Abstract
Background
The laboratory rat is one of the most popular experimental models for the experimental surgery of the liver. The objective of this study was to investigate the morphometric parameters, physiological data, differences in configuration of liver lobes, biliary system, and vasculature (arteries, veins, and lymphatic vessels) of the liver in laboratory rats. In addition, this study supports the anatomic literature and identified similarities and differences with human and other mammals.
Material/Methods
Forty laboratory rats were dissected to prepare corrosion casts of vascular system specimens (n=20), determine the lymph vessels and lymph nodes (n=10), and for macroscopic anatomical dissection (n=10) of the rat liver. The results are listed in percentages. The anatomical nomenclature of the liver morphology, its arteries, veins, lymph nodes, and lymphatic vessels are in accordance with Nomina Anatomica Veterinaria.
Results
We found many variations in origin, direction, and division of the arterial, venous, and lymphatic systems in rat livers, and found differences in morphometric parameters compared to results reported by other authors. The portal vein was formed by 4 tributaries in 23%, by 3 branches in 64%, and by 2 tributaries in 13%. The liver lymph was drained to the 2 different lymph nodes. The nomenclature and morphological characteristics of the rat liver vary among authors.
Conclusions
Our results may be useful for the planing of experimental surgery and for cooperation with other investigation methods to help fight liver diseases in human populations.
MeSH Keywords: Anatomy, Comparative; Hepatic Artery; Hepatic Veins; Liver Transplantation; Models, Animal
Background
There have recently been rapid development and new advances in experimental medicine. Diseases of the gastrointestinal tract (e.g., the syndrome of short small intestine in newborns and adults, pancreas dysfunction, and end-stage liver diseases) and their treatment account for much medical investigation in experimental surgery [1,2]. These illnesses can only be treated by transplantation. The experimental transplantations of digestive organs are accomplished using different methods and various combinations of donors and recipients, but the vasculature and lymphatic drainage of these organs are similar [3]. Knowledge of anatomical variations is quite important for experimental investigation and surgical practice. The investigation of anatomy, comprising the morphology of the vessels in laboratory animals, is vital for managing of ischemia and transplantation of organs.
Future progress in transplantation depends on using the most suitable animal model. The laboratory rat is currently one of the most popular experimental models for research because it is easy to handle and inexpensive. Laboratory rats have anatomical structures of the organs similar to those of humans; therefore, they are the most suitable for anatomical, physiological, and biochemical research on the digestive system. This laboratory species is by far the most commonly used animal model in experimental liver and intestine transplantation [4,5]. Experimental transplantations of the liver have been studied in many mammals, especially in dogs [6,7], swine [8,9], in laboratory animals [10], and in other mammals [11]. The rat liver is the object of extensive investigation because recent evidence demonstrates their involvement in different processes (e.g., secretion, proliferation, absorption, and neoangiogenesis) significant for its pathophysiology. The metabolism and function of the liver are preserved by the hepatic artery, portal vein, and lymphatic vessels, which form a comprehensive network of liver tissue.
The liver is generally the largest group of cells composing homogeneous antigenic matter that can be easily transplanted in the mammal body [12]. The primary function of the liver is detoxification of absorbed substances from organs in the digestive system before their distribution into the bloodstream [13]. The initial hematopoiesis in mammals takes place in the fetal liver [14]. The liver performs many other functions: production of bile, proteins for blood, cholesterol and special proteins to help carry fats, store and release glucose, transformation of harmful ammonia to urea, and creating immune factors.
The aim of this study was to investigate morphometric parameters e.g., (weight, length, and height), differences in configuration of the liver lobes, biliary system, and vasculature (e.g., arteries, veins, and lymphatic vessels) of the liver in laboratory rats. In addition, this study supports to the anatomic literature and identifies similarities and differences with humans and other mammals [15].
The development of the rat liver
On embryonic day 10.5, the rat liver begins its development. Its cells acquire the morphological appearance of immature rat liver hepatoblasts on embryonic day 11.5. The primitive epithelial cells of the foregut form the hepatic bud, which is divided into a smaller caudal part (pars cystica) and a larger cephalic part (pars hepatica). The caudal part gives rise to the gallbladder and cystic duct and the cephalic part gives rise to parts of the parenchyma of the liver, intrahepatic ducts, and both (right and left) hepatic ducts. Except for the cystic bud, most of the structures of the rat embryo are present in the human embryo. Moawad et al. [13] described these changes during prenatal liver development in the rat. On the embryonic day 13 the liver was expanded greatly in size and its position was just caudal to the diaphragm. The venous duct (ductus venosus) was observed draining into the post-hepatic caudal vena cava (v. cava caudalis). On coronal section, many interlobar spaces were found at the same time. These spaces divided the liver into 4 main lobes: median, right, left, and caudate lobes. The median lobe was divided into the right and left parts. The right lobe was subdivided into the right cranial and caudal lobes. The caudate lobe was visible on the side of the stomach and the esophagus. On embryonic day 15, the venous duct, post-hepatic caudal vena cava, and portal vein appear in transverse section and the liver lobes are demarcated in coronal section. On embryonic day 17, the liver lobes are growing and hepatocytes are more mature than on embryonic day 15. On embryonic day 19, there is a marked increase in the number and size of hepatocytes in the rat liver tissue. The blood spaces are irregularly distributed between the cords and hepatocytes and they are smaller than in the previous phase. On embryonic day 21, the liver achieves its mature adult architecture. The hepatic lobule consists of the central vein (v. centralis) with the portal triad in its margin. The sinusoids of the blood are thin irregular structures between the cords of the liver cells. There is continuation between the central vein (v. centralis) and sinusoids of the blood located between the hepatic cords. The rat liver lacks a gall bladder, which is not observed in any available stained fetuses or liver sections. Many authors reported that there is not cystic bud in rats [13,16,17].
Material and Methods
Forty Wistar laboratory rats (Rattus norvegicus f. domestica) of both sexes, aged 1 year and weighing approximately 350–520 g in standard breeding condition were used. All animals were obtained from the accredited Laboratory of Research Biomodels, University of P.J. Šafárik in Kosice. The experiment on rats was performed with approval of the Ethics Committee of the University of Veterinary Medicine and Pharmacy in Kosice and the State Veterinary and Food Institute in Bratislava (No. SK P 12004), following Slovakian protocols for ethical standards for the use of laboratory animals. The first group of rats (10 animals) was used for making corrosion casts of the arteries, the second group (10 animals) was used for making corrosion casts of the veins, the third group (10 animals) was used for making images of the lymphatic vessels and lymph nodes, and the last group (10 animals) was used for macroscopic anatomical dissection of the liver lobes, ligaments, and biliary system.
The preparing of the corrosion casts specimens of the arterial system
Anesthesia of animals was induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg, Thiopental Valeant, Valeant Czech Pharma, Czech Republic). After anesthetizing the rats, we dissected the left ventricle of the heart. We implemented a cannula into the aorta through the left ventricle while the cannula was supported by a ligature. A portion of the venous system was opened to ensure a good distribution of the perfusion medium; the right auricular appendage served this purpose. Vessels were perfused with 0.9% isotonic saline physiological solution at a low flow rate (about 10 ml/min) for 30 s through the left cardiac ventricle. An improved method for the washing out of clotted blood from the vessels was achieved by the addition of 0.05% NaOH (Mikrochem, Slovakia) into the perfusion medium. The perfusion pressure was approximately 200–250 mmHg (2.6–3.25 m H2O). The success of the perfusion was indicated by the uniform fading of the tissues seen during the procedure. We mixed the injection media in stoichiometric rates. The corrosion casts were prepared with Duracryl Dental® resin (Spofa-Dental, Czech Republic). Suitable color tone was achieved by addition of 2–3 drops of red (oil–red paint 0). After proper mixing of all components, we applied this mass into the arterial system through the left ventricle of the heart. After vascular casting with the resin is complete, it (and the animal) must not be manipulated for 30 min., after which the casts are submersed in water at a temperature ranging from 40°C to 60°C for a period of between 30 min and 24 h for full polymerization of resin [18]. The maceration of the soft tissues was carried out in 2–4% solution of KOH (Mikrochem, Slovakia) at 60–70 °C. The maceration took approximately 2–3 days. Prior to the outset of the drying process, the corroded specimens were submersed in water and dried at room temperature.
