shows that each drug treatment provokes a dramatic accumulation of cyclin B1-Cdk1-p21 complexes (Figure 2A). Note that p21 binds both hyperphosphorylated (3) and hypophosphorylated (1) Cdk1 isoforms but very little intermediate and partially active Thr14-dephosphorylated isoform 2 (Borgne and Meijer, 1996) is found to be associated with this inhibitor (see below and Figure 6 for a more detailed analysis). Moreover, Western blot analysis of the extracts carried after p21 immunodepletion shows that after exposure to both drugs p21 targets a significant population of cyclin B1-Cdk1 molecules (Figure 2B). These results are consistent with the proposal that binding of p21 to cyclin B1-Cdk1 complexes leads to their nuclear retention. This, in turn, would block recruitment of cyclin B1-Cdk1 to the centrosome and its activation by Cdc25 family phosphatases, which are mostly cytoplasmic in G2 phase. Alternatively, p21 could prevent directly Cdc25-dependent dephosphorylation of Cdk1 as shown for cyclin-Cdk2 complexes (Saha et al., 1997). If this hypothesis is true, cyclin B1 would not accumulate in the nucleus of cells lacking p21 after treatment with genotoxic drugs, and consequently, activation of cyclin B1-Cdk1 at the centrosome would not be hampered.
Nuclear Accumulation of Cyclin B1 Is p21 Dependent
To check this hypothesis, we studied human fibroblasts expressing the HPV16-E6 oncogene (referred to as E6 cells), which contain very low amounts of p21 (see also Figure 5A; Baus et al., 2003). We have previously shown that in the presence of ICRF-193, E6 cells transiently arrest in G2 phase cells but eventually enter mitosis with active cyclin B1-Cdk1 (Baus et al., 2003). We examined the localization of cyclin B1 in synchronized E6 cells untreated (G2/M) or exposed to ICRF-193 (10 h). In contrast to wild-type cells (Figure 1A), cyclin B1 failed to accumulate in the nucleus of E6 cells arrested in G2 phase. Instead, it was mostly localized in the cytoplasm but was readily detectable at the centrosomes (Figure 3, A and B). Most importantly, as determined by immunostaining with an anti-PS133 antibody (Jackman et al., 2003), these centrosomal cyclin B1-Cdk1 complexes seemed to be active (Figure 3C). In contrast, no PS133 signal could be detected at the centrosomes of ICRF-193-arrested wild-type NHFs. Centrosomal staining with an anti-PS133 signal together with an elevated Mpm2 signal in drug-treated E6 cells led us to conclude that these cells were not truly arrested in G2 phase but were in fact undergoing early mitotic events (Figure 3D). This is in agreement with the observation that in drug-arrested E6 cells, cyclin B1-associated kinase activity, albeit weak in comparison with mitotic cells, is significantly higher than that of cyclin B1 complexes in G2-arrested wild-type NHFs (Dulic et al., 1998; Passalaris et al., 1999; Baus et al., 2003).
To eliminate the possible contribution of other p53 (or E6) targets, we studied cyclin B1 localization in response to DNA damage (ICRF-193 and γ-irradiation) in asynchronously growing p21 null fibroblasts (Wei et al., 2001). Unlike their wild-type counterparts (LF1), p21-/- NHF failed to block pRb phosphorylation and efficiently inactivate cyclin B1-associated kinase activity in the presence of ICRF-193 (Figure 4, A and B), thus confirming our earlier observation in E6 cells (Baus et al., 2003). As shown by Western blot analysis of cyclin B1 immunoprecipitates (Figure 4B), cyclin B1 complexes from ICRF-193-treated wild-type cells contained increased levels of p21 as well as both unphosphorylated (isoform 1) and hyperphosphorylated (isoform 3) Cdk1. Although in ICRF-193-treated p21-/- NHF most of cyclin B1 was associated with hyperphosphorylated Cdk1 (isoform 3), elevated cyclin B1-associated kinase activity further confirmed that p21 also participates in inactivation of Cdk1.
