Abstract
Rho-family GTPases Cdc42p and Rho1p play critical roles in the budding process of the yeast Saccharomyces cerevisiae. However, it is not clear how the functions of these GTPases are coordinated temporally and spatially during this process. Based on its ability to suppress cdc42-Ts mutants when overexpressed, a novel gene PXL1 was identified. Pxl1p resembles mammalian paxillin, which is involved in integrating various signaling events at focal adhesion. Both proteins share amino acid sequence homology and structural organization. When expressed in yeast, chicken paxillin localizes to the sites of polarized growth as Pxl1p does. In addition, the LIM domains in both proteins are the primary determinant for targeting the proteins to the cortical sites in their native cells. These data strongly suggest that Pxl1p is the “ancient paxillin” in yeast. Deletion of PXL1 does not produce any obvious phenotype. However, Pxl1p directly binds to Rho1p-GDP in vitro, and inhibits the growth of rho1-2 and rho1-3 mutants in a dosage-dependent manner. The opposite effects of overexpressed Pxl1p on cdc42 and rho1 mutants suggest that the functions of Cdc42p and Rho1p may be coordinately regulated during budding and that Pxl1p may be involved in this coordination.
INTRODUCTION
Rho-family GTPases regulate cytoskeletal remodeling, vesicular trafficking, and cell cycle progression in eukaryotic cells. The budding yeast Saccharomyces cerevisiae genome encodes six Rho-family members, Cdc42p and Rho1p-Rho5p, all of which are involved in some aspects of cell polarization (Cabib et al., 1998; Johnson, 1999; Pruyne and Bretscher, 2000; Roumanie et al., 2001; Schmitz et al., 2002). Cdc42p plays an essential role in the establishment of cell polarity by regulating actin and septin organization and secretion (Johnson, 1999; Adamo et al., 2001; Zhang et al., 2001; Gladfelter et al., 2002; Caviston et al., 2003). Temperature-sensitive (Ts) cdc42 mutant, such as cdc42-1 (Adams et al., 1990), is unable to polarize cell growth to the presumptive bud site at the restrictive temperature. As a result, cells grow isotropically and arrest with large, round, and unbudded cell morphology. Thus, Cdc42p is thought to function at the very early stage of the budding process through a host of its effectors including the p21-activated kinases (PAKs), Ste20p, Cla4p, and Skm1p; the formin, Bni1p; the IQGAP, Iqg1p/Cyk1p; the exocyst component, Sec3p; and a pair of yeast-specific, CRIB-containing proteins, Gic1p/Gic2p (Cvrckova et al., 1995; Brown et al., 1997; Chen et al., 1997; Evangelista et al., 1997; Osman and Cerione, 1998; Holly and Blumer, 1999; Weiss et al., 2000; Zhang et al., 2001).
Rho1p also plays an essential role in the budding process. Conditional inactivation of Rho1p function by Ts mutations or depletion results in major defects in cell wall integrity and also in polarized actin organization and secretion (Yamochi et al., 1994; Drgonova et al., 1996; Qadota et al., 1996; Guo et al., 2001). As a result, rho1 mutants are arrested at early stages of the budding process, with either unbudded or small-budded cell morphology that is often associated with cell lysis. The functions of Rho1p in the budding process are mediated by some of its effectors including Bni1p; Sec3p; the protein kinase C, Pkc1p; and the cell-wall remodeling enzymes, Fks1p/Fks2p (Mazur et al., 1995; Nonaka et al., 1995; Kohno et al., 1996; Imamura et al., 1997; Andrews and Stark, 2000; Guo et al., 2001; Dijkgraaf et al., 2002). Even though Cdc42p and Rho1p are involved in related processes during budding and share some effectors, its is not clear how the functions of these two GTPases are coordinated temporally and spatially during polarized growth to ensure successful budding.
A number of proteins that regulate cell proliferation, differentiation, and cell motility, contain LIM (Lin-11, Isl-1, Mec-3) domains, which generally comprise ∼50-60 amino acids and are thought to mediate protein-protein interactions (Dawid et al., 1998). Through the chelation of one zinc molecule by four amino acids including cysteine, histidine, or aspartic acid, each LIM domain forms two zinc finger structures separated by two amino acids, [CXXCX16-23 HXX(H/C)]-XX-[CXXCX16-21CXX(D/H/C)] (X is any amino acid; Freyd et al., 1990; Michelsen et al., 1993). In cultured fibroblasts, many LIM proteins in the zyxin/paxillin family, including zyxin, paxillin, Hic-5, and LPP, all target to focal adhesions, which are membrane attachment sites to the extracellular matrix, and participate in integrin signaling (Turner, 2000a, 2000b).
We have identified a novel LIM domain-containing protein, Pxl1p in S. cerevisiae, from our genetic screens. Overexpression of PXL1 on high-copy plasmids suppressed the Ts growth of cdc42 mutants, suggesting Pxl1p may function in Cdc42p signaling pathway. We also show that Pxl1p directly binds to Rho1p-GDP in vitro and downregulates Rho1p function. Thus, our findings suggest that the functions of Cdc42p and Rho1p may be coordinately regulated during budding, which may involve the function of Pxl1p.
MATERIALS AND METHODS
Strains and Growth Conditions
Yeast strains used in this study are listed in Table 1. Standard culture media and genetic techniques were used (Guthrie and Fink, 1991). Escherichia coli strains DH12S (Life Technologies, Gaithersburg, MD) and BL21 (DE3; Novagen, Madison, WI) were used as a plasmid host and for protein expression, respectively.
Table 1.