The preparing of the corrosion casts specimens of the venous system
In the second group of animals, anesthesia was induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg, Thiopental Valeant, Valeant Czech Pharma, Czech Republic). Under anesthesia and after heparin administration (50 000 IU/kg; Heparin Léčiva, Zentiva, Czech Republic), rats were exsanguinated from the jugular vein before they could regain consciousness. The application of an anticoagulant is a key requirement for high-quality vascular casting. After lateral thoracotomy, the caudal vena cava was cannulated, and the liver was perfused manually with 0.9% physiologic saline. Saline was perfused continually until casting, to remove the fixative and increase the permeability of the resin. The cranial caval veins, pulmonary trunk, thoracic part of caudal caval vein, and aorta were ligated. Colored latex and the corrosion cast method were used to visualize the liver venous system. Colored latex (Het, Ohníč u Teplic, Czech Republic) was injected into the caudal vena cava. After perfusion, the liver was removed and cleaned of connective tissue. Corrosion casts were prepared by using a self-curing adhesive resin (Spofacryl, SpofaDental, Jičín, Czech Republic). A plastic cannula was inserted and fixed in the caudal vena cava, and the casting medium was injected. After vascular casting with the resin was complete, rats were left undisturbed for 30 min, after which they were submersed in water at 40–60°C for 30 min to 24 h to achieve full polymerization of the resin. Soft tissues were macerated by immersion in KOH solution (2–4%) at 60–70°C for 3–6 days. Detergents (0.5%) were used to facilitate tissue removal by KOH. Prior to the outset of the drying process, the corroded specimens were submersed in water and dried at room temperature. The liver veins were evaluated macroscopically and by using an operating microscope (model M 320, Leica).
The preparation and application of the injection medium to determine the lymph vessels and lymph nodes
In the third group of rats under sodium pentobarbital anesthesia (50 mg/kg, Thiopental Valeant, Valeant Czech Pharma, Czech Republic), we opened the abdominal cavity via the linea alba. We performed the application of the tracer material at the different sites of the liver parenchyma. To monitor the lymphatic progress of the dye, we used ink of various colors (black, blue, and green). The injected medium was drawing ink (Koh-i-noor, Hardmuth, Czech Republic). The dye particles of selected tracer material must be at least 10 nm diameter because smaller molecules are preferentially reabsorbed by blood vessels. About 0.3–0.5 ml of color medium was repeatedly injected to predetermined sites of the liver (into the connective tissue space of the porta of liver and into the subcapsular places on diaphragmatic surface and visceral surface) by means of a fine metal needle (BD Micro-Fine Plus, BD Medical-Diabetes Care, USA). Subsequently, animals were exsanguinated from the jugular vein without regaining consciousness. The lymphatic drainage of the liver region was investigated at about 60 min after injection. The lymph nodes and the drainage area were evaluated macroscopically and by using an operating microscope (model M 320, Leica).
The macroscopic anatomical dissection of the rat liver
In the fourth group of rats under sodium pentobarbital anesthesia (50 mg/kg, Thiopental Valeant, Valeant Czech Pharma, Czech Republic), we performed macroscopic anatomical dissection of the liver lobes, ligaments, and biliary tree, and assessed features compared to humans and other mammals. The abdominal cavity was opened by midlaparotomy through the abdominal wall in the midline (linea alba) from the caudal end of the sternum (processus xiphoideus) to the pubic bone (pecten ossis pubis). The abdominal wall was cut on both sides, cranially along the last rib and caudally along the inguinal region. Intestines were shifted on the left side and the principal bile duct was ligated to image the biliary system and then dissection was performed [19]. Cotton-tipped sticks, forceps, and a stereotactic microscopic (Leica M 320) were used for anatomical dissection. Images were taken with a digital camera adapted to the microscope. The results are listed in percentages. The anatomical nomenclature of the liver morphology, its arteries, veins, lymph nodes and lymphatic vessels is in accordance with the Nomina Anatomica Veterinaria [20].
Results
Topography, morphological, and physiological parameters of the rat liver
The rat liver is the most cranial structure on the right side of the abdominal cavity, in the intrathoracic portion, coming in intimate contact with the diaphragm. The liver of the rat is a multilobulated organ. The mass of the rat liver accounts for about 6% of the total body weight. The results of morphological and physiological parameters are shown in Table 1.
Table 1.
Morphometric and physiological parameters and associated structures of rat liver.
| Liver weight | 15.5 g |
| Volume of liver | 22.6 ml |
| Transverse diameter of liver | 8.5–9.0 cm |
| Dorsoventral diameter of liver | 4.8–5.2 cm |
| Craniocaudal diameter of liver | 3.2–3.5 cm |
| Blood flow of liver | 13.8 ml/min |
| Blood flow through hepatic artery | 2.0 ml/min |
| Blood flow through portal vein | 9.8 ml/min |
| Bile flow of liver | 22.5 ml/day |
Surfaces and margins of the rat liver
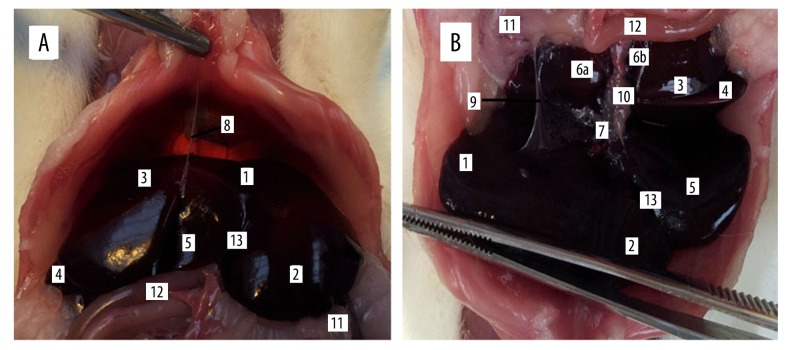
When we opened the abdominal cavity through the linea alba, the rat liver had generally 2 surfaces: diaphragmatic and visceral. While the rat liver is a multilobulated organ, it has about the same surfaces as lobes positioned flat against each other. The diaphragmatic (Figure 1) convex surface (facies diaphragmatica) is in contact with the diaphragm and right abdominal wall. This surface is covered by the peritoneum, in addition part of the attachment of the falciform ligament (Figure 1). The part of the convex surface that is without peritoneum is referred to as the bare area of the liver (area nuda). This surface includes part of the left lateral and medial lobes of the liver. The visceral concave surface (facies visceralis) (Figure 1) is very rugged, because it is in relation to the guts (stomach, descending duodenum, right colic flexure, jejunum, spleen, pancreas, right kidney, and suprarenal gland). These structures indent the liver and, according to the organ involved, produce impressions of the stomach (impressio gastrica), duodenum (impressio duodenalis), colon (impressio colica), and kidney (impressio renalis). The whole visceral surface is embedded into the peritoneum. The porta of the liver (porta hepatis) is located on the visceral surface. The porta hepatis goes through the portal vein, hepatic artery and nerves, lymphatic vessels, and common hepatic duct. A very well-defined border divides the convex from the concave surface. There are 4 such borders in the rat liver: right, left, ventral, and dorsal. The left, right, and ventral border are very sharp but the dorsal border is oblique. The left border (margo sinister) is between the convex and concave surface on the side of the left lateral and medial lobes of liver. The right border (margo dexter) is between the concave and convex surfaces on the right side of the liver, where the right lateral and medial lobes of liver were situated. The ventral border (margo ventralis) is between the concave and convex surface, and is formed by the left and right medial lobes and quadrate lobe of the liver. The dorsal border (margo dorsalis) consists of part of the left and right lateral lobes and caudate lobe. Situated on the dorsal border of the rat liver are the esophageal impression (impressio esophagea) and the caudal vena cava (v. cava caudalis), located completely inside the liver tissue. The caudal vena cava receives segmental veins before its exit on the craniodorsal aspect of the caudate lobe. The groove for the caudal vena cava is termed the sulcus venae cavae.