In addition, immunofluorescence experiments clearly showed that in p21-/- NHF cyclin B1 remained in the cytoplasm both after the treatment with ICRF-193 and γ-rays (Figure 4C). Moreover, like in E6 cells, in most of the p21-/- cells cyclin B1 was strongly localized at the centrosome and cells undergoing mitosis could be readily observed. Counterstaining with anti-γ-H2AX antibody revealed numerous DNA-damage-resulting foci that were present even in mitotic cells (Figure 4B).
Together, these results strongly imply that p21 exerts a direct role in negatively regulating mitotic entry by interfering with recruitment of active cyclin B1-Cdk1 complexes to the centrosome. They are also consistent with the possibility that, by associating to p21, cyclin B1-Cdk1 could not be activated in the cytoplasm or in the nucleus.
Cyclin B1 Nuclear Sequestration Increases in Cells with High p21 Levels
To further strengthen our hypothesis that, in response to genotoxic stress, nuclear sequestration of cyclin B1-Cdk1 by p21 impedes its activation, we investigated cyclin B1 localization in NHFs containing high amounts of endogenous p21. To this end, we used NHF expressing HPV16-E7 oncoprotein (E7 cells, described in Baus et al., 2003; Figure 5A) that degrades pocket proteins of the retinoblastoma family (Munger et al., 2001). Despite high p21 levels (Figure 5A), E7 cells proliferate well because this inhibitor is virtually absent from S- and M-phase cells (Figure 5C; our unpublished data). Thus, the E7 cell model provides a clear advantage in comparison to cell lines that ectopically overexpress p21, because the latter cannot proliferate (Smits et al., 2000). Moreover, we have recently shown that in E7 cells p21 is fully functional, because after treatment with genotoxic agents, G2 arrest and Cdk inactivation correlated with a strong association of p21 with cyclin-Cdk complexes (Baus et al., 2003), Figure 5A).
In most untreated E7 cells, the centrosomal and nuclear localization of cyclin B1 correlated well with mitotic entry as monitored by the DNA condensation pattern and increasing Mpm2 signal (Figure 5B). In E7 cultures, one also could observe some cells containing nuclear cyclin B1 (Figure 5B, cell 1), which are most likely arrested in G2 phase as indicated by a weak Mpm2 signal, the presence of p21 (our unpublished data), and a lack of chromosome condensation. In striking contrast to E6 cells, in response to ICRF-193 cyclin B1 mainly accumulated in the nucleus of E7 cells (Figure 5, B and C). Moreover, these cells were arrested in G2 phase as documented by Mpm2 staining (Figure 5B), lack of DNA condensation, and absence of cyclin B1 at unseparated centrosomes (our unpublished data). Importantly, cyclin B1 nuclear accumulation closely correlated with a dramatically elevated p21 signal (Figure 5C).
p21 Binds to Inactive Cyclin B1-Cdk1 Complexes
This exceptionally strong nuclear colocalization of cyclin B1 and p21 in G2 arrested E7 cells allowed us to test whether cyclin B1 nuclear accumulation is concomitant to binding to p21. We first compared cyclin B1 immunoprecipitates isolated from E7 cells to those from E6 cells, which have low p21 levels (Baus et al., 2003). In both E6 and E7 cells, the presence of ICRF-193 induced strong accumulation of cyclin B1-Cdk1 complexes but, in comparison with drug-treated E6 cells, in E7 cells cyclin B1-associated kinase activity was extremely low (Figure 6A). As judged by the results shown in Figure 6, A and B, the low kinase activity of these complexes in E7 cells could be attributed to their increasing association with p21.
To determine more precisely the proportion of cyclin B1-Cdk1 complexes in ICRF-193-arrested E7 cells that is actually bound to p21, we compared cyclin B1 immunoprecipitates isolated before and after removal of all p21-associated proteins by immunodepletion. As shown in Figure 6, B and C, in drug-treated cells, the majority of cyclin B1-Cdk1 complexes was bound to and thus most likely kept inactive by p21. This is also the first evidence showing that cyclin B1-Cdk1 can massively associate with normally regulated p21 even in the absence of ectopic p21 overexpression. Furthermore, together with the previous observation that cyclin B1 and p21 colocalize in some cells even in untreated E7 cultures (Figure 5B), these results provide an explanation for the low cyclin B1-associated kinase activity observed in these cells (Figure 6A).