Saccharomyces cerevisiae strains used in this study
| Name | Genotype | Source |
|---|---|---|
| YEF473 | a/α his3/his3 leu2/leu2 lys2/lys2 trp1/trp1 ura3/ura3 | Bi and Pringle (1996) |
| YEF473A | ahis3 leu2 lys2 trp1 ura3 | Bi and Pringle (1996) |
| YEF2258 | ahis3 leu2 lys2 trp1 ura3 cdc42-201 | Zhang et al. (2001) |
| JPC241 | ahis3 leu2 lys2 trp1 ura3 cdc42G60D | Caviston et al. (2002) |
| JPC261 | As YEF473 except pxl1Δ::kanMX6/PXL1 | This study |
| JPC262 | As YEF473 except PXL1-GFP-kanMX6/PXL1 | This study |
| JPC265 | ahis3 leu2 trp1 ura3 lys2 pxl1Δ::kanMX6 | This study |
| JPC266 | α his3 leu2 trp1 ura3 lys2 pxl1Δ::kanMX6 | This study |
| JPC267 | ahis3 leu2 trp1 ura3 lys2 PXL1-GFP-kanMX6 | This study |
| JPC272 | ahis3 leu2 trp1 ura3 lys2 pxl1Δ::His3MX6 | This study |
| JPC273 | α his3 leu2 trp1 ura3 lys2 pxl1Δ::His3MX6 | This study |
| NY2284 | α ura3 leu2 trp1 his3 ade2 lys2 rho1::HIS3 ade3::[pRHO1-RHO1:LEU2] | Guo et al. (2001) |
| NY2285 | aura3 leu2 trp1 his3 ade2 lys2 rho1::HIS3 ade3::[pRHO1-rho1-2:LEU2] | Guo et al. (2001) |
| NY2286 | α ura3 leu2 trp1 his3 ade2 lys2 rho1::HIS3 ade3::[pRHO1-rho1-3:LEU2] | Guo et al. (2001) |
| NY2287 | aura3 leu2 trp1 his3 ade2 lys2 rho1::HIS3 ade3::[pRHO1-rho1-5:LEU2] | Guo et al. (2001) |
Construction of Plasmids
Plasmids used in this study include YEp24 (2 μ, URA3), YEp352 (2 μ, URA3), pFA6a-kanMX6, pFA6a-His3MX6, and pFA6a-GFP (S65T, F64L)-kanMX6 (Longtine et al., 1998), pUG36 (CEN, URA3, pMET25-yEGFP3; kindly supplied by Dr. J. H. Hegemann). YEp24-PXL1 was isolated from the genetic screen. PXL1 full-length and various fragments were amplified from the YEp24-PXL1 plasmid by PCR and subsequently cloned into pUG36 vector. Details for these constructs are available upon request. A BamHI-EcoRI fragment containing the full-length chicken paxillin (1-559) and a PstI-EcoRI fragment containing the C-terminal portion of paxillin (309-559) from plasmid pcDNA3-paxillin (Brown et al., 1996) were cloned into pUG36. The N-terminal portion of paxillin (1-325) was amplified by PCR as a BamHI-EcoRI fragment and cloned into pUG36. YEp24-GAL-PXL1 was constructed by tagging of PXL1 in YEp24 with the kanMX6-pGAL1 module by a PCR-mediated method (Longtine et al., 1998). To construct pMAL-c2X-PXL1 that expresses an MBP-Pxl1p fusion protein, a 2.1-kb DNA fragment containing PXL1 gene flanked by two SalI sites was generated by PCR, and inserted into SalI-digested pMAL-c2X vector (New England Biolabs, Beverly, MA). The constructs expressing GST-Cdc42p and GST-Rho1p fusion proteins were kindly provided by Dr. Wei Guo (University of Pennsylvania; Guo et al., 2001; Zhang et al., 2001). Galactoseinducible overexpression of Cdc42p and Rho1p was achieved by using YEp352-GAL-CDC42 and pYES2-RHO1 plasmids. pYES2-RHO1 was constructed by cloning PCR-amplified BamHI-EcoRI fragment of RHO1 into pYES2 (Invitrogen, Carlsbad, CA).
Construction of Yeast Strains
To make strains carrying pxl1Δ, one copy of PXL1 in strain YEF473 was replaced by a kanMX6 module by a PCR-based method (Longtine et al., 1998). The resulting diploid strain JPC261 was sporulated and tetrads were dissected to generate the haploid strains JPC265 and JPC266. The KanMX6 module in strains JPC265 and JPC266 was later substituted with a His3MX6 module to generate JPC272 and JPC273, respectively. To construct a C-terminal PXL1-GFP fusion on the chromosome, one copy of PXL1 in strain YEF473 was tagged with GFP (S65T, F64L)-kanMX6 module (Longtine et al., 1998). The resulting diploid strain JPC262 was sporulated and tetrads were dissected to generate JPC267.
Genetic Screens
In the first screen, yeast strain JPC241 (a cdc42G60D) was transformed with a YEp24-based genomic DNA library (Carlson and Botstein, 1982) and grown on SC-Ura plates at 35°C. From ∼82,000 transformants screened, 75 suppressor-carrying plasmids were isolated and determined to carry two known genes, CDC42 (71×) and MSB1 (2×), and one novel gene, PXL1 (2×). Among the latter group, further subcloning and deletion analysis showed that PXL1 was solely responsible for the suppression. In the second screen, strain YEF2258 (a cdc42-201) was transformed with the same DNA library and grown on SC-Ura plates containing 1 M Sorbitol at 35.5°C. From ∼65,000 transformants, 10 suppressor-carrying plasmids were isolated and determined to carry CDC42 (3×), MSB1 (3×), YHR149C (2×), MSB3 (1×), and PXL1 (1×).
Microscopy and Immunoblotting
For localization of GFP-tagged proteins, cells were grown exponentially in YPD or SC-Ura media at 24°C. Differential interference contrast (DIC) and fluorescence microscopy were performed using Nikon Microscope ECLIPSE E800 (Nikon Corporation, Tokyo, Japan) with a 60× Plan Apo objective. The images were acquired using Phase 3 Imaging Systems (Glen, PA).
For immunoblotting, the mouse monoclonal anti-GFP antibody (BAbCO, Richmond, CA) and horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Roche Molecular Biochemicals, Indianapolis, IN) were used. Proteins were detected with the ECL Western blotting detection reagents.
