Abstract
Background
This study examined the effect of kaempferol on uterine fibroids in vitro and the underlying mechanism, and investigated the potential of kaempferol as a clinical drug for the treatment of uterine fibroids.
Material/Methods
Uterine fibroid tissue and surrounding smooth muscle tissue were collected for primary culture. Different concentrations of kaempferol (12 μM, 24 μM, and 48 μM) were used to treat the cells for 24, 48, and 72 hours. Ethanol was used in the control group. A CCK-8 colorimetric assay was used to detect cell proliferation. Real-time PCR and immunoblot were used to detect estrogen receptor (ER), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF) levels in mRNA and protein.
Results
The differences in proliferation at different time points and concentrations of kaempferol were statistically significant. The inhibitory effect of kaempferol on mRNA levels of ER and IGF, and protein levels of ER, VEGF, and IGF-1 were positively correlated with kaempferol concentration. Changes in kaempferol concentration showed no effect on VEGF mRNA expression. Treatment with kaempferol significantly lowered myocardin levels in uterine fibroid tissue compared to normal uterine smooth muscle (P<0.05).
Conclusions
Kaempferol might be used for clinical treatment of uterine fibroids due to its inhibitory effect on the proliferation of uterine fibroids cells.
MeSH Keywords: Embryo Implantation, Delayed; Kaempferols; Uterine Artery Embolization
Background
Uterine fibroids are benign tumors targeting childbearing women of 30 to 50 years old. Although it is considered a hormone-dependent disease, the causes of uterine fibroids remain unclear [1]. Surgical resection is widely used for treatment of uterine fibroids, but surgical resection definitely affects women’s health. Non-surgical therapies have not shown satisfactory effects [2,3]. The rising incidence of uterine fibroids in recent years has triggered studies of the efficacy of various drugs on uterine fibroids and their working mechanisms. Flavonoids became one of the natural substances of interest because they have anti-inflammatory and anti-tumor effects and can be easily found in nature. Flavones and flavonols are two well-known monomers of flavonoids [4]. As an important agent with abundant flavonols, kaempferol is the most widely-used drug for the study of uterine fibroids [5]. Previous studies showed that kaempferol not only inhibited the proliferation of cancer cells, but also induced apoptosis [6]. Moreover, kaempferol was shown to have promising efficacy on multiple cancers [7], but it was still unclear how kaempferol influenced uterine fibroids.
Considering the complicated mechanisms underlying the inhibitory effects of drugs on cancer, various biomarkers will be considered in our study. According to previous findings [4], we proposed multiple factors might be involved in the efficacy of kaempferol, including ER, VEGF, IGF-1, and myocardin.
In summary, the present study focused on the effect of kaempferol on uterine fibroids in vitro to explore biomarkers related to such effects and demonstrate the underlying mechanisms.
Material and Methods
Material
Tissue collection
Uterine fibroid tissue and surrounding smooth muscle were collected from thirty women with uterine fibroids hospitalized in the Second People’s Hospital of Liaocheng who underwent subtotal hysterectomy or total hysterectomy from Oct. 2013 to Oct. 2014. Their average age was 47.7±2.3 years. The study was approved by the medical ethics committee of the Second People’s Hospital of Liaocheng. All women participating in this study signed a patient consent form.
Inclusion criteria: Women were included in this study if they 1) were diagnosed with uterine fibroids; 2) without hormone treatment within three months; 3) married; and 4) middle aged (45 to 50 years).
Exclusion criteria: Women were excluded from this study if they 1) were suffering from other gynecological diseases other than uterine fibroids; and 2) were suffering other medical conditions that might affect this study, such as hypertension and diabetes.
Reagents and instruments
Reagents: High glucose (10%) DMEM medium, 0.25% trypsin, FBS, CDT-FBS, type II collagenase, kaempferol (School of Public Health, Southeast University), PVDF membrane, mouse anti-human smooth muscle actin monoclonal antibodies, BCA protein assay kit, CCK-8 proliferation kit, protein extraction kit, tetrabromoethane buffer, ER antibody, IGF-1 antibody, VEGF antibody, ECL substrate kit, and β-actin monoclonal antibodies. Proteins were detected using horseradish peroxidase (HRP)-labeled goat anti-rabbit and mouse secondary antibodies and protein size markers. β-actin was detected as a loading control.