In addition, p21 immunodepletion experiments allowed us to identify the specific cyclin B1-Cdk1 complexes that are targeted by p21 and thus presumably retained in the nucleus. As shown in Figure 6C, p21-bound cyclin B1 complexes contain both hyperphosphorylated (isoform 3) and hypophosphorylated (isoform 1) Cdk1. Isoform 3 is phosphorylated on Thr161 by CAK (Cdk-activating kinase) and on Thr14 and on Tyr15 by Myt1 and Wee1/Mik1 kinases, respectively (Porter and Donoghue, 2003). Its accumulation in inactive cyclin B1 complexes implies that p21 blocks, directly or indirectly by nuclear sequestration, dephosphorylation of Cdk1 by Cdc25 (also see Figure 2B). Isoform 1 would correspond, a priori, to an active enzyme (Figure 6D, arrow) whose Thr14 and Tyr15 residues are dephosphorylated by Cdc25, whereas Thr161 remains phosphorylated (Morgan, 1995). However, our results suggest that this fastest migrating isoform corresponds mainly to unphosphorylated Cdk1 because it is only weakly phosphorylated on Thr161 in comparison with E6 cells, where this isoform is much less abundant (Figure 6E). Note that the intermediate and partially active Thr14-dephosphorylated isoform 2 of Cdk1 (Borgne and Meijer, 1996) is largely absent in inactive cyclin B1 complexes isolated from G2-arrested cells (Figure 2A). Although the complexes between cyclin B1 and unphosphorylated Cdk1 have not been described in vivo (Ducommun et al., 1991), they are probably stabilized in the nucleus in the presence of elevated p21 levels (cf. Smits et al., 2000; Baus et al., 2003). Together, these results further support our hypothesis that, by binding to cyclin B1-Cdk1 complexes, p21 causes their nuclear retention and blocks the activation of Cdk1 at different stages.
P21 Blocks Activation of Cyclin B1-Cdk1 by Cdc25
Whereas inhibition of CAK-dependent phosphorylation of different Cdks by p21 is well documented (Aprelikova et al., 1995; Kaldis et al., 1998), only one report shows that p21 could interfere with activation of cyclin-Cdk2 by Cdc25 in vitro (Saha et al., 1997). Our results showing that p21 associates with hyperphosphorylated Cdk1 (Figures 2 and 6) raised a possibility that this inhibitor also could prevent activation of cyclin B1-Cdk1 by Cdc25-dependent dephosphorylation. To explore this possibility, we exposed cyclin B1 immunoprecipitates isolated from different cells arrested in S or G2 phases to recombinant Cdc25B and analyzed them subsequently for Cdk1 phosphorylation status and associated histone H1 kinase activity (Figure 7). Indeed, Cdc25B could readily activate cyclin B1-Cdk1 complexes isolated from p21-deficient cells such as HeLa cells arrested in S phase by thymidine block (Figure 7A, lanes 1 and 2), E6-expressing NHFs arrested in G2 phase by ICRF-193 (lanes 3 and 4) or in S/G2 by bleomycin (lanes 5 and 6). Note a diminution of isoform 3 and an increase of isoforms 1 and 2 in Cdc25-treated immunoprecipitates.
In contrast, Cdc25B was unable to dephosphorylate Cdk1 isoform 3 and activate cyclin B1 immunocomplexes isolated from ICRF-treated E7-expressing fibroblasts (Figure 7B, lanes 1 and 2) and p21-bound cyclin B1-Cdk1 complexes isolated from ICRF-treated wild-type NHF cultures (lanes 5-8). On the other hand, under these experimental conditions Cdc25B could readily activate cyclin B1-Cdk1 from untreated wild-type NHFs (WT-CT), which lacked p21 (lanes 3 and 4). These results point toward a novel way by which p21 could sustain G2 arrest and prevent efficiently (and permanently) mitotic progression.