Figure 1.
The intrathoracic position of the liver, diaphragmatic (A), and visceral (B) surface of the rat liver. 1. Left lateral lobe (lobus hepatis sinister lateralis), 2. Left medial lobe (lobus hepatis sinister medialis), 3. Right lateral lobe (lobus hepatis dexter lateralis), 4. Right medial lobe (lobus hepatis dexter medialis), 5. Quadrate lobe (lobus quadrates), 6. Caudate lobe (lobus caudatus), 6a. Preventricular part of the papillary process (pars preventricularis processus papillaris), 6b. Retroventricular part of the papillary process (pars retroventricularis processus papillaris), 7. Porta of the liver (porta hepatis), 8. Falciform ligament of liver (lig. falciforme), 9. Hepatogastric ligament (lig. hepatogastricum), 10. Common hepatic duct (ductus hepaticus communis), 11. Stomach (ventriculus), 12. Intestine (intestinum), 13. Notch for round ligament (incisura ligamenti teretis).
Lobes and notches of the rat liver
We described 6 lobes on the rat liver: left medial (smaller), left lateral (greater) right medial (smaller), right lateral (greater), caudate, and quadrate lobe (lobus hepatis sinister medialis, lateralis et lobus hepatis dexter medialis, lateralis, lobus caudatus et quadratus, respectively). The caudate lobe is between the caudal vena cava and the left branch of the portal vein. The caudate lobe has 2 processes: papillary and caudate (processus papillaris et caudatus, respectively). The papillary process (processus papillaris) is the smaller and projects more to the left side of the liver, arising from the left of the porta. In rats, this process is divided into 2 parts: preventricular (pars preventricularis) and retroventricular (pars retroventricularis) (Figure 1). The large caudate process extends to the right side, covering much of the visceral surface of the right lobe of liver, at the renal impression (impressio renalis). The lateral, greater lobes of the liver are joined to the others parts only by means of interstitial tissue and vessels. The medial, smaller lobes on the right and left side of the liver are situated more cranial to the lateral part. Deep interlobar notches (incisurae interlobares) are located between the lateral and medial part of the left and right lobes and between the right, medial, left, medial, and quadrate lobes. Between the left, medial lobe and quadrate lobe is the deep notch for the round ligament (incisura ligamenti teretis), which contains the round ligament (lig. teres hepatis). On the opposite side, between the right, medial lobe and quadrate lobe, is a hole, which contains the gall bladder (vesica fellea) in other mammals, but is absent in the laboratory rat.
Ligaments of the rat liver
The rat liver ligaments are very thin. The falciform ligament of the liver (lig. falciforme) is a thin peritoneal fold attached to the convex surface of the diaphragm and the caudal surface of the right abdominal muscles. This ligament originates from the peritoneum on the convex surface of the liver and runs toward the coronary ligament, which inserts around the exit of the caudal vena cava. The coronary ligament of liver was divided into 2 parts: right and left (lig. coronarium dextrum et sinistrum). These ligaments attach the liver to the diaphragm on a line from the right triangular ligament of liver (lig. triangulare dextrum) along the right side of the caudal vena cava and around the ventral margin of the caval opening (foramen venae cavae) to the left triangular ligament (lig. triangulare sinistrum). The right triangular ligament joins the right lobe to the dorsal abdominal wall. The left triangular ligament attaches the caudolateral angle of the left lobe to the same part of the abdominal wall. The round ligament (lig. teres hepatis) is a negligible thickening of the caudal free edge of the falciform ligament. The round ligament of liver I a vestige of the umbilical vein. The greater omentum (omentum majus) continues to the greater curvature of the stomach as the gastrosplenic ligament (lig. gastrolienale). The hepatogastric and hepatoduodenal ligaments (lig. hepatogastricum et hepatoduodenale) are part of the lesser omentum (omentum minus) and extend from the area of the porta to the lesser curvature of the stomach and the proximal part of the duodenum.
The biliary system
Laboratory rats have no gallbladder or common bile duct (ductus choledocus); they only have a common hepatic duct (ductus hepaticus communis), which is formed by right and left hepatic duct (ductus hepaticus dexter et sinister) in the portal area, superficially to the portal and arterial branches, because this is an extrahepatic biliary system. Each lobe of the liver has its own biliary ducts, presenting as intrahepatic biliary tracts. The right hepatic duct is formed by the confluence of the duct from the caudate and right lobe. The left hepatic duct drains the bile from the left and quadrate lobe of the liver. In some cases, the quadrate lobe is drained by ducts of the right or left lateral lobe. Caudate process drain bile into the duct of the right lateral lobe and the caudate lobe, or directly into the main biliary tract. All hepatic ducts are fused and form a common hepatic duct (ductus hepaticus communis), which leads to the duodenum (Figure 2). The common hepatic duct is situated ventrally and to the right of the portal vein (v. portae).
Figure 2.
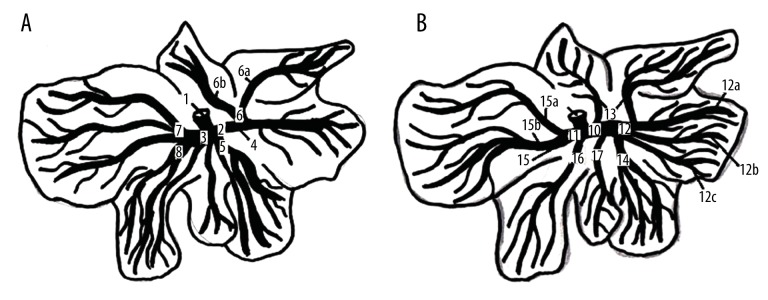
The division of the hepatic artery (A) and portal vein (B) in the liver parenchyma of the laboratory rat. 1. Hepatic artery (a. hepatica), 2. Right branch of the hepatic artery (r. dexter), 3. Left branch of the hepatic artery (r. sinister), 4. Right lateral branch (r. dexter lateralis), 5. Right medial branch (r. dexter medialis), 6. Artery of caudate lobe (a. lobi caudati), 6a. Branch for papillary process, 6b. Branch for caudate process, 7. Left lateral branch (r. sinister lateralis), 8. Left medial branch (r. sinister medialis), 8a. Branch for quadrate lobe, 9. Portal vein (v. portae), 10. Right branch of the portal vein (r. dexter), 11. Left branch of the portal vein (r. sinister), 12. Right lateral branch (r. dexter lateralis), 12a. Dorsal branch of the right lateral lobe (r. dorsalis lobi lateralis dextri), 12b. Interomedial branch of the right lateral lobe (r. intermedius lobi lateralis dextri), 12c. Ventral branch of the right lateral lobe (r. ventralis lobi lateralis dextri), 13. Branches of the caudate lobe (rr. lobi caudati), 14. Right medial lobe (r. dexter medialis), 15. Left lateral branch (r. sinister lateralis), 15a. Dorsal branch of the left lateral lobe (r. dorsalis lobi lateralis sinistri), 15b. Ventral branch of the left lateral lobe (r. ventralis lobi lateralis sinistri), 16. Right medial branch (r. dexter medialis), 17. Branches of the quadrate lobe (rr. lobi quadrati).