Instruments: ELISA microplate reader, fluorescence quantitative PCR cycler, UV spectrometer.
Methods
Cell culture [6]: After the removal of the uterus, fibroid tissue and neighboring normal tissue (1 cm3) were taken by the same operator under sterile condition. Three pieces of tissue were sampled for each case. The tissues were transferred to 50 mL centrifuge tubes with an appropriate amount of type II collagenase and incubated in a 37°C shaker for 2–6 hours until the tissues were completely digested, centrifuged at 1000 rpm for 5 minutes, and supernatant collected. Cells were resuspended with high glucose DMEM with 10% FBS at a density of 5×105 cells/ml and cultured in a 37°C incubator with 5% CO2. Trypsin (0.25%) was used to passage the cells at a 1:2 ratio when they reached 90% confluency. Cells at 2–5 generations were used in this study.
Experimental groups: the experimental cells were treated with kaempferol dissolved in absolute ethanol (12 μM, 24 μM, 48 μM) as an intervention. Control cells were treated with absolute ethanol alone.
Real-time quantitative RT-PCR [8]: Total RNA of cultured uterine leiomyoma cells was extracted with Trizol. The integrity and purity of the RNA was measured by agarose gel electrophoresis and UV spectrometry. RT-PCR was performed according to kit instructions to produce cDNA. The real-time PCR reaction system was as follows: 10 μL SYBR Green Mix, 10.6 μL primer 1, 20.6 μL primer 2, 2 μL cDNA solution, 6.8 μL H2O. The reaction conditions were: 2 minutes 94°C, followed by 35 cycles of 94°C 45 seconds, 56°C 45 seconds, 72°C 45 seconds. The final extension was 72°C, 7 minutes. Primer sequences used in this study are shown in Table 1.
Table 1.
Primer sequences for RT-PCR.
| mRNA | DNA sequences | PCR product |
|---|---|---|
| ER | F 5′-TGCCAAGGAGACTCGCTAG-3′ R 5′-CCTCTTCGTCTTTTCGTATCC-3′ |
249bp |
| IGF-1 | F 5′-GCTGGTGGATGCTCTTCAGTTC-3′ R 5′-AGCTGACTTGGCAGGCTTGAG-3′ |
184bp |
| VEGF | F 5′-GCAGAATCATCATCACGAAGTGGT-3′ R 5′-TGAAGATGTACTCGATCTCATCA-3′ |
253bp |
CCK-8 assay [9]: 100 μL of 3–5-generation cells were seeded to three 96-well plates at a density of 2×105/mL. Culture medium was removed after attachment, then 12, 24, or 48 μmol/L of kaempferol was used to treat the cells. Each concentration had 5 replicates. CCK-8 reagent (10 μL) was added to each well 2 hours before the end of the experiment and an ELISA microplate reader was used to read the absorbance at 450 nm. The proliferation index (PI%) was calculated as the average absorbance difference between experimental and control groups divided by the average absorbance of the control group.
Immunoblot [10]: equal amounts of protein extract were used for agarose gel electrophoresis according to their concentrations. The gel was soaked in transfer buffer for 10~20 minutes, and the proteins transferred to a nitrocellulose membrane which was soaked in deionized water for 5 minutes. Ponceau S was used to stain the nitrocellulose membrane and marker pen to mark the protein molecular weight standards. The nitrocellulose membrane was then blocked in 10% skim milk for 2 hours at room temperature. Primary antibody and HRP-conjugated secondary antibody were diluted with 5% nonfat milk and incubated with the membrane for 24 hours at 4°C and 2 hours at room temperature, respectively. The membrane was then exposed, developed, and fixed in a dark room. The protein expression level was quantified with statistical software.
Statistical analysis
SPSS13.0 was used for statistical analysis. All data were expressed as mean ±SD. The chi-square test was used for the study of count data. A paired t-test was used for analysis between groups. P<0.05 was considered significant.