DISCUSSION
The results presented in this article describe the molecular mechanisms underlying long-term inactivation of cyclin B1-Cdk1 in response to DNA damage in NHFs. The activity of cyclin B1-Cdk1, which plays a crucial role in controlling the initiation and progression of mitosis, is tightly regulated at multiple levels. In cycling cells, cyclin B1-Cdk1 complexes are kept inactive during S and G2 phases by inhibitory phosphorylations on Cdk1 at Thr14 and Tyr15 (by Myt1 and Wee1 kinases), and they are retained in the cytoplasm due to an active nuclear export (Hagting et al., 1998; Yang et al., 1998). The onset of prophase correlates with dephosphorylation of Cdk1 by Cdc25 family phosphatases, phosphorylation of cyclin B1, and a rapid nuclear accumulation of active cyclin B1-Cdk1 (rev. in (Porter and Donoghue, 2003). In the presence of DNA damage, activation of cyclin B1-Cdk1 and mitotic entry are initially blocked by Chk1/2-dependent inhibition of Cdc25 members, an event that does not require functional p53 (Taylor and Stark, 2001). G2 arrest is further assured by nuclear exclusion of cyclin B1-Cdk1 (Jin et al., 1998; Yang et al., 1998, 2001; Deming et al., 2001), which is achieved by binding to the adaptor protein 14-3-3σ, a transcriptional target of p53 (Hermeking et al., 1997; Chan et al., 1999; van Hemert et al., 2001). However, because 14-3-3σ is mainly expressed in epithelial cells (Hermeking et al., 1997), it is likely that alternative mechanisms may contribute to sustain cyclin B1-Cdk1 inactivation in other cell types.
Another p53 target, p21Waf1/Cip1 (p21), does not seem to control directly the initiation of DNA damage-induced G2 arrest, but accumulating evidence suggests that this Cdk inhibitor plays a major role in its maintenance (Bunz et al., 1998; Ohi and Gould, 1999; Taylor and Stark, 2001). We have recently shown that this could be due to its ability to block the Cdk-dependent phosphorylation of pocket proteins (Baus et al., 2003). Interestingly, in spite of its strong association with cyclin A-Cdk1 (Baus et al., 2003), data suggest that cyclin B1-Cdk1 complexes are not an important target of p21 (Winters et al., 1998; Passalaris et al., 1999; Flatt et al., 2000; Smits et al., 2000; Deming et al., 2001).
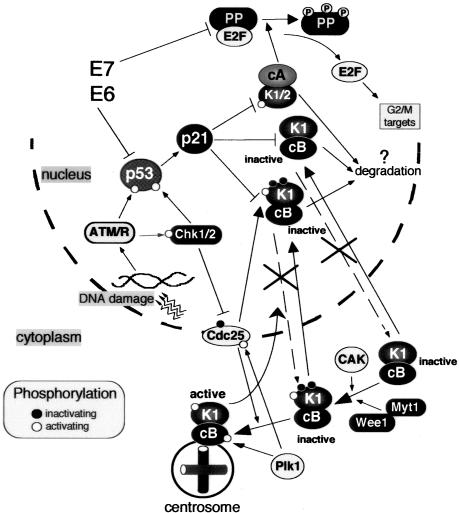
However, in this article we present evidence that in NHFs, p21 actually could play a direct role in preventing activation of cyclin B1-Cdk1 in response to genotoxic stress. We show that in the presence of nonrepairable DNA damage occurring in S and G2 phases NHF accumulate cyclin B1 in the nucleus without apparent signs of mitotic events. Moreover, our results suggest that this nuclear retention of inactive cyclin B1-Cdk1 complexes is caused by binding to p21. This observation gains its importance in view of the recent reports showing that in proliferating cells cyclin B1-Cdk1 is already activated at the centrosomes (Hirota et al., 2003; Jackman et al., 2003), implying that some early mitotic events could be initiated while cyclin B1 is still mainly in the cytoplasm (Pines and Rieder, 2001). In light of the latter observation, it may seem contradictory that cyclin B1 could localize in the nucleus without initiation of mitosis. This apparent contradiction could be resolved by our results showing that, by binding to and sequestering cyclin B1-Cdk1 in the nucleus, p21 inhibits their activation which takes place either in the cytoplasm/centrosome (De Souza et al., 2000) or/and in the nucleus. In this way, the cells expressing p21 could efficiently block initiation of early mitotic events (Figure 8).