The vessels of the liver
The liver has a typical dual blood supply, which is the common feature of the hepatic vasculature and clearly determines the regulation and distribution of the blood flow. There is a very close relationship between the 2 vascular systems of the rat liver: hepatic arterial and venous blood supply. The hepatic artery is the vessel of resistance, but extrahepatic and hepatic veins are vessels of capacities. The hepatic arterial blood flow carries well-oxygenated blood to the liver. The portal veins are partly deoxygenated, but nutrient-rich splanchnic blood flows from the capillary system of the unpaired organs of the digestive system. In rats there are no identified variations in vessel origins, but we described much variability of the liver vasculature.
The hepatic arterial system
Our results show that the rat is supplied by the hepatic artery (a. hepatica), which is a branch of the celiac artery (a. ceoliaca).
The Arteria coeliaca is the first visceral, unpaired branch, which leaves the ventral wall of the abdominal aorta, just below the diaphragm pillars. It has a short, unpaired trunk, which arises at the level of the third lumbar vertebra. This artery supplies the stomach, spleen, liver, pancreas, and cranial part of the duodenum, and it branches into the splenic artery (a. lienalis), left gastric artery (a. gastrica sinistra), and hepatic artery (a. hepatica).
The main trunk of the hepatic artery (Figure 3) continues cranially between portal vein (v. portae) and right side of the papillary process (processus papillaris) to the porta (porta hepatis). Before the hepatic artery passes the lesser curvature of the stomach (curvatura ventriculi minor), it gives rise to the gastroduodenal artery (a. gastroduodenalis). This gastroduodenal artery is divided into the cranial pancreaticoduodenal artery (a. pancreaticoduodenalis cranialis) of the pancreas and duodenum, and the second branch of the gastroduodenal artery is the right gastroepiploic artery (a. gastroepiploica dextra). This branch is linked by anastomosis with the left gastroepiploic artery (a. gastroepiploica sinistra) on the greater curvature of the stomach (curvatura ventriculi major). Then the hepatic artery runs caudally across the pylorus to the greater curvature of the stomach; we named this branch for the pylorus of the right gastric artery (a. gastrica dextra). The right gastric artery is linked by anastomosis with the left gastric artery (a. gastrica sinistra) on the lesser curvature of the stomach. From the hepatic artery there arise many small branches to the pancreas (rr. pancreatici). One of the branches from the terminal division of the hepatic artery supplies the caudal part of the esophagus with its cranial and caudal branches.
Figure 3.
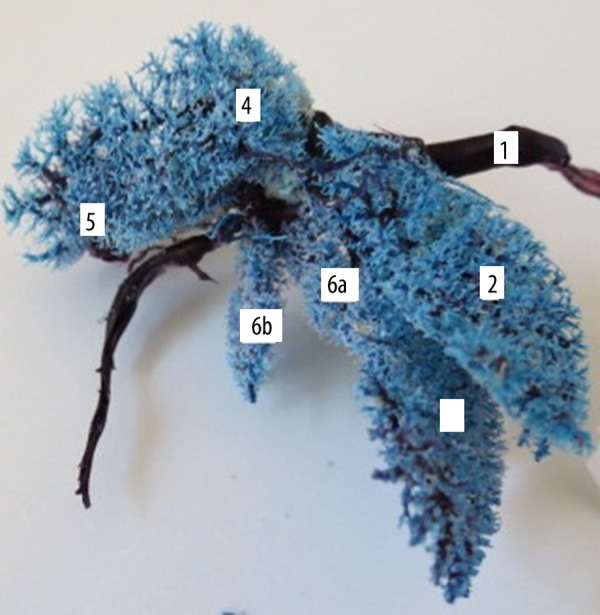
Intrahepatic venous division in rat liver, corrosion cast (ventral view, casting medium Duracryl Dental®). 1. Caudal vena cava (v. cava caudalis), 2. Left lateral branch (r. sinister lateralis), 3. Left medial branch (r. sinister medialis), 4. Right lateral branch (r. dexter lateralis), 5. Right medial branch (r. dexter medialis), 6a. Branches of retroventricular part of the papillary process, 6b. Branches of the caudate lobe of preventricular part of the papillary process.
The hepatic artery continues at the posterior surface of the portal vein (v. portae). At the portal of the liver, the hepatic artery is divided into right and left branches (r. dexter et sinister). The arterial limbs continue on the anterior surface of the portal vein, pass behind the common hepatic duct (ductus hepaticus communis), and accompany a parallel course with the branches of the portal vein as they divide into their main lobar division (Figure 4). In some cases, 2 or 3 lobar arteries follow a single lobar branch of the portal vein. The right branch of the hepatic artery is divided into the right lateral branch (r. dexter lateralis) and right medial branch (r. dexter medialis). The right lateral branch supplies the right lateral lobe (lobus hepatis dexter lateralis) and includes the caudate lobe (lobus caudatus), which is supplied by the caudate lobe artery (a. lobi caudati). The caudate lobe artery is a branch of the right lateral branch. This artery supplies both processes of the caudate lobe, and the caudate and papillary process (processus caudatus et papillaris). The right medial branch is represented by 2 or more vessels supplying the right medial lobe (lobus hepatis dexter medialis), dorsal part of the quadrate lobe (lobus quadratus), and part of the left medial lobe (lobus hepatis sinister medialis). The left branch of the hepatic artery is divided into the left lateral and medial branch (r. sinister lateralis et medialis). The left lateral branch is represented by more vessels, supplying the large, left lateral lobe of the liver (lobus hepatis sinister lateralis). The many left medial branches supply the left medial and quadrate lobes of the liver (lobus hepatis sinister medialis et lobus quadratus) (Figure 4).
Figure 4.
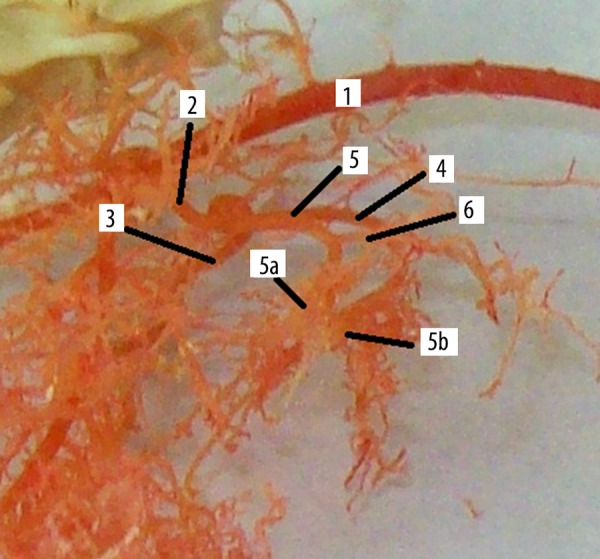
Origin and division of hepatic artery of rat liver, corrosion cast (lateral view, casting medium Duracryl Dental®). 1. Abdominal aorta (aorta abdominalis), 2. Celiac artery (a. coeliaca), 3. Left gastric artery (a. gastrica sinistra), 4. Splenic artery (a. lienalis), 5. Hepatic artery (a. hepatica), 5a. Right branch of the hepatic artery (r. dexter), 5b. Left branch of the hepatic artery (r. sinister), 6. Intestinal branch of the hepatic artery.
We measured the length and diameter of the hepatic artery (a. hepatica) and our results are listed in Table 2.
Table 2.