Results
Effects of kaempferol on the proliferation of uterine fibroids
Kaempferol significantly inhibited the proliferation of uterine fibroid cells (P<0.05). The inhibitory effect was increased with increasing concentration and treatment time of kaempferol and reached a peak at 48 hours (Table 2).
Table 2.
Effects of kaempferol on the proliferation of uterine fibroids.
| Group | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|
| A450 | PI | A450 | PI | A450 | PI | |
| Control | 0.857±0.051 | – | 0.872±0.055 | – | 0.916±0.028 | – |
| Kaempferol group | ||||||
| 12 μml/L | 0.867±0.033* | 89.7 | 0.659±0.052*,& | 75.7 | 0.762±0.059*,&,@ | 83. 4 |
| 24 μml/L | 0.657±0.038*,** | 78.1 | 0.622±0.029*,& | 72.4 | 0.690±0.035*,**,@ | 75. 6 |
| 48 μml/L | 0.549±0.051*,**,# | 64.5 | 0.603±0.023*,** | 68.3 | 0.619±0.034*,**,#,@ | 68. 4 |
Compared to control group, P<0.05;
Compared to the group of 12 μmol/L with the same treatment time, P<0.05;
Compared to the group of 24 μmol/L with the same treatment time, P<0. 05;
Compared to the group of 24 hours treatment, P<0.05;
Compared to the group of 24 hours treatment, P<0.05.
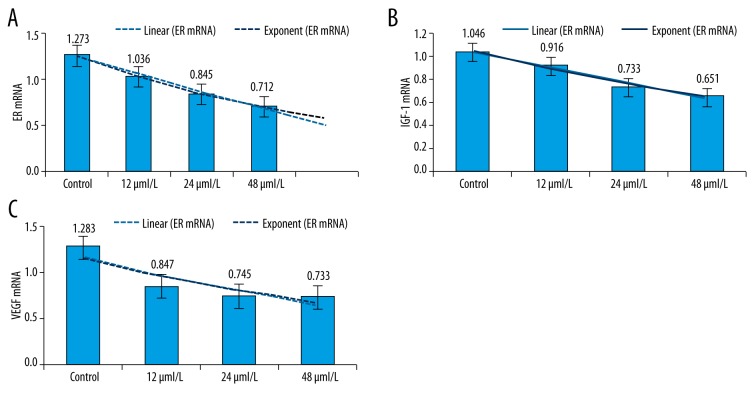
Effect of kaempferol on the mRNA expression of ER, IGF-1, and VEGF
Kaempferol treatment significantly lowered the mRNA levels of ER, IGF-1, and VEGF. The inhibitory effect of kaempferol on the mRNA levels of ER and IGF-1 was increased with the increase of kaempferol concentration (P<0.05). However, the inhibitory effect of kaempferol on the mRNA level of VEGF showed no change with the increase of kaempferol concentration (P>0.05) (Table 3, Figure 1).
Table 3.
Effect of kaempferol on the mRNA expression of ER, IGF-1, and VEGF.
| Group | ER mRNA | IGF-1 mRNA | VEGF mRNA |
|---|---|---|---|
| Control | 1.273±0.026 | 1.046±0.028 | 1.283±0.016 |
| Kaempferol group | |||
| 12 μml/L | 1.036±0.013* | 0.916±0.026 | 0.847±0.062*,& |
| 24 μml/L | 0.845±0.050* | 0.733±0.060*,** | 0.745±0.034*,& |
| 48 μml/L | 0.712±0.053*,**,# | 0.651±0.013*,** | 0.733±0.045*,& |
Compared to control group, P<0.05;
Compared to 12 μmol/L group, P<0.05;
Compared to 24 μmol/L group, P<0.05;
No significant difference between groups of different concentrations of kaempferol, P>0.05.
Figure 1.
Effect of kaempferol on the mRNA levels of ER, IGF-1, and VEGF. (A) ER mRNA; (B) IGF-1 mRNA; (C) VEGF mRNA.