In Vitro Protein-binding Assay
The recombinant MBP-Pxl1p, GST-Cdc42p and GST-Rho1p fusion proteins were purified from E. coli strain BL21. GST fusion proteins of Cdc42p and Rho1p were first stripped off nucleotide and then loaded with either GDP or GTP-γ-S. To do this, 15 μg of GST alone or a GST fusion protein was first incubated in the preloading buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 5 mM DTT, and 10 mM GDP or GTP-γ-S) at 30°C for 30 min, followed by the addition of MgCl2 to a final concentration of 25 mM, and the reaction was incubated at 24°C for 15 min. After nucleotide loading, GST fusion proteins were added to the Amylose beads containing 5-10 μg of MBP-Pxl1p fusion protein. After incubation at 4°C for 2 h, samples were washed five times with washing buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 5 mM DTT, and 0.1% Triton X-100), and the bound proteins were eluted from the beads with SDS sample buffer. Samples were analyzed by a 12% SDS-PAGE, and proteins were detected by Coomassie blue staining and immunoblotting with an anti-GST antibody.
RESULTS
Isolation of PXL1 as a Multicopy Suppressor of cdc42-Ts Alleles
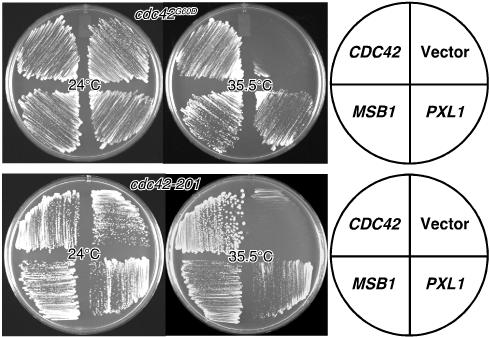
To identify additional components involved in Cdc42p signaling pathway, we performed two independent genetic screens with two recessive, Ts alleles of CDC42, cdc42-201 (Zhang et al., 2001) and cdc42G60D (Caviston et al., 2002; see details of the screens in Materials and Methods). The cdc42-201 allele encodes a protein with four amino acid changes (C6S, D31V, D65G, and F90G) and cells carrying this allele are elongated at the permissive temperature and are defective in the localization of the exocyst component Sec3p at the nonpermissive temperature (Zhang et al., 2001). In contrast, cdc42G60D cells form multiple buds per mother per nuclear cycle at the permissive temperature and the mutant Cdc42p appears to be hyperactive (Caviston et al., 2002). Because these cdc42 alleles cause distinct morphological defects at the permissive temperature, we thought that we might be able to identify some allele-specific multicopy suppressors. Screens with both alleles led to the identification of a novel gene, PXL1 (YKR090W). Multicopy PXL1 or MSB1, another gene isolated from both screens and a known multicopy suppressor of loss-of-function cdc24-Ts and cdc42-Ts alleles (Bender and Pringle, 1989), suppressed both cdc42-201 and cdc42G60D mutants effectively at 35.5°C (Figure 1). The suppression by MSB1 suggests that both cdc42 mutants behave as loss-of-function alleles at the nonpermissive temperature. We also found that multicopy PXL1 did not suppress the morphological defects of either cdc42 mutant significantly at the permissive temperature (our unpublished data). In addition, multicopy PXL1 failed to suppress temperature-sensitive growth phenotype of three other cdc42-Ts alleles, cdc42-1 (Adams et al., 1990), cdc42V36G (Caviston et al., 2003), and cdc42-13 (Zhang et al., 2001; our unpublished data), suggesting that the suppression of cdc42 mutants by PXL1 is allele-specific. Together, these results suggest that PXL1 is somehow involved in a Cdc42p-mediated function.
Figure 1.
Multicopy PXL1 suppresses two temperature-sensitive cdc42 mutants. Yeast strains JPC241 (cdc42G60D) and YEF2258 (cdc42-201) were transformed with YEp24 empty vector, or YEp24 carrying CDC42, PXL1, or MSB1. Transformants were streaked onto SC-Ura plates and incubated at 24°C or 35.5°C for 3 days.
Pxl1p Is a Novel LIM Domain-containing Protein
Pxl1p, a protein of 706 amino acids, contains two LIM domains at its C-terminus (Figure 2A). Database search revealed that Pxl1p displays amino acid sequence homology and similar structural organization with paxillin (Figure 2), an adaptor protein involved in focal adhesion signaling (Turner, 2000a). Pxl1p and chicken paxillin share 15% identity and 26% similarity over their entire length and both contain tandem LIM domains at their C-termini separated by an eight-amino acids linker (Figure 2). There are two leucine-rich stretches in Pxl1p, 340VEDLSLDGL348 and 372VEQLIAQLDDVSL384, which resemble the LD motifs of paxillin (Figure 2B; Brown et al., 1996, 1998; Tumbarello et al., 2002). The strongest homology between these two proteins resides in their LIM domains (Figure 2C), with 30% identity. Both LIM1 (556-612) and LIM2 (621-672) of Pxl1p resemble the LIM3 of paxillin the most in terms of the composition of eight zinc-chelating amino acids (Figure 2C). Homologous proteins with C-terminal LIM domains are also present in other fungal, including SPBC4F6.12 in Schizosaccharomyces pombe and IPF9950 in Candida albicans.
Figure 2.
Pxl1p is a LIM-domain protein and shares sequence homology with paxillin. (A) The schematic diagram of domain structure in Pxl1p and chicken paxillin, which contains five LD motifs and four LIM domains. (B) Alignment of the N-terminal portion of Pxl1p (37-555) with that of chicken paxillin (1-325) using MacVector software. Identical and similar residues are highlighted with bold letters in dark shade and regular letters in gray shade, respectively. The two lines above the sequence indicate LD motif-like sequences in Pxl1p. (C) Alignment of individual LIM domains from Pxl1p and chicken paxillin using MacVector software. Residues conserved in more than half of the aligned sequences are shaded. The eight residues that form the core of double zinc-fingers are indicated with the boxes.