Figure 8.
Role of p21 in establishing irreversible cell cycle arrest in G2 after genotoxic stress. In response to DNA damage, G2 arrest is initiated by Chk1/2-dependent inactivation of Cdc25 family phosphatases (Cdc 25A, B, C), thus preventing activation of cyclin B1-Cdk1. The maintenance of G2 arrest is assured by p21-dependent inactivation of mitotic Cdks (cyclin B1-Cdk1 and cyclin A-Cdk1) and their eventual degradation. Cyclin B1-Cdk1 complexes are maintained in an inactive state by association with p21, which provokes nuclear retention of cyclin B1-Cdk1 and prevents their activation at the centrosome and, possibly, in the cytoplasm and nucleus. By binding to cyclin B1-Cdk1, p21 prevents both activation by CAK-dependent phosphorylation of Thr161 (white circle) and Cdc25-dependent dephosphorylation of Thr14 and Tyr15 (black circles). For the sake of simplicity, Wee1 and CAK, which are predominantly in the nucleus, are placed in cytoplasm. Additionally, p21 inactivates cyclin-Cdk complexes (such as cyclin A-Cdk2) responsible for phosphorylation of pocket proteins (PP; pRB, p107, and p130) during S/G2/M progression. Subsequent accumulation of active pocket proteins blocks expression of genes involved in G2/M progression (Ren et al., 2002; Baus et al., 2003). Inactivation of either p53 or pocket proteins by expression of viral oncogenes (such as HPV16-E6 and HPV16-E7), does not interfere with the cell cycle arrest in G2 but compromises the irreversible cell cycle exit. Black and white circles denote inactivating and activating phosphorylations (P), respectively.
Biochemical analysis of cyclin B1-Cdk1 complexes isolated from arrested cells supports entirely the hypothesis that p21 interferes with both CAK-mediated phosphorylation of Thr161 and with Cdc25-mediated dephosphorylation of Thr14 and Tyr15 (Figure 7 and cf. Baus et al., 2003). Moreover, our results showing that p21 directly blocks the latter event are in agreement with an earlier work indicating that Cdc25A-cyclin-Cdk2 association is inhibited by p21 (Saha et al., 1997).
The critical role of p21 in nuclear sequestration and blocking activation of cyclin B1-Cdk1 is underscored by our results showing that exposure of p21-deficient or null fibroblasts to genotoxic stress does not induce nuclear retention of cyclin B1. As a consequence, these cells fail to prevent efficiently centrosomal recruitment and activation of cyclin B1-Cdk1 and are unable to undergo a permanent G2 arrest (cf. Passalaris et al., 1999; Baus et al., 2003). Conversely, in HPV16-E7-expressing fibroblasts containing high endogenous levels of p21, after exposure to genotoxic drugs virtually all cyclin B1-Cdk1 complexes are associated with p21 and remain inactive in the nucleus. Although previous work indicated that, in carcinoma cells with inducible p53 or p21 levels, DNA damage also could lead to nuclear accumulation of cyclin B1 (Winters et al., 1998; Smits et al., 2000), these experiments failed to show a significant association between p21 and cyclin B1-Cdk1. Therefore, this is the first report showing that, in response to a genotoxic insult, endogenous p21 could massively bind to cyclin B1-Cdk1. Our previous results, obtained in synchronized NHFs, implied that premitotic nuclear accumulation of cyclin B1 and p21 could be an integral part of G2/M progression (Dulic et al., 1998). Because this event is rarely observed in asynchronously proliferating early-passage wild-type NHFs (Baus and Dulic, unpublished data), we assume that its occurrence has been provoked by increased levels of p21 induced by hydroxyurea or aphidicolin used in the synchronization protocol.