Measured dates (length and diameter) of the hepatic artery and portal vein in laboratory rat.
| Number of animals | Length (mm) | Diameter (mm) |
|---|---|---|
| Hepatic artery | ||
| 4 | 4–5 | 0.2–0.3 |
| 3 | 3–6 | 0.3–0.5 |
| 3 | 4–7 | 0.5–0.7 |
| Portal vein | ||
| 3 | 3–6 | 1–3 |
| 4 | 4–8 | 3–4 |
| 3 | 3–7 | 4–5 |
The extrahepatic venous system
The venous system is more important, along with the portal venous circulation, which is formed by the portal vein (v. portae) and its tributaries. The v. porta is a valveless afferent vessel which drains the splanchnic blood flow from the capillary system of the unpaired organs of the abdominal cavity to the liver. The extrahepatic part of the portal vein is located on the posterior and lateral to the hepatic artery and common hepatic duct. We found many variations of tributaries in the extrahepatic part of portal vein.
Our results indicate many variations in confluence of v. portae. Table 3 showed our findings in tributaries of extrahepatic portal venous system.
Table 3.
Variations in confluence of extrahepatic part of portal vein in laboratory rat.
| Four tributaries (23%) | Splenic vein |
| Cranial pancreaticoduodenal vein | |
| Cranial mesenteric vein | |
| Left gastric vein | |
| Three tributaries (64%) | Splenic vein |
| Cranial pancreaticoduodenal vein | |
| Cranial mesenteric vein | |
| Two tributaries (13%) | Splenic vein |
| Cranial mesenteric vein |
Eighty percent of the splenic vein (v. lienalis) is formed by 3 tributaries: the left gastric vein (v. gastrica sinistra), right gastroepiploic vein (v. gastroepiploica dextra) or left gastroepiploic vein (v. gastroepiploica sinistra), and cranial pancreaticoduodenal vein (v. pancreaticoduodenalis cranialis).
The left gastric vein (v. gastrica sinistra) is a 23% independent branch of the portal vein, 73% is tributary to the splenic vein (v. lienalis), and 4% goes into a branch of the cranial pancreaticoduodenal vein (v. pancreaticoduodenalis cranialis).
The cranial pancreaticoduodenal vein (v. pancreaticoduodenalis cranialis) is an 87% independent branch of the portal vein, 9% is tributary to the splenic vein (v. lienalis), and 4% of the cranial pancreaticoduodenal vein (v. pancreaticoduodenalis cranialis) enters into the left gastric vein (v. gastrica sinistra).
The cranial mesenteric vein (v. mesenterica cranialis) is formed by branches: the cranial pancreaticoduodenal vein (v. pancreaticoduodenalis cranialis), jejunal veins (vv. jejunales), ileocolic vein (v. ileocolica), right colic vein (v. colica dextra), and middle colic vein (v. colica media).
In the course of the jejunal veins (vv. jejunales), we found many variations. Jejunal veins are formed by the fusion of the venous arcades, which drain the blood from the wall of the jejunum. Venous arcades were fused into the common trunks (trunci jejunales), which enter into the cranial mesenteric vein (v. mesenterica cranialis). There are 10–16 (10 in 12%, 11 in 25%, 13 in 12%, 15 in 38%, and 16 in 12%) in the jejunal trunks. We measured the length and diameter of the portal vein (v. portae) in rats and found some differences listed in Table 2.
The caudal mesenteric vein (v. mesenterica caudalis) is formed by the left gastric vein (v. colica sinistra) and cranial rectal vein (v. rectalis cranialis) in all cases.
The intrahepatic venous system
The venous branches continue in the liver lobes and follows a parallel course with the branches of the hepatic artery as they divide into their main lobar divisions. In some lobes, we found not just one, but many, tributaries in liver lobes. All of this depends on the animals. The main trunk of the portal vein is ramified into the right and left tributary (r. dexter et sinister).
The right branch (r. dexter) of the portal vein has tributaries for lateral and medial parts of the right lobe of liver and for the caudate lobe.
The dorsal branch of the right lateral lobe (r. dorsalis lobi lateralis dextri) passes within the parenchyma of the dorsal part of the right lateral lobe of the liver, between the lateral lobe and caudate process of the liver. This branch has 2 terminal tributaries that ramify within the right dorsal angle of the right lateral lobe.
The intermediate branch of the right lateral lobe of liver (r. intermedius lobi lateralis dextri) is considered as the ventral continuation of the r. dorsalis lobi lateralis dextri. This branch is ramified into 2 or 3 tributaries in the middle part of the right lateral lobe.
The ventral branch of the right lateral lobe of liver (r. ventralis lobi lateralis dextri) has 3 tributaries. Along its course, it ramifies into 4 or 6 collateral branches for the residual for the right lateral lobe of liver. In some cases, another small branch, which enters directly to the ventral side of the portal vein, drains blood from the ventral part of the right lateral lobe.
The branches of the caudate lobe (rr. lobi caudati) are tributaries of the r. dorsalis lobi lateralis dextri or v. portae (Figure 5). The first, smaller branch is a branch of the portal vein or a branch of r. dorsalis lobi lateralis dextri, and it drains blood from the minor part of this lobe from the papillary process. The second, larger branch is always a tributary of the r. dorsalis lobi lateralis dextri, and this branch drains blood from the dorsal major part of the caudate lobe of liver from the caudate process.
Figure 5.
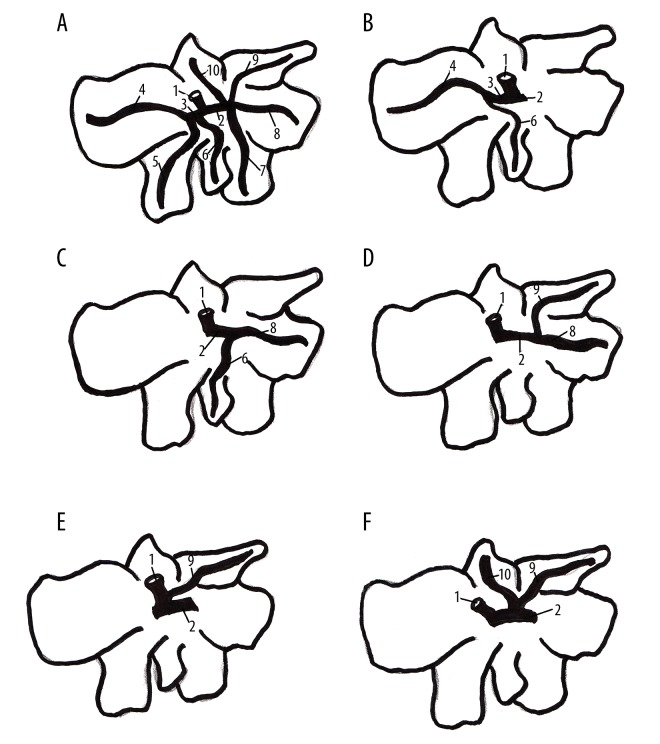
(A) Normal drainage of bile in rat liver, (B, C) Variations of bile drainage from quadrate lobe, (D–F) Variations of bile drainage from caudate lobe. 1. Common hepatic duct (ductus hepaticus communis), 2. Right hepatic duct (ductus hepaticus dexter), 3. Left hepatic duct (ductus hepaticus sinister), 4. Duct of left lateral lobe, 5, Duct of right lateral lobe, 6. Duct of quadrate lobe, 7, Duct of right medial lobe, 8. Duct of right lateral lobe, 9. Duct of caudate process, 10. Duct of papillary process.
The branch of the right medial lobe (r. lobi medialis dextri) is a tributary of the portal vein, close to the entry of the dorsal branch of the right lateral lobe. The ventral branch of the right medial lobe proceeds ventrally within the hepatic tissue of the right medial lobe. Along its course, it ramifies into several branches for the dorsal, middle, and ventral part of the right medial lobe.