Pxl1p Is Not Essential for Cell Viability and Localizes to the Sites of Polarized Growth
PXL1 is not essential for vegetative growth, as pxl1Δ cells are viable in our strain background and in the systematic deletion project (Saccharomyces Genome Database). In addition, we failed to detect any obvious morphological and growth abnormalities associated with pxl1Δ cells at temperatures of 20-37°C on plates with or without 1 M sorbitol (our unpublished data). Moreover, there was no enhanced polarity defect observed between pxl1Δ and deletion of a number of polarity genes including msb1Δ, msb3Δ msb4Δ, gic1Δ gic2Δ, bni1Δ, pea2Δ, bud6Δ, and ste20Δ.
To gain some hint on how Pxl1p might function in Cdc42p-mediated pathway, we examined the localization of Pxl1p in yeast cells. We tagged the chromosomal copy of PXL1 with GFP to its C-terminus. The PXL1-GFP construct thus functioned as the sole source of PXL1 gene under the control of its own promoter. Similar to Cdc42p and other polarity proteins, Pxl1p-GFP localized to the sites of polarized growth in a cell cycle-regulated manner (Figure 3A). It first appeared at the presumptive bud site. After bud emergence, it formed a cap at the tip of a small bud. As the bud enlarged, the Pxl1p-GFP cap gradually diffused and eventually became invisible. At the time of cytokinesis, Pxl1p-GFP appeared at the mother-bud neck.
Figure 3.
Pxl1p and paxillin both localize to the sites of polarized growth. (A) Cells of strain JPC267 (PXL1-GFP) were grown in YPD media and incubated at 24°C for 12-16 h before visualization. (B) The localization of GFP-paxillin fusion protein in yeast cells. The pUG36 vector carrying full-length chicken paxillin was transformed into strain JPC272 (pxl1Δ). Cells were grown in SC-Ura media and visualized for GFP.
In cultured fibroblasts, paxillin localizes to focal adhesions (Brown et al., 1996). We expressed a GFP-paxillin fusion protein in yeast cells and found that paxillin displayed an essentially identical localization pattern with Pxl1p (Figure 3B). These findings suggest that the mechanisms underlying the targeting of Pxl1p and paxillin to discrete cortical regions of the cell may be conserved, and that certain similarity exists between the bud tip of a yeast cell and the focal adhesion of a mammalian cell, two seemingly different structures.
Identification of LIM Domains as the Primary Targeting Element in Pxl1p
To determine which portion of the protein is responsible for targeting Pxl1p to the sites of polarized growth, we generated N-terminal GFP-PXL1 fusion constructs by cloning different fragments of PXL1 into pUG36 vector and monitored the localization of these GFP-tagged proteins in pxl1Δ cells. All these fusion proteins were expressed well as determined by immunoblotting with an anti-GFP antibody (Figure 4A). The full-length GFP-Pxl1p, not any of its truncated derivatives, expressed from the MET25 promoter in pUG36 was at least partially functional, because pUG36-PXL1 complemented cdc42-201 mutant at 30°C (Figure 4B), but not at 35.5°C. In contrast, GFP-tagged full-length or C-terminal portion of paxillin expressed from pUG36 plasmid failed to complement cdc42-201 at 30°C (Figure 4B). The full-length GFP-Pxl1p expressed from pUG36-PXL1 showed an identical localization pattern with the full-length GFP-Pxl1p expressed from its native promoter (Figure 4C). The N-terminal portion (1-555) of Pxl1p was capable of targeting itself to the sites of polarized growth. However, its targeting capacity was greatly reduced. In contrast, the C-terminal portion (548-706) of Pxl1p, particularly the two LIM domains (548-680) showed an identical localization pattern with the full-length protein (Figure 4, C and D). These results indicate that the primary element involved in targeting Pxl1p to the sites of polarized growth resides in the two LIM domains. When analyzed separately, each LIM domain showed much reduced targeting capacity, especially for LIM2 (Figure 4, C and D), suggesting that the two LIM domains may work synergistically to promote maximal targeting.
Figure 4.
Pxl1p localizes to the sites of polarized growth through its C-terminal LIM domains. (A-D) The pUG36 vector carrying the full-length Pxl1p (FL, 1-706), N-terminal portion (N-term, 1-555), C-terminal portion (C-term, 548-706), two LIM domains (LIM1 + 2, 548-680), LIM1 (548-620), and LIM2 (613-680), along with the full-length chicken paxillin (FL, 1-559) or its C-terminal portion (C-term, 309-559) was transformed into JPC272 (pxl1Δ) cells. Cells were grown in SC-Ura media. (A) Expression of the GFP-fusion proteins. Cell lysates prepared from the strains described above were separated by a 10% SDS-PAGE and immunoblotted with an anti-GFP antibody. The position of different GFP-fusion proteins on the blot is indicated by an asterisk. (B) Overexpression of GFP-Pxl1p fusion protein suppresses cdc42-201 mutant. Yeast strain YEF2258 (cdc42-201) carrying plasmids pUG36, pUG36-PXL1, pUG36-PXL1 N-term, pUG36-PXL1 C-term, pUG36-PXL1-LIM1 + 2, pUG36-PXL1-LIM1, pUG36-paxillin, and pUG36-paxillin C-term was streaked onto SC-Met-Ura plate and incubated at 30°C for 4 days. Control SC-Ura plate was incubated at 24°C for 4 days. (C) Localization of different GFP-tagged Pxl1p fragments. (D) A summary on the localization of GFP-tagged Pxl1p fragments. Strength in targeting capacity is indicated from strong (+++++) to very weak (+/-) or no detection (-). (E) Localization of paxillin N-term and paxillin C-term fused with GFP. (F) GFP-Pxl1p-LIM2 and GFP-paxillin C-term localize to the nucleus. Yeast strain JPC272 (pxl1Δ) carrying pUG36-PXL1-LIM2 and pUG36-paxillin C-term was fixed with formaldehyde and stained for DNA.