The question is why, in addition to Cdc25 pathway, the cells also would need p21 to prevent cyclin B1-Cdk1 activation? One possibility is that, by (permanently?) locking cyclin B1-Cdk1 in nonactivable complexes (bound for degradation?), normal fibroblasts take advantage of an additional level of security thereby ensuring the irreversibility of the G2 arrest (Figure 8). As mentioned above, in other cell types a stable G2 arrest was proposed to be achieved by a 14-3-3σ-mediated nuclear exclusion of cyclin B1-Cdk1 complexes (Jin et al., 1998; Toyoshima et al., 1998; Chan et al., 1999). Our results, which were obtained in normal fibroblasts with a priori intact cell cycle control pathways, indicate that p21-dependent nuclear sequestration of cyclin B1-Cdk1 may have evolved as an alternative safeguard mechanism to block permanently mitotic entry. The respective contribution of p21 and 14-3-3σ-dependent mechanisms to this block should be further evaluated in normal cell types of different origin.
In conclusion, we propose that p21 exerts a dual role in mediating stress-induced cell cycle arrest and irreversible exit before mitosis (Figure 8). Its first role would be to stably prevent activation of mitotic cyclin-Cdk complexes by directly inactivating cyclin A-Cdk1/2, by maintaining the inactive state of cyclin B1-Cdk1 by nuclear sequestration, and by inhibiting CAK- and Cdc25-dependent activation. Indeed, it has been shown that p21 interferes with activation of cyclin A-Cdk2 (Aprelikova et al., 1995; Saha et al., 1997; Kaldis et al., 1998). The second role of p21 would be to block inactivating phosphorylations of pocket proteins by Cdks (Figure 8). Subsequent accumulation of active pocket proteins would drive permanent cell cycle exit, at least partially by sequestering E2F factors playing a role in the G2/M transition (Ren et al., 2002; Baus et al., 2003). Thus, in addition to its well-established role in the G1/S cell cycle checkpoint, p21 seems to be a crucial element for G2 arrest, the ultimate barrier to the entry of cells with damaged chromosomes into mitosis.
Supplementary Material
Acknowledgments
We thank Dr. Marcel Dorée for encouragement and invaluable comments in the course of this work; and Drs. Daniel Fisher, Naomi Taylor, Jean-Claude Labbé, and Annick Péléraux for critically reading the manuscript. Special thanks go to Dr. Pierre Travo (Platform for Imagery, Centre de Recherche de Biochimie Macromoléculaire) for enthusiastic help in immunofluorescence experiments and Dr. N. Taylor for the 2% O2 chamber. We also are grateful to Drs J. Shay and D. Galloway for reagents used in retroviral transfections, to Dr. A.M. Creighton for the generous gift of ICRF-193, to Drs. M. Jackman and J. Pines for phosphospecific anti-cyclin B1 antibodies, and to Dr. V. Baldin for providing recombinant Cdc25B-GST. F.B. was recipient of a fellowship from the Ministère de l'Education Nationale et de la Recherche, and V.G. is recipient of a fellowship from the Association de la Recherche sur le Cancer. This work was supported by grants from La Ligue nationale contre le cancer (équipe labelisée), Association de la Recherche sur le Cancer (to J.P), and Ministère de l'Education Nationale et de la Recherche (to J.P).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-12-0871. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0871.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Aprelikova, O., Xiong, Y., and Liu, E.T. (1995). Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270, 18195-18197. [DOI] [PubMed] [Google Scholar]
- Barboule, N., Lafon, C., Chadebech, P., Vidal, S., and Valette, A. (1999). Involvement of p21 in the PKC-induced regulation of the G2/M cell cycle transition. FEBS Lett. 444, 32-37. [DOI] [PubMed] [Google Scholar]
- Baus, F., Gire, V., Fisher, D., Piette, J., and Dulic, V. (2003). Permanent cell cycle exit in G2 phase after DNA damage in normal human fibroblasts. EMBO J. 22, 3992-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, L.D., and Gould, K.L. (1996). Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog. Cell Cycle Res. 2, 99-105. [DOI] [PubMed] [Google Scholar]