Effect of kaempferol on the protein levels of ER, IGF-1, and VEGF
The effect of kaempferol on the protein levels of ER, IGF-1, and VEGF is shown in Figure 1. The protein band density is shown in Table 4, normalized to the band density of β-actin set to 1. Results indicated that the inhibitory effect of kaempferol on the protein levels of ER, IGF-1, and VEGF was increased with the increase of kaempferol concentration (P<0.05).
Table 4.
Effect of kaempferol on the protein levels of ER, IGF-1, and VEGF.
| Protein | Control | 12 μml/L | 24 μml/L | 48 μml/L |
|---|---|---|---|---|
| VEGF | 1 | 0.735±0.055* | 0.626±0.036* | 0.396±0.033*,**,# |
| IGF | 1 | 0.615±0.064* | 0.464±0.055*,** | 0.203±0.025*,**,# |
| ER | 1 | 0.926±0.047* | 0.876±0.012* | 0.624±0.066*,** |
| β-actin | 1 | 1 | 1 | 1 |
Compared to control group, P<0.05;
Compared to 12 μmol/L group, P<0.05;
Compared to 24μmol/L group, P<0.05.
Expression of myocardin in uterine fibroids and its surrounding smooth muscle tissue
Results showed that the expression of myocardin in uterine fibroids is significantly lower than that in surrounding smooth muscle tissue (Figures 2, 3).
Figure 2.
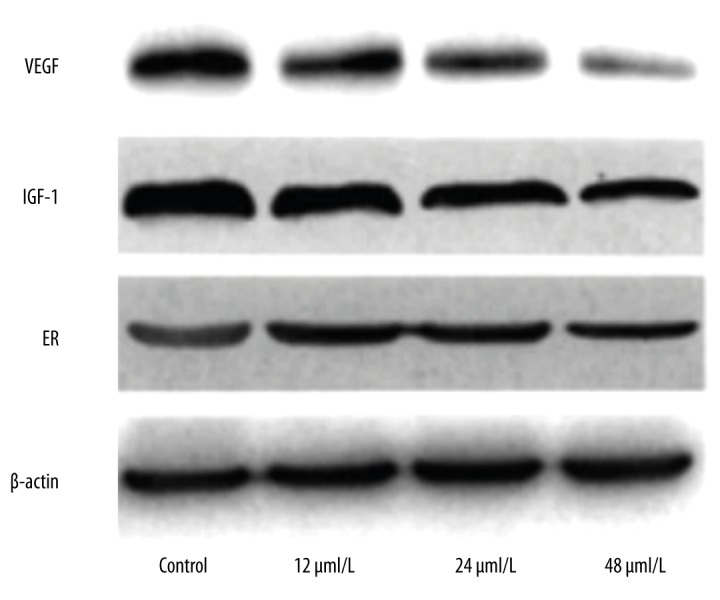
Effect of kaempferol on the protein levels of ER, IGF-1, and VEGF.
Figure 3.
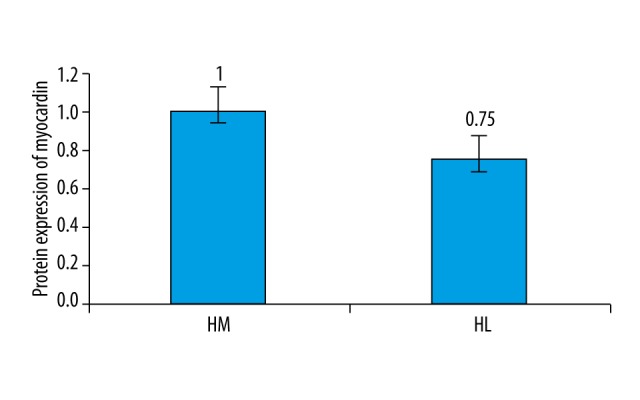
Myocardin has a lower expression level in uterine fibroids. HM – human uterine smooth muscle tissue; HL – human uterine fibroids.