Besides the localization to the cortical sites, a pool of full-length Pxl1p also localized to the nucleus in a few cells (our unpublished data; Figure 4D). The N-terminal portion of Pxl1p did not localize to the nucleus. In contrast, all the Pxl1p fragments containing the LIM domains, particularly LIM2 alone, displayed strong nuclear localization (Figure 4, D and F), suggesting that the nuclear localization of Pxl1p may also be mediated by its LIM domains.
Because the C-terminal LIM domains of paxillin are the primary targeting element to focal adhesions in fibroblasts (Brown et al., 1996; Thomas et al., 1999), we asked whether these LIM domains also mediate the localization of paxillin to the sites of polarized growth in yeast. In contrast to the full-length protein, the LIM domains of paxillin mainly localized to the nucleus (Figure 4, E and F). Its targeting ability to the sites of polarized growth was greatly reduced (Figure 4E) and its localization pattern largely resembled that of the LIM2 of Pxl1p. It is not clear whether the reduced localization to the sites of polarized growth for the LIM2 of Pxl1p or the LIM domains of paxillin is due to their decreased affinity for a cortical protein or due to their increased nuclear targeting activity in the absence of other portions of the molecules. On the other hand, the N-terminal portion of paxillin targeted to the sites of polarized growth very efficiently (Figure 4E), suggesting that this portion of paxillin is capable of interacting with protein(s) localized at the sites of polarized growth.
Pxl1p Binds to Rho1p and Downregulates Its Function
Besides Pxl1p, the budding yeast genome encodes three additional LIM domain-containing proteins: Rga1p (Stevenson et al., 1995; Chen et al., 1996), Rga2p (Gladfelter et al., 2002; Smith et al., 2002), and Lrg1p (Muller et al., 1994; Watanabe et al., 2001). All three contains at least two LIM domains at their N-termini and a Rho GAP (GTPase-activating protein) domain at their C-termini, and they are indeed GAPs for Rho-family GTPases including Cdc42p and Rho1p (Chen et al., 1996; Watanabe et al., 2001; Gladfelter et al., 2002; Smith et al., 2002). Because some LIM domains are involved in protein dimerization (Sanchez-Garcia et al., 1993; Xue et al., 1993; Feuerstein et al., 1994), we speculated that Pxl1p might regulate Cdc42p and Rho1p function through interacting with either Rga1p, Rga2p, or Lrg1p. However, we failed to detect any interactions between Pxl1p and Rga1p, Rga2p, or Lrg1p with two-hybrid assays. In addition, a GFP-Pxl1p fusion protein localized well in rga1Δ rga2Δ lrg1Δ cells (our unpublished data).
To understand the molecular basis for the observed genetic interaction between PXL1 and CDC42, we asked whether Pxl1p binds directly to Cdc42p in vitro. We performed a protein-binding assay using purified MBP-Pxl1p and GST-Cdc42p expressed in E. coli. GST-Rho1p was included as a control. We did not observe any significant interaction between Pxl1p and Cdc42p that was stripped off nucleotide with EDTA or loaded with either GDP or GTP-γ-S (Figure 5A). Surprisingly, we found that Pxl1p bound preferentially to Rho1p-GDP, indicating that Pxl1p interacts directly with the inactive form of Rho1p.
Figure 5.
Physical and genetic interactions between Pxl1p and Rho1p. (A) In vitro binding assay was performed using MBP-Pxl1p, GST-Cdc42p, and GST-Rho1p. Equal amount of purified GSTCdc42p, GST-Rho1p, or GST alone, was either stripped off nucleotide (-nt) or loaded with GDP or GTP-γ-S, and then incubated with MBP-Pxl1p bound to Amylose resin. After washing, flow-through and bound fractions were collected and detected by immunoblotting with an anti-GST antibody. (B) Overexpression of Pxl1p inhibits the growth of rho1-2 and rho1-3 cells. Wild-type (WT, NY2284), rho1-2 (NY2285), rho1-3 (NY2286), and rho1-5 (NY2287) strains were transformed with plasmid YEp24 (Vec), or YEp24-GAL1-PXL1. Transformants were streaked onto SC-Ura plates containing dextrose (Dextrose) and SC-Ura plates containing 2% galactose plus 1% raffinose (Galactose), and incubated at 30°C for 3 days.
To determine the functional consequences of the Pxl1p-Rho1p interaction, we examined the overexpression effect of Pxl1p on the growth of several rho1-Ts mutants. Mild overexpression of PXL1 under its own promoter carried on highcopy plasmids appeared to weakly inhibit the growth of rho1-2 and rho1-3 mutants (our unpublished data). When PXL1 was placed under the control of a galactose-inducible promoter pGAL1, overexpression of Pxl1p strongly inhibited the growth of rho1-2 and rho1-3 mutants at 30°C, but not rho1-5 and wild-type cells (Figure 5B). These findings suggest that Pxl1p downregulates Rho1p function by directly binding to its inactive form. However, it is not clear whether the suppression of cdc42-Ts mutants by Pxl1p is due to its inhibitory effect on the Rho1p function.
Functional Interactions between Cdc42p and Rho1p Signaling Pathways
The observations that overexpression of Pxl1p suppressed cdc42-Ts, but inhibited rho1-Ts mutants suggest that Cdc42p and Rho1p signaling pathways might be antagonistic to each other during budding and that Pxl1p is involved in coordinating this relationship between Cdc42p and Rho1p. If so, overexpression of Cdc42p might inhibit Rho1p function, and vice versa. Indeed, overexpression of Cdc42p from a galactose-inducible promoter inhibited the growth of rho1-2 and rho1-3 mutants at 30°C, a semipermissive temperature for both mutants (Figure 6A). Reciprocally, overexpression of Rho1p from a galactose-inducible promoter inhibited the growth of cdc42-201 mutant, but not cdc42G60D mutant or the wild-type strain at 24°C and at 30°C (Figure 6B). Because both the wild-type strain and the hyperactive cdc42G60D mutant could tolerate increased expression of Rho1p at and below 30°C, these findings suggest that overexpression of Rho1p inhibits cell growth only when Cdc42p function was compromised.