The left branch (r. sinister) of the portal vein has tributaries for the lateral and medial part of the left lobe and for the quadrate lobe. The left branch is considered the main continuation of the portal vein. It passes in a ventral direction within the hepatic tissue of the dorsal part of the quadrate lobe and then goes between the quadrate and left medial lobes.
The dorsal branch of the left lateral lobe (r. dorsalis lobi lateralis sinistri) enters into the dorsal aspect of the left branch of the portal vein. This branch has several terminal tributaries that ramify within the parenchyma of the dorsal portion of the left lateral lobe of the liver.
The ventral lobe of the left lateral lobe (r. ventralis lobi lateralis sinistri) has 3 or 4 tributaries, which proceed laterally, crossing the corresponding branches of the hepatic arteries and draining blood from the ventral portion of the left lateral lobe.
The branches of the left medial lobe (rr. lobi mediales sinistri) are tributaries of the left branch of the portal vein. Only one large dorsal branch drains blood from the dorsal part of the left medial lobe of the liver. Other branches are smaller and they drain blood from the remaining part of the left medial lobe of the liver.
The branches of the quadrate lobe (rr. lobi quadrati) enter to the left branch of the portal vein opposite the entry of the branches for the left medial lobe of liver. We found 3 or 4 branches: the first 3 branches are smaller and drain blood from the dorsal portion of the quadrate lobe, while the last, larger, branch is the tributary of the remaining terminal part of the quadrate lobe (Figure 4).
The hepatic lymphatic system
The results of our study of liver lymphatic drainage were as follows. The lymph of the liver is drained in 2 ways. Lymph from the portal connective tissue drains to the hepatic lymph nodes (lnn. hepatici). The lnn. hepatici are located between the porta of liver and the trifurcation of the coeliac artery. There is usually a group of 3 lymph nodes. The lymph from the lnn. hepatici empties into the cisterna chyli (cisterna chyli) via the celiac trunk (truncus coeliacus). This lymphatic trunk accompanies the great vessels in the porta of the liver (v. portae, a. hepatica), nerves, and the hepatic duct. These lymph nodes also receive subcapsular applied dye from the visceral surface of the liver, identified by using dyes of different colors, showing that most of the lymph coming from the liver leads to the thoracic duct (ductus thoracicus). The second (and probably less significant) route of extrahepatic lymphatic drainage. The ink (another color) was applied under the liver capsule on diaphragmatic surface was found in the parathymic nodes. These lymph nodes (2 to 3 in number) in rats are situated on the lateral aspect of the thymic capsule. The lymph travels through the falciform ligament of the liver. Subsequently, the lymph from the parathymic lymph nodes flows into the subclavian veins (on both sides) through the mediastinal trunk.
Discussion
It is fundamental for experimental liver surgery to have complete information on not only the general liver anatomy, but also variations in morphological and vascular anatomy of the rat liver. The knowledge of the anatomical variability and an understanding of rat liver embryology would help to avoid critical complications when performing experimental surgery on this organ. The rigorous description of rat liver morphology and its vasculature is essential for experimental surgery in these laboratory animals. Many previous publications and descriptions have mentioned only partial results of rat liver anatomy [21,22]. We have submitted well-knit knowledge about morphology and vasculature of a rat liver, which is more important for performing experiments in this model of laboratory animal. A description of the nervous system of the liver in the rat has been left out, but can be found in other studies [23].
Many authors use different anatomical nomenclature of the rat liver [13,24–27]. Recent studies have compared the rat and human livers, showing that the lobes of the rat liver correspond to human liver divisions [28] (Table 4). Martins et al. [19], in a basic study, described and named these divisions of the rat liver: middle lobe, left and right lateral lobe, right lobe, and caudate lobe spiegel lobe, which is divided into anterior and posterior portions. According to Madrahimov et al. [22], the rat liver consists of 4 lobes, and Couinaud [28] reported that the human liver is divided into 8 segments. Research on the horizontal liver cleft by Ting and Lim illustrated the difficulties in liver segmentation using Couinaud’s classification [29]. The classical description of the pig liver by Popesko [30] divided the liver into a right medial and lateral hepatic lobe, left medial and lateral lobe, and quadrate and caudate lobe. The caudate lobe presented only the caudate process. Human and pig anatomy is very similar; therefore, the pig is now the most commonly studied potential source of organs and cells for use in humans [31,32] to overcome the severe shortage of human material for clinical transplantation [33].
Table 4.
The comparison of the rat and human liver divison, (Kogure et al., 1999).
| Rat liver division | Human liver division |
|---|---|
| The caudate lobe, paracaval potion | Segment I and VIII |
| The left lobe | Segment II |
| The middle lobe | Segment III, IV, V |
| The right lobe | Segment VI and VII |
The morphological relationship between the human and rat liver is still relatively undefined. Among laboratory animals, rat morphology is very similar to humans. The anatomy of the pig is very different from other domestic animals. More than 80 million years of evolution separates primates from rodents [18]. The functional liver morphology of the rat differs from that of human but is similar to that of the pig [26,34].
The above-described nomenclature and division of the rat liver is closely related to fissures of the liver. Many authors have described special notches or fissures in the hepatic parenchyma [16,19,27–29].
A study by Martins et al. [19] reported that the intrahepatic bile ducts in rats are the same as the bile ducts in human. The extrahepatic bile ducts are more superficial and the common hepatic duct is longer in rats than in the human common bile duct. There are many morphological variations in secondary branches for the liver lobes. In contrast to other laboratory animals, pigs, and humans, the rat liver does not have a gallbladder. Some other mammals, such as horses and deer, also lack a gallbladder [19].
The results of research by Martins et al. [19] show that the rat liver, which weighs about 13.6 g, accounts for approximately 5% of the total body weight, which averages about 250–300 g. A study by Yeakel [35] of increased weight of the liver in rats with induced and transplanted tumors, showed that in female rats weighing 81–342 g, the weight of the liver was 7.04–19.12 g. In male rats weighing 89–332 g, the liver weight was 4.93–17.81 g. Others authors reported the weight of the rat liver at 9.7–9.9 g in rats weighing 255.7–259.8 g [36]. Martins et al. [19] described different values for morphometric dates. Davies et al. [37], in a study comparing laboratory animals and human physiological parameters, showed that the volume of the rat liver was approximately 19.6 ml. The blood flow through the rat liver, through the hepatic artery and portal vein, was similar with our results. The weight of the liver in carnivores is 3–4% of body weight, about 2% in omnivores, and about 1–1.5% in herbivores [38].
The origin, division, and course of the celiac trunk observed in our study was also reported by Hebel et al. [16], Nejedlý [39], Popesko et al. [25], and Baláž et al. [40]. Pigs have a similar division of the celiac trunk [29]. Lenemann et al. [41] named the branch of the celiac trunk for the oesophagus a. eosphagea. The celiac trunk and its branches are similar to those of humans, but the area supplying single arteries is different. Anatomically, the celiac trunk in humans is subject to a wide range of variation [42–44]. The division of the celiac trunk into 3 branches in humans was first described by Haller [45]. The anatomic variations of the celiac trunk in humans have been described by many authors [46,47]. In 1928 Adachi [48] studied the celiac trunk of human cadavers and classified 6 types with 28 forms. An anatomical examination by Malnar et al. [49] showed that in most of cases, the celiac trunk in rats is divided into the splenic artery and the common hepatic artery, while the left gastric artery arises as a first branch and originates between aorta, all over the celiac trunk, up to a bifurcation.
The division of the hepatic artery outside the liver observed in the present was also reported by many authors [16,39,40,50]. Osman et al. [51] and Nickel et al. [52] described some differences in division of the hepatic artery in pigs. Many investigators, however, revealed that the basic arterial structures in the liver are quite similar among several species, including humans [53]. Differences in the blood supply of the human liver were described by many authors [47,54].