Discussion
Uterine fibroids are one of the most common benign tumors of the female reproductive system. They are mainly found in women of reproductive age, and during and after menopause. Androgens, such as methyl testosterone tablets or testosterone propionate injections are widely used clinically as treatment for this condition. However, androgen, if used improperly, can easily cause women’s masculinization. Natural active flavonoids (mainly monomers, such as flavones and flavonols) are of high interest for treatment of uterine fibroids, and kaempferol is one of the most broadly studied flavonols with a plant source. Studies have proved that kaempferol effectively inhibits the proliferation of cancer cells including liver cancer, stomach cancer, and cervical cancer. The kaempferol used in this study was extracted and purified from the Chinese herbal medicine Huaijiao (Sophora japonica-Leguminosae). Previous studies showed that there is a positive correlation between the inhibitory effect of kaempferol on cell proliferation and kaempferol concentration [11,12]. In this study, we showed that cell proliferation varied with time of treatment and concentration, showing that kaempferol has an inhibitory effect on uterine fibroid cell proliferation and this inhibition is positively correlated with kaempferol concentration.
Previous studies showed that the inhibitory effect of kaempferol on tumor cell proliferation may be due to its induction of tumor cell apoptosis [13–15]. This study showed that kaempferol significantly lowered the levels of both mRNA and protein of ER, IGF-1, and VEGF. Estrogen not only promotes normal uterine smooth muscle cell mitosis, but also inhibits apoptosis of uterine fibroids. IGF-1 is one of the mediators of estrogen4[16], and has positive effects on the development of uterine fibroids. VEGF could promote ECM deposition to stimulate the growth of uterine fibroids. The experimental results showed that kaempferol treatment inhibited ER, IGF-1, and VEGF at both mRNA and protein levels, and thats suppression of ER, IGF-1, and VEGF increased with the increase of kaempferol concentration (P<0.05). The results indicated that kaempferol treatment not only inhibited the proliferation of human uterine fibroid cells in vitro, but also reduced the expression of ER, IGF-1, and VEGF at both mRNA and protein levels. The effect of kaempferol on apoptosis of human uterine fibroid cells is currently not known and needs to be further explored.
Previous studies showed that that the level of estrogen receptors (ER) in uterine fibroids is higher than in normal smooth muscle tissue, and mifepristone showed good therapeutic effect clinically, indicating that estrogen has a great influence on the development of uterine fibroids. IGF-1 has been shown to promote the growth of tumors through induction of mitogenesis. Myocardin is one of the key factors for the differentiation of cardiac and smooth muscle cells with the cooperation of SRF-CArG [17,18]. Myocardin not only exists in the cardiac muscle cells, but is also present in arteries, bladder tissue, intestinal tissue (both small intestine and colon), and uterus, among other tissues. In addition, it also exists in human normal muscle tissue and female uterine fibroids, suggesting that myocardin is involved in the occurrence and development of uterine fibroids. Therefore, detection of myocardin, ER, IGF-1, and VEGF can help explain the effect of kaempferol on the proliferation of uterine fibroids cells. Kaempferol can inhibit smooth muscle cell proliferation, so that the expression of uterine fibroid marker genes is suppressed. This leads to changes in cell structure and cell shape, loss of contractile functions, and abnormal cell proliferation. It is known that myocardin is a major cofactor of the SRF transcription factor and plays a very important role in the differentiation of smooth muscle cells [19,20]. This study also showed that the level of myocardin in uterine fibroids was significantly lower than that of smooth muscle tissue. Kaempferol treatment significantly lowered the level of myocardin in uterine fibroids.
Conclusions
Kaempferol can suppress the proliferation of uterine fibroid cells, lower the levels of ER, IGF-1, VEGF, and myocardin in vitro. Kaempferol has inhibitory effects on fertility, but it can be used for treating uterine fibroids in patients without fertility requirements and menopausal women.