Figure 6.
Antagonistic relationship between Cdc42p and Rho1p signaling pathways. (A) Overexpression of Cdc42p inhibits the growth of rho1-Ts mutants. Yeast strains NY2284 (WT), NY2285 (rho1-2) and NY2286 (rho1-3) carrying plasmid YEp352 (Vec) or YEp352-GAL-CDC42 were streaked onto SC-Ura plates containing dextrose (Dextrose) and SC-Ura plates containing 2% galactose and 1% raffinose (Galactose), and incubated at 30°C for 3 days. (B) Overexpression of Rho1p inhibits the growth of cdc42-201, but not cdc42G60D mutant. Yeast strains YEF473A (WT), YEF2258 (cdc42-201), and JPC241 (cdc42G60D) carrying plasmid pYES2 (Vec) or pYES2-RHO1 (GAL-RHO1) were streaked onto SC-Ura plates containing 2% galactose and 1% raffinose (Galactose), and incubated at 24 or 30°C for 3 days. Control SC-Ura plate containing dextrose (Dextrose) was incubated at 24°C for 3 days.
We also found that overexpression of Cdc42p inhibited pxl1Δ rho1-2 and pxl1Δ rho1-3 mutants at 30°C, and that overexpression of Rho1p inhibited pxl1Δ cdc42-201 mutants at 24°C or at 30°C (our unpublished data). These results suggest that Pxl1p is not the sole mediator between Cdc42p and Rho1p signaling pathways, which is consistent with the fact that deletion of PXL1 did not produce any obvious phenotype.
DISCUSSION
Pxl1p Is a Zyxin/Paxillin-family Protein
Proteins that contain LIM domains can be roughly classified into five groups: LIM homeodomain proteins, LIM only proteins, LIM kinases, GTPase-activating proteins (GAPs), and zyxin/paxillin-family proteins. In S. cerevisiae, four proteins, Rga1p, Rga2p, Lrg1p, and Pxl1p, contain LIM domains. The first three are Rho GAPs and they have a similar structural organization, with the LIM domains at their N-termini and the Rho GAP domain at their C-termini (Muller et al., 1994; Stevenson et al., 1995; Chen et al., 1996; Watanabe et al., 2001; Gladfelter et al., 2002; Smith et al., 2002). Several lines of evidence suggest that the novel Pxl1p belongs to the zyxin/paxillin family. First, the LIM domains of Pxl1p are located at its C-terminus, resembling zyxin/paxillin-family proteins. Second, Pxl1p shares overall sequence homology with paxillin. Third, Pxl1p and paxillin display a similar localization pattern in yeast. Finally, like paxillin and Hic-5 (Brown et al., 1996; Thomas et al., 1999), the LIM domains of Pxl1p act as the major targeting element for its localization to the sites of polarized growth. This similarity in targeting paxillin-like proteins to a cortical site in yeast and mammalian cells suggests that the yeast Pxl1p can be used as a model to address the important question in paxillin biology: how is paxillin initially recruited to the focal adhesion site?
Coordination between Cdc42p and Rho1p GTPases during Budding
We isolated PXL1 as a multicopy suppressor of cdc42-Ts mutants from two independent screens. One interpretation for this genetic interaction is that Pxl1p is a direct regulator or downstream effector of Cdc42p. However, we failed to detect any significant interaction between Pxl1p and Cdc42p regardless of its nucleotide-bound state (Figure 5A). We also failed to detect any interactions by two-hybrid assays between Pxl1p or its N- and C-terminal fragments and Cdc42p, or a number of Cdc42p regulators such as its GAPs, Rga1p and Rga2p; the scaffold protein Bem1p; and its effectors such as the formin Bni1p; and PAKs Ste20p, Cla4p, and Skm1p (our unpublished data). In addition, overexpression of Pxl1p did not suppress cdc24-Ts mutants, cdc24-4 and cdc24-11, which are defective in the guanine-nucleotide-exchange factor (GEF) activity for Cdc42p (Zheng et al., 1994; our unpublished data), suggesting that the suppression of cdc42 mutants by Pxl1p is not mediated by Cdc42p GEF. Thus, it remains an open question how Pxl1p mechanistically regulates Cdc42p signaling pathway.
We also showed here that Pxl1p binds to Rho1p-GDP in vitro and that overexpression of Pxl1p inhibited the growth of rho1 mutants. Such a functional connection between Pxl1p and Rho1p is supported by additional genetic interactions observed between Pxl1p and other components of the Rho1p signaling pathway such as the Rho1p GAP, Bem2p (Zheng et al., 1993), and the Rho1p effector, Fks1p (Drgonova et al., 1996; Qadota et al., 1996; Mackin et al., 2004). Together, these results suggest that Pxl1p might coordinate Cdc42p and Rho1p signaling pathways by exerting opposite effects on them. These data also raise the possibility that Cdc42p and Rho1p might be functionally antagonistic during budding. Indeed, we observed that overexpression of Cdc42p inhibited the growth of some rho1 mutants, and vice versa. Thus, it appears that Pxl1p might mediate this functional relationship between Cdc42p and Rho1p. However, Pxl1p cannot be the sole linker between these GTPases, because deletion of PXL1 did not produce any obvious defect in polarized growth and, in addition, did not affect the mutual inhibition displayed by overexpression of Cdc42p or Rho1p in the relevant mutants. Currently, we are trying to identify genes that act in parallel to PXL1 in coordinating the functions of Cdc42p and Rho1p during budding.