- Borgne, A., and Meijer, L. (1996). Sequential dephosphorylation of p34(cdc2) on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 271, 27847-27854. [DOI] [PubMed] [Google Scholar]
- Bunz, F., Dutriaux, A., Lengauer, C., Waldman, T., Zhou, S., Brown, J.P., Sedivy, J.M., Kinzler, K.W., and Vogelstein, B. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497-1501. [DOI] [PubMed] [Google Scholar]
- Cans, C., Sert, V., De Rycke, J., Baldin, V., and Ducommun, B. (1999). Use of CDC2 from etoposide-treated cells as substrate to assay CDC25 phosphatase activity. Anticancer Res. 19, 1241-1244. [PubMed] [Google Scholar]
- Chan, T.A., Hermeking, H., Lengauer, C., Kinzler, K.W., and Vogelstein, B. (1999). 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature 401, 616-620. [DOI] [PubMed] [Google Scholar]
- Chan, T.A., Hwang, P.M., Hermeking, H., Kinzler, K.W., and Vogelstein, B. (2000). Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 14, 1584-1588. [PMC free article] [PubMed] [Google Scholar]
- Davis, F.M., Tsao, T.Y., Fowler, S.K., and Rao, P.N. (1983). Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA 80, 2926-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, C.P., Ellem, K.A., and Gabrielli, B.G. (2000). Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events. Exp. Cell Res. 257, 11-21. [DOI] [PubMed] [Google Scholar]
- Deming, P.B., Cistulli, C.A., Zhao, H., Graves, P.R., Piwnica-Worms, H., Paules, R.S., Downes, C.S., and Kaufmann, W.K. (2001). The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA 98, 12044-12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes, C.S., Clarke, D.J., Mullinger, A.M., Gimenez-Abian, J.F., Creighton, A.M., and Johnson, R.T. (1994). A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature 372, 467-470. [DOI] [PubMed] [Google Scholar]
- Ducommun, B., Brambilla, P., Felix, M.A., Franza, B.R., Jr., Karsenti, E., and Draetta, G. (1991). cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 10, 3311-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic, V., Stein, G.H., Far, D.F., and Reed, S.I. (1998). Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18, 546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt, P.M., Tang, L.J., Scatena, C.D., Szak, S.T., and Pietenpol, J.A. (2000). p53 regulation of G(2) checkpoint is retinoblastoma protein dependent. Mol. Cell. Biol. 20, 4210-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting, A., Jackman, M., Simpson, K., and Pines, J. (1999). Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 9, 680-689. [DOI] [PubMed] [Google Scholar]
- Hagting, A., Karlsson, C., Clute, P., Jackman, M., and Pines, J. (1998). MPF localization is controlled by nuclear export. EMBO J. 17, 4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.W., et al. (1995). Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6, 387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald, R., McLoughlin, M., and McKeon, F. (1993). Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell 74, 463-474. [DOI] [PubMed] [Google Scholar]
- Hermeking, H., Lengauer, C., Polyak, K., He, T.C., Zhang, L., Thiagalingam, S., Kinzler, K.W., and Vogelstein, B. (1997). 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1, 3-11. [DOI] [PubMed] [Google Scholar]
- Hirota, T., Kunitoku, N., Sasayama, T., Marumoto, T., Zhang, D., Nitta, M., Hatakeyama, K., and Saya, H. (2003). Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 114, 585-598. [DOI] [PubMed] [Google Scholar]
- Jackman, M., Lindon, C., Nigg, E.A., and Pines, J. (2003). Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5, 143-148. [DOI] [PubMed] [Google Scholar]
- Jin, P., Hardy, S., and Morgan, D.O. (1998). Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 141, 875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldis, P., Russo, A.A., Chou, H.S., Pavletich, N.P., and Solomon, M.J. (1998). Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol. Biol. Cell 9, 2545-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levedakou, E.N., Kaufmann, W.K., Alcorta, D.A., Galloway, D.A., and Paules, R.S. (1995). p21CIP1 is not required for the early G2 checkpoint response to ionizing radiation. Cancer Res. 55, 2500-2502. [PubMed] [Google Scholar]