The origin and course of the rat extrahepatic venous system are similar to humans, but the branching of the rat portal vein is different from humans. In the rat, the portal vein is trifurcated, while in humans it is bifurcated [19,55]. There is not communication between the portal and venous system of the rat liver, but each of the 6 sectors receives a portal branch and has its own venous tributary [26]. Malinovsky et al. [56] described differences in the extrahepatic division of the portal vein in rats. Many authors have described 4 main branches of the portal vein in pigs [51,52].
The hepatic lymphatic system is an integral part of the liver microcirculation with immunological control system [57,58]. The liver produces a large volume of lymph, which is estimated to be 25% to 50% of the lymph flowing through the thoracic duct, 80% or more of hepatic lymph drains into portal lymphatic vessels, while the remainder drains through sublobular and capsular lymphatic vessels traveling in a cranial direction into the thoracic cavity (along the internal thoracic vessels to the parathymic nodes) through the ligaments attaching the liver to the diaphragm [59]. Lymphatics accompany the hepatic veins in humans, leaving the liver as lymphatic vessels that continue in the wall of the inferior vena cava [60]. Although experimental studies by some authors [61] reported that some of the lymphatics traverse the diaphragm with the v. cava caudalis and enter the retrocaval, para-aortic, or mediastinal lymph nodes.
Many authors have used different nomenclature for the lymphatic system [62–65]. The lymphatic vascular system and its role is closely related with/in many pathological conditions. An impairment of its function causes lymphedema and ascites [57]. Another important fact is that liver is one of the most frequent sites of tumor formation [64]. Tumor staging prognosis and therapeutic approaches for cancer are most often based on the extent of involvement of regional lymph nodes [59]. Therefore, detailed knowledge of the hepatic lymphatic drainage and lymphatic communications from the liver to the adjacent lymph nodes is crucial.
Conclusions
Results of our research show that knowledge of morphology and vasculature of the rat liver help to improve the possible in development of experimental surgery, organ transplantation, and education in this sector of medicine. This information is useful for the planning of experimental surgery and for cooperation with other investigation methods to fight human liver diseases.
Footnotes
Conflict of interests
The authors declare no conflict of interests.
Source of support: The study was supported by the project of the Slovak Ministry of Education, grant number VEGA 1/0046/16. This work was realized within the framework of the project ITMS No. 26220120066 “Center of excellence for biomedical technologies”, which is supported by the Operational Program “Research and Development” financed through the European Regional Development Fund
References
- 1.Ma Y, Guo ZY. Use of surgical techniques in the rat pancreas transplantation model. Hepatobiliary Pancreat Dis Int. 2008;7:156–60. [PubMed] [Google Scholar]
- 2.Majorová E, Mráz M, Drahovský P. Syndróm krátkeho čreva. Pediatrie Pro Praxi. 2004;4:209. [in Czech] [Google Scholar]
- 3.Li YX, Li JS, Li N. Improved technique of vascular anastomosis for small intestinal transplantation in rats. World J Gastroenterol. 2000;2:259–62. doi: 10.3748/wjg.v6.i2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes FSC, Cartucho DJF, Cabrita AMS, Patritio JAB. Techniques of intestinal transplantation in rat. Microsurg. 1998;7:424–29. doi: 10.1002/(sici)1098-2752(1998)18:7<424::aid-micr7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Galvao FHF, Santos RMN, Machado MAC, et al. Simplified rat model of intestinal transplantation. Transplant. 2005;10:1522–23. doi: 10.1097/01.tp.0000184448.86704.c8. [DOI] [PubMed] [Google Scholar]
- 6.Welch CW. A note on the transplantation of the whole liver in dogs. Transplant Bull. 1955;2:54–55. [Google Scholar]
- 7.Denmark SW, Shaw BW, Jr, Starzi TE, Griffith BP. Veno – venous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum. 1983;34:380–82. [PMC free article] [PubMed] [Google Scholar]
- 8.Swindle MM, Smith AC, Hepburn BJ. Swine as model in experimental surgery. J Invest Surg. 1988;1:65–79. doi: 10.3109/08941938809141077. [DOI] [PubMed] [Google Scholar]
- 9.Kelly DM, Demetris AJ, Fung JJ, et al. Porcine partial liver transplantation: A novel model of the “small-for-size” liver graft. Liver Transpl. 2004;10:253–63. doi: 10.1002/lt.20073. [DOI] [PubMed] [Google Scholar]
- 10.Overturf K, al-Dhalimy M, Ou CN, et al. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;5:1273–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Arey LB. Throttling veins in the livers of certain mammals. Anat Rec. 1941;1:21–33. [Google Scholar]
- 12.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;3:374–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Moawad RS, Hegab AS, Barey MAE, Sabry RM. Prenatal development of the liver in albino rat. Brit J Sci. 2014;1:1–20. [Google Scholar]
- 14.Enzan H, Takahashi H, Kawakami M, et al. Light and electron microscopic observations of hepatic hematopoiesis of human fetuses II. Megakaryocytopoiesis. Acta Pathol Jpn. 1980;6:937–54. doi: 10.1111/j.1440-1827.1980.tb03282.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoldas A, Dayan MO. Morphological characteristics of renal artery and kidney in rats. ScientificWorldJournal. 2014;10:1–7. doi: 10.1155/2014/468982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebel R, Stromberg MW. Digestive system. In: Hebel R, Stromberg MW, editors. Anatomy of the Laboratory Rat. Baltimore: Williams and Wilkins Co.; 1986. pp. 46–57. [Google Scholar]
- 17.Godlevwski G, Gaubert-Cristol R, Prudhomme M, et al. Comparison of the liver and biliary duct development in man and in the rat at the end of the embryonic period. Morphol. 1998;258:11–14. [PubMed] [Google Scholar]
- 18.Lametschwandner A, Lametschwandner U, Weiger T. Scanning electron microscopy of vascular corrosion casts-technique and applications: Updated review. Scan Micro. 1990;4:889–940. [PubMed] [Google Scholar]
- 19.Martins PNA, Neuhaus P. Surgical anatomy of the liver, hepatic vasculature and bile ducts in the rat. Liver Int. 2007;3:384–92. doi: 10.1111/j.1478-3231.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- 20.Danko J, Šimon F, Artimová J. Apparatus Digestorius, Angiologia, Systema Lymphaticum. In: Danko J, Šimon F, Artimová J, editors. Nomina Anatomica Veterinaria. Košice: UVLF; 2012. pp. 72–150. [Google Scholar]
- 21.Martins PNA, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int. 2008;1:3–11. doi: 10.1111/j.1478-3231.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- 22.Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuneki K, Ichibara K. Electron microscope study of vertebrate liver innervations. Arch Histol Jpn. 1981;1:1–13. doi: 10.1679/aohc1950.44.1. [DOI] [PubMed] [Google Scholar]
- 24.Flower MW. Sur la disposition et la nomenclature des lobes du foi chez les mammiferes. J de Zool. 1872;1:420–25. [in French] [Google Scholar]