Conditional inactivation of Cdc42p blocks bud emergence, resulting in large, round, and unbudded cells. Thus, Cdc42p is generally believed to act at the very early stage and be continuously required during the budding process (Adams et al., 1990; Johnson, 1999; Gladfelter et al., 2002). In contrast, conditional inactivation of Rho1p results in cell lethality with cells arrested predominantly as small-budded cells (Yamochi et al., 1994). Thus, it appears that Cdc42p and Rho1p act sequentially during bud formation. However, this simple interpretation is complicated by the fact that certain alleles of RHO1 also cause defects at bud emergence as well as in actin organization in a similar way as most cdc42 mutants do (Guo et al., 2001). In addition, Cdc42p and Rho1p share some effectors, such as Bni1p and Sec3p (Kohno et al., 1996; Evangelista et al., 1997; Guo et al., 2001; Zhang et al., 2001); thus, the relationship between Cdc42p and Rho1p may be more complex than previously envisioned. We speculate that Cdc42p and Rho1p may act cyclically to promote bud growth. Cdc42p may promote the initial membrane expansion and the loosening of the cell wall at the bud tip by controlling actin dynamics very much in the same way as Cdc42 and Rac promote the initial membrane expansion and/or cell spreading in animal cells. On the other hand, Rho1p might fix the expanded membrane at the bud tip by regulating cell wall synthesis through its effector Pkc1p and Fks1p/Fks2p and/or by controlling the delivery of cell wall materials to the bud tip via its effector Bni1p-nucleated actin cables. This hypothesis reconciles the “sequential” and “mutually inhibitory” relationships between Cdc42p and Rho1p. Further studies must be carried out to determine how Cdc42p and Rho1p are coordinated at the molecular level during bud formation.
Studies in mammalian cells also indicate a coordinated regulation of small GTPases in specific cellular functions. During cell spreading, the binding of integrins to the extracellular matrix triggers the rearrangement of the actin cytoskeleton by regulating the activity of Rho family GTPases, Cdc42, Rac1, and RhoA. A transient localized downregulation of RhoA activity is critical for the initial phase of cell adhesion (Ren et al., 1999; Arthur et al., 2000). A recent study showed that paxillin plays a role in the local suppression of RhoA activity by binding to p120RasGAP, which releases its normal binding partner p190RhoGAP to downregulate RhoA activity (Tsubouchi et al., 2002). These and our studies suggest that the inhibitory function of paxillin and its yeast homologue, Pxl1p, on Rho GTPases appears to be an evolutionarily conserved feature, although the molecular details may differ.
Acknowledgments
We thank Dr. W. Guo for providing rho1-Ts mutants, GST-Cdc42p, and GST-Rho1p fusion constructs; Dr. J.H. Hegemann for pUG36 plasmid; Dr. C. Turner for pcDNA3-paxillin plasmid; and Dr. S. Erdman for sharing results before publication. This work was supported by National Institutes of Health Grant GM59216 to E.B.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0079. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0079.
References
- Adamo, J.E., Moskow, J.J., Gladfelter, A.S., Viterbo, D., Lew, D.J., and Brennwald, P.J. (2001). Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155, 581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, A.E.M., Johnson, D.I., Longnecker, R.M., Sloat, B.F., and Pringle, J.R. (1990). CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111, 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, P.D., and Stark, M.J. (2000). Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J. Cell Sci. 113, 2685-2693. [DOI] [PubMed] [Google Scholar]
- Arthur, W.T., Petch, L.A., and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722. [DOI] [PubMed] [Google Scholar]
- Bender, A., and Pringle, J.R. (1989). Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA 86, 9976-9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, E., and Pringle, J.R. (1996). ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 5264-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.L., Jaquenoud, M., Gulli, M.P., Chant, J., and Peter, M. (1997). Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 11, 2972-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M.C., Curtis, M.S., and Turner, C.E. (1998). Paxillin LD motifs may define a new family of protein recognition domains. Nat. Struct. Biol. 5, 677-678. [DOI] [PubMed] [Google Scholar]
- Brown, M.C., Perrotta, J.A., and Turner, C.E. (1996). Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135, 1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib, E., Drgonova, J., and Drgon, T. (1998). Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67, 307-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M., and Botstein, D. (1982). Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell 28, 145-154. [DOI] [PubMed] [Google Scholar]
- Caviston, J.P., Longtine, M., Pringle, J.R., and Bi, E. (2003). The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston, J.P., Tcheperegine, S.E., and Bi, E. (2002). Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA 99, 12185-12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G.-C., Kim, Y.-J., and Chan, C.S.M. (1997). The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 11, 2958-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G.-C., Zheng, L., and Chan, C.S.M. (1996). The LIM domain-containing Dbm1 GTPase-activating protein is required for the normal cellular morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 1376-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrckova, F., De Virgilio, C., Manser, E., Pringle, J.R., and Nasmyth, K. (1995). Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9, 1817-1830. [DOI] [PubMed] [Google Scholar]
- Dawid, I.B., Breen, J.J., and Toyama, R. (1998). LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 14, 156-162. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf, G.J., Abe, M., Ohya, Y., and Bussey, H. (2002). Mutations in Fks1p affect the cell wall content of beta-1,3- and beta-1,6-glucan in Saccharomyces cerevisiae. Yeast 19, 671-690. [DOI] [PubMed] [Google Scholar]
- Drgonova, J., Drgon, T., Tanaka, K., Kollar, R., Chen, G.C., Ford, R.A., Chan, C.S., Takai, Y., and Cabib, E. (1996). Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272, 277-279. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Blundell, K., Longtine, M.S., Chow, C.J., Adames, N., Pringle, J.R., Peter, M., and Boone, C. (1997). Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118-122. [DOI] [PubMed] [Google Scholar]