- Mikhailov, A., Cole, R.W., and Rieder, C.L. (2002). DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr. Biol. 12, 1797-1806. [DOI] [PubMed] [Google Scholar]
- Morgan, D.O. (1995). Principles of CDK regulation. Nature 374, 131-134. [DOI] [PubMed] [Google Scholar]
- Munger, K., Basile, J.R., Duensing, S., Eichten, A., Gonzalez, S.L., Grace, M., and Zacny, V.L. (2001). Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20, 7888-7898. [DOI] [PubMed] [Google Scholar]
- Norbury, C., Blow, J., and Nurse, P. (1991). Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 10, 3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, R., and Gould, K.L. (1999). Regulating the onset of mitosis. Curr. Opin. Cell Biol. 11, 267-273. [DOI] [PubMed] [Google Scholar]
- Passalaris, T.M., Benanti, J.A., Gewin, L., Kiyono, T., and Galloway, D.A. (1999). The G(2) checkpoint is maintained by redundant pathways. Mol. Cell. Biol. 19, 5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines, J., and Hunter, T. (1989). Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58, 833-846. [DOI] [PubMed] [Google Scholar]
- Pines, J., and Rieder, C.L. (2001). Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3, E3-E6. [DOI] [PubMed] [Google Scholar]
- Porter, L.A., and Donoghue, D.J. (2003). Cyclin B1 and Cdk 1, nuclear localisation and upstream regulators. Prog. Cell Cycle Res. 5, 335-447. [PubMed] [Google Scholar]
- Ren, B., Cam, H., Takahashi, Y., Volkert, T., Terragni, J., Young, R.A., and Dynlacht, B.D. (2002). E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 16, 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca, J., Ishida, R., Berger, J.M., Andoh, T., and Wang, J.C. (1994). Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc. Natl. Acad. Sci. USA 91, 1781-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou, E.P., Boon, C., Redon, C., and Bonner, W.M. (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, P., Eichbaum, Q., Silberman, E.D., Mayer, B.J., and Dutta, A. (1997). p21CIP1 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol. Cell. Biol. 17, 4338-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, V.A., Klompmaker, R., Vallenius, T., Rijksen, G., Makela, T.P., and Medema, R.H. (2000). p21 inhibits thr161 phosphorylation of cdc2 to enforce the G2 DNA damage checkpoint [In Process Citation]. J. Biol. Chem. 275, 30638-30643. [DOI] [PubMed] [Google Scholar]
- Taylor, W.R., and Stark, G.R. (2001). Regulation of the G2/M transition by p53. Oncogene 20, 1803-1815. [DOI] [PubMed] [Google Scholar]
- Toyoshima, F., Moriguchi, T., Wada, A., Fukuda, M., and Nishida, E. (1998). Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 17, 2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert, M.J., Steensma, H.Y., and van Heusden, G.P. (2001). 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays 23, 936-946. [DOI] [PubMed] [Google Scholar]
- Wei, W., Hemmer, R.M., and Sedivy, J.M. (2001). Role of p14(ARF) in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21, 6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf, J.M., Rao, P.N., and Gerace, L. (1994). Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc. Natl. Acad. Sci. USA 91, 714-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters, Z.E., Ongkeko, W.M., Harris, A.L., and Norbury, C.J. (1998). p53 regulates Cdc2 independently of inhibitory phosphorylation to reinforce radiation-induced G2 arrest in human cells. Oncogene 17, 673-684. [DOI] [PubMed] [Google Scholar]
- Yang, J., Bardes, E.S., Moore, J.D., Brennan, J., Powers, M.A., and Kornbluth, S. (1998). Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 12, 2131-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Song, H., Walsh, S., Bardes, E.S., and Kornbluth, S. (2001). Combinatorial control of cyclin B1 nuclear trafficking through phosphorylation at multiple sites. J. Biol. Chem. 276, 3604-3609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.