- 25.Popesko P, Rajtová V, Horák J. The rat. In: Popesko P, Rajtová V, Horák J, editors. Colour Atlas of Anatomy of Small Laboratory Animals. Bratislava: Príroda; 1989. pp. 46–103. [Google Scholar]
- 26.Lorente MA, Aller J, Rodriguez J, et al. Surgical anatomy of the liver in Wistar rats. Sur Res Comm. 1995;17:113–21. [Google Scholar]
- 27.Kongure K, Ishizaki M, Nemoto M, et al. A comparative study of the anatomy of the rat and human liver. J HepatoBiliary Pancreat Surg. 1999;2:171–75. doi: 10.1007/s005340050101. [DOI] [PubMed] [Google Scholar]
- 28.Couinaud C. The paracaval segments of the liver. J HepatoBiliary Pancreat Surg. 1994;2:145–51. [Google Scholar]
- 29.Ting YH, Lim KS. Horizontal liver cleft: A rare anatomic variant. Am J Case Rep. 2014;15:401–3. doi: 10.12659/AJCR.892274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popesko P. Apparatus digestorius. In: Popesko P, editor. Anatómia hospodárskych zvierat. Bratislava: Príroda; 1992. pp. 299–304. [in Czech] [Google Scholar]
- 31.Cooper DKC, Gollackner B, Sachs DH. Will pig solve the transplantation backlog. Annu Rev Med. 2002;53:133–47. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DKC. Outwitting evolution – progress towards clinicl xenotransplantation. J Surg. 2003;1:9–14. [Google Scholar]
- 33.Mollnes TE, Fiane AE. Perspectives on complement in xenotransplantation. Mol Immunol. 2003;40:135–43. doi: 10.1016/s0161-5890(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 34.Zanchet DJ, Montero EFZ. Pig liver sectorization and segmentation and virtual reality depiction. Acta Chir Bras. 2002;6:381–87. [Google Scholar]
- 35.Yeakel EH. Increased weight of the liver in wistar albino rats with induced and transplanted tumors. Cancer Res. 1948;8:392–96. [Google Scholar]
- 36.Noorafshan A, Karbalay-Doust S, Ardekani FM. High doses of nandrolone decanoate reduce volume of testis and length seminiferous tubules in rats. Acta Path Micro et Im Scan. 2005;2:122–25. doi: 10.1111/j.1600-0463.2005.apm1130205.x. [DOI] [PubMed] [Google Scholar]
- 37.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharmaceut Res. 1993;7:1093–95. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 38.Mc Gavin MD, Zachary JF. Liver, biliary system and exocrine pancreas. In: McGavin MD, Zachary JF, editors. Pathologic basis of veterinary disease. St Louis, Missouri: Mosby Elsevier; 2007. pp. 414–22. [Google Scholar]
- 39.Nejedlý K. Cévní soustava. In: Nejedlý K, editor. Biologie a soustavná anatomie laboratorních zvířat. Praha: SPN; 1965. pp. 328–40. [in Czech] [Google Scholar]
- 40.Baláž JP, Mergental H. Cévní system. In: Baláž JP, Mergental H, editors. Transplantace v experiment. Praha: Galén; 2006. pp. 25–29. [in Czech] [Google Scholar]
- 41.Lenemann F, Burton S. The hepato-esophageal artery of the rat: Brief report. Acta Anat. 1967;68:334–43. doi: 10.1159/000143038. [DOI] [PubMed] [Google Scholar]
- 42.Watson WC, Sadikali F. Celiac axis compression. Experience with 20 patient and a critical appraisal of the syndrome. Ann Inter Med. 1977;86:278–84. doi: 10.7326/0003-4819-86-3-278. [DOI] [PubMed] [Google Scholar]
- 43.Bergman RA, Thompson SA, Afifi AK, Saadeh FA. Celiac trunk. In: Bergman RA, Thompson SA, Afifi AK, Saadeh FA, editors. Compendium of human anatomic variation Catalog, Atlas and World Literature. Munich: Urban & Schwarzenberg; 1988. [Google Scholar]
- 44.Bech FR. Celiac artery compression syndromes. Surg Clin North Am. 1997;2:409–24. doi: 10.1016/s0039-6109(05)70558-2. [DOI] [PubMed] [Google Scholar]
- 45.Haller A. Icones anatomicae in quibus praecipae partes corporis humani delineate proponuntur et arteriarum potissimum histiria continetur. Gottingen. Vandenhoeck. 1756;8:270. [in Latin] [Google Scholar]
- 46.Nelson TM, Pollak R, Jonasson O, Abcarian H. Anatomic variations of the celiac, superior mesenteric and inferior mesenteric arteries and their clinical relevance. Clin Ann. 1988;2:75–91. [Google Scholar]
- 47.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;1:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adachi B. Das Arteriensystem der Japaner. Verlag der Kaiserlich-Japanischen Universitat zu Kyoto. 1928;2:18–71. [in German] [Google Scholar]
- 49.Malnar D, Klasan GS, Miletić D. Properties of the celiac trunk – anatomical study. Coll Antropol. 2010;34:917–21. [PubMed] [Google Scholar]
- 50.Brand MI, Kononov A, Vladisavljevic A, Milson JW. Surgical anatomy of the celiac artery and portal vein of the rat. Lab Anim Sci. 1995;45:76–80. [PubMed] [Google Scholar]
- 51.Osman FA, Wally YR, El Nady FA, Rezk HM. Gross anatomical studies on the portal vein, hepatic artery and bile duct in the liver of the pig. J Vet Anat. 2008;1:59–72. [Google Scholar]
- 52.Nickel R, Schummer A, Seiferle E. A. hepatica. In: Nickel R, Schummer A, Seiferle E, editors. The circulatory system, the skin and the cutaneous organs of the domestic mammals. Berlin-Hamburg: Verlag Paul Parey; 1981. pp. 162–63. [Google Scholar]
- 53.Takasaki S, Hano H. Three-dimensional observations of the human hepatic artery (arterial system in the liver) J Hepatol. 2001;34:455–66. doi: 10.1016/s0168-8278(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 54.Pujahari AK. Problem of a rare anomalous hepatic artery during whipple procedure. Saudi J Gastroenterol. 2010;16:122–23. doi: 10.4103/1319-3767.61243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elias H, Petty D. Gross anatomy of the blood vessels and ducts within the human liver. Am J Anat. 1952;90:59–111. doi: 10.1002/aja.1000900104. [DOI] [PubMed] [Google Scholar]
- 56.Malinovský L, Navrátilová E. Origin of the v. portae and variability of its tributaries in laboratory animals III. The laboratory rat (Rattus norvegicus var. alba) F Morph. 1990;4:366–75. [PubMed] [Google Scholar]
- 57.Chung Ch, Iwakin Y. The vascular system in liver diseases: Its role in ascites formation. Clin Mol Hepatol. 2013;2:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec. 2008;6:643–52. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 59.Lupinacci RM, Paye F, Coelho FF, et al. Lymphatic drainage of the liver and its implications in the management of colorectal cancer liver metastases. Surg. 2014;4:239–45. doi: 10.1007/s13304-014-0265-0. [DOI] [PubMed] [Google Scholar]
- 60.Trutmann M, Sasse D. The lymphatics of the liver. Anat Embryol. 1994;3:201–9. doi: 10.1007/BF00234299. [DOI] [PubMed] [Google Scholar]
- 61.Szabó G, Magyar Z, Jakab F. The lymphatic drainage of the liver capsula and hepatic parenchyma. Res Exp Med. 1975;2:193–200. doi: 10.1007/BF01851185. [DOI] [PubMed] [Google Scholar]
- 62.Tilney NL. Patterns of lymphatic dranaige in the adult laboratory rat. J Anat. 1971;3:369–83. [PMC free article] [PubMed] [Google Scholar]
- 63.Blau JN, Gaugas JM. Parathymic lymph nodes in rats and mice. Immun. 1968;14:763–65. [PMC free article] [PubMed] [Google Scholar]
- 64.Banfalvi G. Role of parathymic lymph nodes in metastatic tumor development. Cancer Metastasis Rev. 2012;31:89–97. doi: 10.1007/s10555-011-9331-y. [DOI] [PubMed] [Google Scholar]
- 65.Sarsilmaz M, Gumusalan Y, Celík HH, et al. Classification of the thoracic and abdominal lymph nodes of the rat. Turk J Med Res. 1994;5:185–91. [Google Scholar]