- Feuerstein, R., Wang, X., Song, D., Cooke, N.E., and Liebhaber, S.A. (1994). The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc. Natl. Acad. Sci. USA 91, 10655-10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyd, G., Kim, S.K., and Horvitz, H.R. (1990). Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature (London) 344, 876-879. [DOI] [PubMed] [Google Scholar]
- Gladfelter, A.S., Bose, I., Zyla, T.R., Bardes, E.S.G., and Lew, D.J. (2002). Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 156, 315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., Tamanoi, F., and Novick, P. (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3, 353-360. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (1991). Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology, Vol. 194, San Diego, CA: Academic Press. [PubMed]
- Holly, S.P., and Blumer, K.J. (1999). PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J. Cell Biol. 147, 845-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, H., Tanaka, K., Hihara, T., Umikawa, M., Kamei, T., Takahashi, K., Sasaki, T., and Takai, Y. (1997). Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D.I. (1999). Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, H. et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 6060-6068. [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A., III, DeMarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Mackin, N.A., Sousou, T.J., and Erdman, S.E. (2004). The PXL1 gene of Saccharomyces cerevisiae encodes a paxillin-like protein functioning in polarized cell growth. Mol. Biol. Cell 15, 1904-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur, P., Morin, N., Baginsky, W., el-Sherbeini, M., Clemas, J.A., Nielsen, J.B., and Foor, F. (1995). Differential expression and function of two homologous subunits of yeast 1,3-beta-D-glucan synthase. Mol. Cell. Biol. 15, 5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen, J.W., Schmeichel, K.L., Beckerle, M.C., and Winge, D.R. (1993). The LIM motif defines a specific zinc-binding protein domain. Proc. Natl. Acad. Sci. USA 90, 4404-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, L., Xu, G., Wells, R., Hollenberg, C.P., and Piepersberg, W. (1994). LRG1 is expressed during sporulation in Saccharomyces cerevisiae and contains motifs similar to LIM and rho/racGAP domains. Nucleic Acids Res. 22, 3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, H., Tanaka, K., Hirano, H., Fujiwara, T., Kohno, H., Umikawa, M., Mino, A., and Takai, Y. (1995). A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14, 5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman, M.A., and Cerione, R.A. (1998). Iqg1p, a yeast homologue of the mammalian IQGAPs mediates Cdc42p effects on the actin cytoskeleton. J. Cell Biol. 142, 443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D., and Bretscher, A. (2000). Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113, 365-375. [DOI] [PubMed] [Google Scholar]
- Qadota, H., Python, C.P., Inoue, S.B., Arisawa, M., Anraku, Y., Zheng, Y., Watanabe, T., Levin, D.E., and Ohya, Y. (1996). Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science 272, 279-281. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumanie, O., Weinachter, C., Larrieu, I., Crouzet, M., and Doignon, F. (2001). Functional characterization of the Bag7, Lrg1 and Rgd2 RhoGAP proteins from Saccharomyces cerevisiae. FEBS Lett. 506, 149-156. [DOI] [PubMed] [Google Scholar]
- Sanchez-Garcia, I., Osada, H., Forster, A., and Rabbitts, T.H. (1993). The cysteine-rich LIM domains inhibit DNA binding by the associated homeodomain in Isl-1. EMBO J. 12, 4243-4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, H.P., Huppert, S., Lorberg, A., and Heinisch, J.J. (2002). Rho5p down-regulates the yeast cell integrity pathway. J. Cell Sci. 115, 3139-3148. [DOI] [PubMed] [Google Scholar]
- Smith, G.R., Givan, S.A., Cullen, P., and Sprague, G.F., Jr. (2002). GTPase-activating proteins for Cdc42. Eukaryotic Cell 1, 469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, B.J., Ferguson, B., De Virgilio, C., Bi, E., Pringle, J.R., Ammerer, G., and Sprague, G.F., Jr. (1995). Mutation of RGA1, which encodes a putative GTPase-activating protein for the polarity-establishment protein Cdc42p, activates the pheromone-response pathway in the yeast Saccharomyces cerevisiae. Genes Dev. 9, 2949-2963. [DOI] [PubMed] [Google Scholar]
- Thomas, S.M., Hagel, M., and Turner, C.E. (1999). Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 112, 181-190. [DOI] [PubMed] [Google Scholar]
- Tsubouchi, A., Sakakura, J., Yagi, R., Mazaki, Y., Schaefer, E., Yano, H., and Sabe, H. (2002). Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J. Cell Biol. 159, 673-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello, D.A., Brown, M.C., and Turner, C.E. (2002). The paxillin LD motifs. FEBS Lett. 513, 114-118. [DOI] [PubMed] [Google Scholar]
- Turner, C.E. (2000a). Paxillin and focal adhesion signalling. Nat. Cell Biol. 2, E231-E236. [DOI] [PubMed] [Google Scholar]
- Turner, C.E. (2000b). Paxillin interactions. J. Cell Sci. 113, 4139-4140. [DOI] [PubMed] [Google Scholar]
- Watanabe, D., Abe, M., and Ohya, Y. (2001). Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-beta-glucan synthesis. Yeast 18, 943-951. [DOI] [PubMed] [Google Scholar]
- Weiss, E.L., Bishop, A.C., Shokat, K.M., and Drubin, D.G. (2000). Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat. Cell Biol. 2, 677-685. [DOI] [PubMed] [Google Scholar]
- Xue, D., Tu, Y., and Chalfie, M. (1993). Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science 261, 1324-1328. [DOI] [PubMed] [Google Scholar]
- Yamochi, W., Tanaka, K., Nonaka, H., Maeda, A., Musha, T., and Takai, Y. (1994). Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125, 1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Bi, E., Novick, P., Du, L., Kozminski, K.G., Lipschutz, J.H., and Guo, W. (2001). Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276, 46745-46750. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Cerione, R., and Bender, A. (1994). Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269, 2369-2372. [PubMed] [Google Scholar]
- Zheng, Y., Hart, M.J., Shinjo, K., Evans, T., Bender, A., and Cerione, R.A. (1993). Biochemical comparisons of the Saccharomyces cerevisiae Bem2 and Bem3 proteins. Delineation of a limit Cdc42 GTPase-activating protein domain. J. Biol. Chem. 268, 24629-24634. [PubMed] [Google Scholar]