Footnotes
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
Source of support: Departmental sources
References
- 1.Liang JF, Wu LN, Xiao H, et al. Use of myocardin in theclassification of mesenchymal tumors of the uterus. Int J Gynecol Pathol. 2010;29(1):55–62. doi: 10.1097/PGP.0b013e3181b1cdb0. [DOI] [PubMed] [Google Scholar]
- 2.Moore AB, Yu L, Swartz CD. Human uterine leiomyoma- derived fibro-blasts stimulate uterine leiomyoma cell proliferation and collagen typeI production,and activate RTKs and TGF beta receptor signaling incoculture. Cell Commun Signal. 2010;8(10):1–12. doi: 10.1186/1478-811X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y, Xu Q, Ren M, et al. Measurement of phenolic environmental estrogens in women with uterine leiomyoma. PLoS One. 2013;8(11):79–83. doi: 10.1371/journal.pone.0079838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Hu J, An W, et al. Detection and occurrence of chlorinated by-products of bisphenol A, nonylphenol,and estrogens in drinking water of China: Comparison to the parent compounds. Environ Sci Technol. 2013;47(19):10841–50. doi: 10.1021/es401504a. [DOI] [PubMed] [Google Scholar]
- 5.Islam MS, Protic O, Giannubilo SR, et al. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013;98(3):921–34. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 6.Shanle EK, Xu W. Review endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24(1):6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Sinha S, Dandre F, et al. Myocardin is a key regulator of Car G dependent transcription of muhiple smooth muscle marker genes. Circ Res. 2003;92(8):856–64. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi A, Agarwal C, Dwyer-Nield L, et al. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2cells. Mol Carcinog. 2012;51(10):832–42. doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- 9.Sosna O, Kolesár L, Slavčev A, et al. Thl/Th2 cytokine gene poly morphisms in patients with uterine fibroid. Folia Biol (Praha) 2010;56(5):206–10. [PubMed] [Google Scholar]
- 10.Kyung-a H, Park SE, Yi BR. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol Ausing microarray analysis. Lab Anim Res. 2011;27(2):99–107. doi: 10.5625/lar.2011.27.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam MS, Protic O, Stortoni P, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013;100(1):178–93. doi: 10.1016/j.fertnstert.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Bidgoli SA, Khorasani H, Keihan H, et al. Role of endocrine disrupting chemicals in the occurrence of benign uterine leiomyomata: Special emphasis on AhR tissue levels. Asian Pacific J Cancer Prev. 2012;13(11):5445–50. doi: 10.7314/apjcp.2012.13.11.5445. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Park SS, Kim WJ, et al. Gleditsia sinensis thorn extract inhibits proliferation and TNF-alpha-induced MMP-9 expression in vascular smooth muscle cells. Am J Chin Med. 2012;40(2):373–86. doi: 10.1142/S0192415X12500292. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Chang PS, Wang Z, et al. Activation of cardinac gene expression by Myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–62. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 15.Liang JF, Wu LN, Xiao H, et al. Use of myocardin in the classification of mesenchymal tumors of the uterus. Int J Gynecol Pathol. 2010;29(1):55–62. doi: 10.1097/PGP.0b013e3181b1cdb0. [DOI] [PubMed] [Google Scholar]
- 16.Cameron DA, Douglas S, Brown JE, et al. Bone mineral density loss during adjuvant chemothe rapy in pre-menopausal women with early breast cancer: Is it dependent on estrogen deficiency. Breast Cancer Res Treat. 2010;123(3):805–14. doi: 10.1007/s10549-010-0899-7. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y, Yin H, Tian RJ, et al. Differential effects of epidermal growth factor on smooth muscle cells derived from human myometrium and leiomyoma. Fertil Steril. 2011;96(4):1015–20. doi: 10.1016/j.fertnstert.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Tang RH, Zheng XL, Thomas EC, et al. Myocardin inhibits cellular proliferation by inhibiting NF-KB(P65)dependent cell cycle progression. Proc Natl Acad Sci USA. 2008;105:3362–67. doi: 10.1073/pnas.0705842105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasunori K, Tsuyoshi M, Ken IH, et al. Myocardin function as an effective inducer of growth arrest and differentiation in human uterine leiomyosarcoma cells. Cancer Res. 2010;70(2):501–11. doi: 10.1158/0008-5472.CAN-09-1469. [DOI] [PubMed] [Google Scholar]
- 20.Chen HM, Lin YH, Cheng YM, et al. Overexpression of integrin-β1 in leiomyoma promotes cell spreading and proliferation. J Clin Endocrinol Metab. 2013;98(5):E837–46. doi: 10.1210/jc.2012-3647. [DOI] [PubMed] [Google Scholar]